Abstract
Gynecological and breast tumors are one of the main causes of cancer-related mortalities among women. Despite recent advances in diagnostic and therapeutic methods, tumor relapse is observed in a high percentage of these patients due to the treatment failure. Late diagnosis in advanced tumor stages is one of the main reasons for the treatment failure and recurrence in these tumors. Therefore, it is necessary to assess the molecular mechanisms involved in progression of these tumors to introduce the efficient early diagnostic markers. Fokhead Box (FOX) is a family of transcription factors with a key role in regulation of a wide variety of cellular mechanisms. Deregulation of FOX proteins has been observed in different cancers. MicroRNAs (miRNAs) as a group of non-coding RNAs have important roles in post-transcriptional regulation of the genes involved in cellular mechanisms. They are also the non-invasive diagnostic markers due to their high stability in body fluids. Considering the importance of FOX proteins in the progression of breast and gynecological tumors, we investigated the role of miRNAs in regulation of the FOX proteins in these tumors. MicroRNAs were mainly involved in progression of these tumors through FOXM, FOXP, and FOXO. The present review paves the way to suggest a non-invasive diagnostic panel marker based on the miRNAs/FOX axis in breast and gynecological cancers.
Similar content being viewed by others
Background
Breast cancer (BC) is the most frequently diagnosed (2.3 million cases or 11.7% of all cancers) and the fifth cancer-related mortality (685,000 death or 6.9% of all cancer) worldwide [1]. Invasive ductal carcinoma is the main histological type of BC with prevalence rate of 50–80% [2,3,4]. Gynecological tumors are one of the most frequent and the major causes of cancer-related death in women worldwide. The five main types of gynecological cancer are cervical, uterine, vaginal, vulvar, and ovarian. Gynecological tumors incidence is about 1.4 of 19 million of all new cases with 0.7 out of 10 million deaths per year. Cervical cancer (CC) is the fourth cancer-related mortality in women globally. It is estimated that there were over 600,000 (3.1%) new cervical cancer cases and 342,000 (3.4%) deaths in 2020. Vaginal cancer is a rare cancer that accounts for 0.1% of new cases deaths globally. Vulvar cancer is also a rare gynecological cancer with an estimated incidence and mortality rate of 0.2% in 2020. Ovarian cancer also accounts for 1.6% of all new cases and 2.1% death worldwide [1].
Chemotherapy is the first therapeutic option for many types of cancers. However, chemo resistance remains still a challenge in tumor therapy [5, 6]. Over 90% of cancer-related deaths occur in patients with drug resistance [7, 8]. More than half of all patients suffering from cancer will undergo chemo-treatment. Resistance to chemotherapy develops in 50–96% of cancer patients within 6–9 months of treatment [9, 10]. This is a major obstacle to achieving a high rate of complete pathological response during cancer treatment [11]. About 85–90% of chemotherapy failures have been reported in breast and ovarian tumors [12]. The 5-year survival rate of BC is about 80–90%, and early diagnosis can give the best treatment results [13]. About 80–90% of endometrial cancers are early stage with good prognosis [14]. Around 20% of endometrial cancer patients who are treated with chemotherapy alone, experience regional recurrence [15]. Early diagnosis improves the survival rates of ovarian cancer patients up to 70% [16]. Absence of specific symptoms in the early stages of ovarian cancer and lack of effective biomarker screening are major reasons for the increasing number of patients being diagnosed with advanced stages and poor prognosis [17]. The 7-year survival rate for patients with end-stage ovarian cancer treated with chemotherapy is only 9% [18]. Tumor relapse due to treatment failure occurs in about 70% of the ovarian cancer cases [19]. About 30–50% of cervical cancer patients develop treatment failure due to regional recurrence [20]. Therefore, early detection of gynecological and breast tumors can be an important way to find the most efficient treatment. To find novel and efficient early detection markers, it is necessary to assess the molecular mechanisms involved in these cancers.
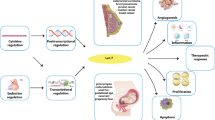
Forkhead box (FOX) transcription factors are involved in regulation of a wide variety of cellular mechanisms, such as cell proliferation, metabolism, migration, and tumor progression [21]. Mammalian FOX proteins are categorized into 19 subgroups including (FOXA to FOXS) based on sequence similarity outside and inside of the forkhead box [22]. Fox proteins possess highly conserved DNA-binding domains (FOX–DBD) but have different properties and functions [23]. Forkhead domain (FHD) structurally contains three β-strands, three n-ter α-helices (H1–3), and two loops, constructing butterfly winged helix in its C-terminal region (W1–2) [24]. FHD could interact with specific sequences, including the major groove of DNA and the H3 helix (recognition helix) [25]. In addition, the specificity of Forkheads DNA-binding is related to the variable region located on the junction of helices H2 and H3 and wings W1 and W2, which links to bases in the minor groove of DNA [26]. Association between FOXA1 and its target sequences has indicated that wings could regulate the DNA-binding affinity and specificity of the nominated domain [27]. This domain is also accountable for nuclear transportation. FOXE1, FOXA2, FOXF2, and FOXP3 have two nuclear localization sequences (NLS) at both ends of the domain site, which were located in H1 and W2 [28, 29]. FOX deregulation can be associated with diabetes mellitus, congenital disorders, and cancers. There was FOXC1 up-regulation in cervical cancer that was correlated with OS, stage, and metastasis. Down-regulation of FOXC1 inhibited cell proliferation and invasion through modulating the AKT cascade [30]. FOXA1 could inhibit the EMT process and angiogenesis by VEGF inhibition in cervical cancer [31]. FOXA2 plays a tumor-suppressive role in endometrial carcinoma which could suppress cell cycle progression through Myc [32]. FOXC2 regulates the MAPK and Akt pathways to down-regulate Bcl-2 while up-regulate Bax and CASP3 that intervene in the CDDP resistance of ovarian cancer [33]. Suppression of FoxM1 is a critical strategy to overcome the metastatic breast cancer progression [34]. MicroRNAs (miRNAs) are non-coding RNAs involved in prost-transcriptional regulation by mRNA degradation or translational inhibition [35]. They are also the key regulators of cell cycle, apoptosis, and differentiation [36]. MiRNAs biogenesis begins in the nucleus with the generation of polyadenylated and capped primary miRNAs (pri-miRNAs) transcripts via RNA polymerase II (PolII) [37]. Pri-miRNAs are further processed via Drosha/DGCR8 complex into single hairpin precursor miRNAs (pre-miRNAs) [38]. Pre-miRNAs are exported into the cytoplasm via the exportin 5 (XPO5) and cleaved by Dicer. This process contains the cleavage of the terminal loop, which leads to forming of a mature miRNA duplex intermediate [39, 40]. Mature miRNA duplex consists of two strands that can be loaded into the Argonaute (AGO) proteins. Moreover, the guide strand is located in the RNA-induced silencing complex (RISC), where it could target the complementary 3′-untranslated regions (UTR) of target mRNAs [41]. MiRNAs deregulations have been reported to be associated with tumor progression and drug resistance [42,43,44]. MiRNAs have a good potential as diagnostic biomarkers for the early detection of cancers [45, 46]. MiRNA profiles can distinguish not only the tissue of origin, but also the various subtypes of a particular cancer [45]. They have also a high stability in serum and blood plasma [47, 48]. Therefore, miRNAs can be used as minimally invasive tumor biomarkers [49, 50]. It has been shown that miRNAs are important regulators of the FOX proteins in cervical and breast tumors [51, 52]. Considering the pivotal role of FOX proteins in gynecological and breast tumors, we discussed the role of miRNAs–FOX axis as an important molecular mechanism involved in progression and metastasis in these tumors (Table 1).
FOXA, C, D, F, and G
FOXA protein family plays pivotal roles in the endoderm and endoderm development [53]. They are expressed in various tissues including the mammary gland, pancreas, liver, and the prostate to regulate cellular differentiation and organ function [54]. FOXA, FOXC, and FOXD have key roles in progression of gynecological and breast tumor cells that can be regulation by miRNAs (Fig. 1). Down-regulation of miR-204 expression was correlated with metastasis and tumor stage in BC. MiR-204 suppressed BC cell proliferation and invasion, while promoted apoptosis by FOXA1 targeting [55]. There was miR-590-3p up-regulation in EOC tumor tissues and plasma samples that was significantly correlated with high-grade tumors. FOXA2 down-regulation and VCAN up-regulation were significantly correlated with reduced survival rates in EOC patients. MiR-590-3p significantly promoted EOC cell proliferation, invasion, and in vivo growth via FOXA2 targeting and VCAN up-regulation [56].
FOXC is involved in promotion of tumor angiogenesis, EMT, and invasion. FOXC2 induces HGF–MET signaling to promote colorectal tumor cell invasion [57]. It is also involved in regulation of tumor glycolysis and lipid metabolism [58, 59]. FOXC proteins are required for the cardiovascular system and kidney development [60]. FOXC1 and FOXC2 deletion has been correlated with abnormal lymphatic remodeling [61, 62]. There were FOXC1 up-regulation and miR-495 down-regulation in endometrial tumor tissues compared with healthy tissue. MiR-495 inhibited cell proliferation and migration in endometrial cancer through apoptosis induction. FOXC1 inhibited the endometrial tumor cell proliferation and migration. There was a negative correlation among miR-495 and FOXC1 and inhibition of endometrial tumor progression [63].
FOXD is a crucial factor during the kidney and neuronal development [64,65,66]. FOXD3 has tumor-suppressive functions and inhibits angiogenesis in neuroblastoma and non-small cell lung cancer; however, its deficiency triggers EMT and promotes aggressiveness in breast tumor cells [67,68,69]. Long non-coding RNAs (lncRNAs) are involved in cell proliferation, apoptosis, and invasion [70, 71]. There was a significant LINC01133 up-regulation in CC tissues compared with paired adjacent normal tissues that was correlated with the increase of T stage and negative HPV infection. LINC01133 enhanced CC cell migration and proliferation by the regulation of miR-30a-5p/FOXD1 axis [72]. LIM domain kinase 1 (LIMK1) plays a key role in cytoskeletal remodeling by stimulation of ROCK1, Rac/p21 activated kinase 1, and CDC42/MRCK signaling pathways [73, 74]. FOXD3-AS1 was significantly up-regulated in CC cells and tissues compared with normal cervical epithelial cell lines and margins, respectively. It was significantly correlated with poor differentiation and lymph node involvement in CC patients. FOXD3-AS1 down-regulation significantly suppressed CC tumorigenic behavior in comparison with the control group. FOXD3-AS1 enhanced the cancerous phenotype of CC cells by miR-128-3p sponging and LIMK1 up-regulation [75].
Forkhead box F2 (FoxF2) is involved in promotion of organ development, extracellular matrix (ECM) synthesis, and EMT. There was significant miR-182 up-regulation in triple negative breast cancer (TNBC) tissues and cells. It increased TNBC cells proliferation and metastasis by CDH1 and FOXF2 targeting [76]. ADAMTS9-AS2 down-regulation was correlated with poor survival rate, advanced FIGO stage, and lymph-node involvement in ovarian cancer (OC) patients. ADAMTS9-AS2 significantly reduced OC cell proliferation and EMT process by miR-182-5p sponging and FOXF2 up-regulation [77].
Transforming growth factor-β (TGF-β) is an important regulator of different biological processes, including self-renewal, tissues homeostasis, and tumor metastasis [78, 79]. It has a dual function as a tumor suppressor in normal cells and early carcinomas, while oncogene in advanced invasive tumor cells [80, 81]. Forkhead Box G1 (FOXG1) plays an important role in cortical development [82, 83]. It acts as an oncogene by suppressing TGF-b-mediated anti-proliferative responses in tumor cancer cells by p21WAF1/CIP1 down-regulation [84, 85]. MiR-200b was up-regulated in cervical tumor tissues compared with normal margins that was associated with tumor progression through FoxG1 targeting [86].
FOXK, M, and N
Forkhead Box Class K (FOXK) proteins subfamily mediate cell proliferation, differentiation, apoptosis, and DNA repair [87]. There was FOXK1 up-regulation in BC tissues and cell lines that was correlated with TNM stage, tumor size, and invasion. FOXK1 induced cell migration by EMT regulation in breast tumor cells. FOXK1 significantly increased breast tumor cell proliferation via facilitating G1/S transition. MiR-365-3p is the negative regulator of FOXK1 during BC progression [88]. FOXM, FOXK, and FOXN have key roles in cell cycle progression in gynecological and breast tumor cells that can be regulation by miRNAs (Fig. 2). FOXM1 is a key regulator of G2/M-specific proteins in different tumor types [89,90,91]. Triple-negative breast cancer is a kind of Basal-like BC without the expression of estrogen receptor (ER), HER2, and progesterone receptor (PR) [92]. TNBC has several clinical manifestations including higher invasiveness, larger tumor size, and tumor load and higher susceptibility to metastasis in comparison with other subgroups [93]. It is more common in young women that accounts 9–16% of cases [94]. FOXM1 is involved in regulation of DNA replication and cell cycle phase transition during normal cell proliferation and tumorigenesis [95,96,97]. FOXM1 is also involved in positive regulation of different transcription factors, including cyclin A, cyclin B, and polo-like kinase1. FOXM1 can also reduce nuclear accumulation of p21cip1 and p27kip1 as the CDK inhibitor proteins through their deterioration [98, 99]. DEP domain containing 1 (DEPDC1) is a transcriptional suppressor that promotes anti-apoptotic pathway by activating the NF-κB pathway [100]. DEPDC1 up-regulation was observed in TNBC tissue and enhanced cell proliferation. MiR-26b functioned as a negative regulator of DEPDC1 in TNBC cells and also FOXM1 enhanced stimulating effects of DEPDC1 on tumor growth. DEPDC1 increased TNBC cell proliferation through FOXM1 up-regulation [101]. Eukaryotic elongation factor 2 kinase (eEF2K) negatively mediates phosphorylation and inactivation of eEF2, the protein that facilitates the elongation step of protein synthesis [102, 103]. eEF2K acts as a negative regulator of cell growth, protein synthesis, and translation. It is highly expressed in different cancers, and also is activated under stress conditions, including energy depletion or nutrient starvation [104]. MiR-34a down-regulation was correlated with longer overall survival of TNBC patients. MiR-34a was negatively associated with the expression of eEF2K which was also correlated with shorter survival of patient. MiR-34a inhibited cell growth and invasion via FOXM1/eEF2K axis in TNBC cells [105]. FOXM1 has a key role in regulation of angiogenesis and EMT [106]. MiR-802 suppressed BC cell proliferation via FOXM1 targeting [107]. Down-regulation of miR-342-3p reduced cervical tumor cell proliferation, growth, and migration by targeting FOXM1 [108]. There was miR-4429 down-regulation in CC tissues that was contributed with poor prognosis. MiR-4429 suppressed the CC cell proliferation and EMT, while promoted apoptosis through FOXM1 targeting [109]. PVT1 up-regulation was correlated with a shorter survival in OC patients. PVT1 suppressed OC cell apoptosis, while promoted cell viability and drug resistance via miR-149-5p/FOXM1 axis [110]. CircCLK3 promoted CC cell proliferation and invasion by miR-320a sponging and FoxM1 up-regulation. There was a significant circCLK3 up-regulation in CC tissues compared with normal samples. CircCLK3 was also significantly correlated with advanced FIGO stages and depth of stromal invasion [111]. There was significant SBF2-AS1 up-regulation in CC cell lines and tissues that was associated with lymph node involvement and progressive FIGO stage. Down-regulation of SBF2-AS1 significantly decreased CC cells survival. SBF2-AS1 suppression led to cell cycle arrest and reduced in-vivo growth, while increased apoptosis in CC cells. SBF2-AS1 promoted the CC progression by miR-361-5p sponging and FOXM1 up-regulation [112]. FBXL19-AS1 was up-regulated in BC cancer cells. Down-regulation of FBXL19-AS1 reduced BC cell proliferation, while increased apoptosis. FBXL19-AS1 promoted BC progression via miR-876/FOXM1 axis [113]. MiR671-5p suppressed cell proliferation and invasiveness by FOXM1 targeting. It down-regulated the genes that were involved in cell proliferation, such as GINS2, CDK2, and MCM10. MiR-671-5p was involved in cell cycle regulation through FOXM1 targeting which suppressed CDK2 and CCNB1 [114]. An inverse association has been reported between miR-216b and FOXM1 expression in CC cells. MiR-216b suppressed the CC cell proliferation by pRb, c-Myc, and CCND1 down-regulations, which were downstream targets of FOXM1 [115]. MiR-197, miR-374b, and miR-320 were also considered as the tumor suppressors that inhibited the CC cell proliferation and motility via FOXM1 suppression [116,117,118]. WT1-AS was significantly down-regulated in CC tissues and cell lines. WT1-AS inhibited the CC cell growth and motility by miR-203a-5p sponging that resulted in FOXN2 up-regulation [119].
FOXO
Forkhead box O (FOXO) protein family are the critical regulators of PI3K/Akt signaling which are involved in cell differentiation, cell cycle regulation, and tumor progression [120,121,122]. FOXO1 acts as a crucial downstream effector in PI3K/Akt signaling pathway. Activation of Akt, leads to phosphorylation of FOXO1 and its localization in the cytoplasm instead of the nucleus, consequently inhibition of FOXO1-regulated genes. FOXO1 target genes are involved in different biological processes, including carcinogenesis and cell cycle modulation (Fig. 3). It has been demonstrated that miR-181a was significantly up-regulated in CC cells in comparison with healthy cervical epithelium cell, and miR-181a played a key regulatory role in growth and invasion of CC cells via the PTEN/AKT/FOXO1 pathway. Inhibition of miR-181a enhanced p21 and p27 expression. Down-regulation of miR-181a significantly inhibited CC cell invasion by enhancing TIMP3 expression and down-regulation of MMP6 expression [123]. CCND1 is a key regulator of cell cycle progression that is also known as a growth-promoting factor in the G1 phase [124]. There was miR-96 up-regulation in CC tissues that was significantly associated with tumor staging, lymph nodes involvement, and differentiation. MiR-96 enhanced G1/S-phase transition, cell proliferation, and colony formation by FOXO1 targeting. Suppression of miR-96 promoted apoptosis and suppressed cell proliferation by up-regulating the p21 and p27 in CC cells [125]. Suppression of miR-135b reduced CC cell growth through FOXO1, p21, and p27 up-regulations while CCND1 down-regulation [124]. There was significant miR-196a up-regulation in CC tissues that was contributed with prognosis and stage. MiR-196a enhanced CC cell proliferation by p21Kip1 and FOXO1 targeting [51]. DNA methyl transferase 3 beta (DNMT3B) is a key factor of epigenetic regulation during embryogenesis and imprinting that is also upregulated in different tumors [126, 127]. MiR-29c down-regulation was reported in BC in comparison with normal tissues. MiR-29c suppressed tumor growth and migration by DNMT3B targeting. DNMT3B was necessary for the methylation and down-regulation of TIMP3, which enhanced BC progression through the TIMP3/STAT1/FOXO1 axis [128]. MiR-9 increased BC cell proliferation and migration through FOXO1 targeting and CDH1 down-regulation [129].
Twist-related protein 1 (TWIST-1) is a transcription factor involved in EMT induction [130, 131]. FOXO3a suppressed EMT and metastasis through regulation of miR-10b and CADM2 expression and TWIST-1 down-regulation in BC cells [132]. Sirtuin 1 (SIRT1) belongs to the class-III histone deacetylase (HDAC) participated in different physiological and pathological processes, such as gene regulation, DNA repair, cell proliferation, aging, and tumorigenesis. It has also critical roles in the epigenetic modulation of tissue homeostasis and various diseases through histone and non-histone deacetylation [133]. It has oncogenic or tumor suppressor functions in different cancers [134, 135]. MiR-506-3p inhibited OC cell proliferation, while induced apoptosis through SIRT1 suppression. FOXO3a and AKT were also the downstream targets of SIRT1. Therefore, miR-506-3p reduced the expression levels of p-AKT and p-FOXO3a in OC cells. Moreover, SIRT1 up-regulation conversed the suppression ability of miR-506-3p on p-AKT and p-FOXO3a expression [136]. It has been demonstrated that miR-940 was markedly up-regulated in BC tissues and cells which was associated with decreased survival in BC patients. MiR-940 enhanced cell invasiveness and proliferation in BC via regulation of FOXO3 [125]. Circ-0025202 promoted cell apoptosis and TAM sensitivity and suppressed BC cell colony formation and proliferation. Down-regulation of circ-0025202 was associated with histological grade and metastasis to lymph nodes, proposing that it functioned as a significant regulator and tumor suppressor in HR-positive breast cancer. Circ-0025202 was involved in tumor progression and regulation of TAM sensitivity through miR-182-5p/FOXO3a axis [137]. MiR-148a inhibited ovarian tumor cell viability and invasion, while induced apoptosis by FOXO3 targeting [138]. A significant miR-96 up-regulation was shown in BC tissues in comparison with normal samples. MiR-96 increased BC cell proliferation by FOXO3a targeting that down-regulated the p27Kip1 and p21Cip1, while up-regulated CCND1 [52]. MiR-150 was significantly over expressed in CC patients compared to normal tissues that were correlated with the processed stages of cancer. MiR-150 enhanced CC cell growth and survival in via FOXO4, BIM, and FASL targeting. It also promoted the cell cycle progression from the G1/G0 to S phase in CC cells by p27 down-regulation while CCND1 up-regulation [139].
FOXP, Q, and R
FOXP subfamily is involved in cancer progression and embryonic development through interacting with noncoding RNAs and signaling pathways [83, 140]. They are one of the main effectors in PI3K/AKT pathway (Fig. 3). FOXP1/2/4 are expressed in brain, while FOXP3 is mainly expressed in T regulatory cells. FOXP family functions as oncogene or tumor suppressor in different types of cancer [21, 141, 142]. LC3 is known as a homologue of yeast ATG8 in mammalian cells [143]. LC3-II ratio and LC-II to LC3-I amount display the quantity and content of autophagy [144]. It was indicated that the elevated LC3-II/LC3-I ration up-regulated the Beclin1 and MDR-1, while down-regulated the p62 in CDDP-resistant cells, indicating that the DDP resistance was correlated with autophagy in ovarian cancer. MiR-29c-3p reduced autophagy and CDDP resistance via FOXP1/ATG14 targeting in OC cells [145].
MiR-374b-5p down-regulation was correlated with poor prognosis in ovarian tumor tissues. MiR-374b-5p played as a tumor suppressor by regulation of ovarian tumor cell proliferation, EMT, and CDDP sensitivity via FOXP1 targeting [146]. A direct association was shown between the miR-449b-5p expression level and overall survival rate of CC patients. MiR-449b-5p suppressed the CC cell proliferation and invasion via FOXP1 targeting [147]. There was TSLNC8 down-regulation in BC cell lines and tissues. TSLNC8 significantly suppressed tumor growth and G1/S phase transition in BC cells by miR-214-3p sponging and FOXP2 up-regulation [148].
SOX2 and CCAT1 up-regulations were observed in CC tissues and cells which were correlated with LNM, tumor size, and advanced FIGO. SOX2 and CCAT1 silencing reduced CC stem cell proliferation and invasion, while promoted apoptosis. CCAT1 inhibited the CC stem cell proliferation and self-renewal by miR-185-3p sponging and FOXP3 up-regulation [149]. There was miR-150-5p/3p down-regulation in OC in comparison with normal tissues. MiR-150 significantly reduced OC cell proliferation and invasion, while increased apoptosis through IGFIR and IRS1 targeting. FoxP3-miR-150 axis inhibited the OC progression through IGF1R/IRS1 feedback loop in which PI3K/AKT pathway reduced the levels of FoxP3 expressions [150]. Tumor necrosis factor receptor-related factors (TRAFs) are a class of cytoplasmic adaptor proteins that link tumor necrosis factors to the Toll-like/IL-1 receptor (TLR/ILR) superfamily [151]. TRAF6 overexpression has been observed in different tumor types which can promote tumor progression by regulating various signaling pathways involved in cell proliferation and invasion [152]. Interleukin-1 receptor-associated kinase (IRAK) is a serine/threonine kinase involved in regulation of the IL-1R signaling pathway. It is also a key effector of the TLR signaling pathway [153, 154]. IRAK1 is participated in the formation and development of different myeloid malignancies or tumors [155,156,157]. It has been revealed that up-regulation of miR-146a/b by FOXP3 led to inhibition of IRAK1 and TRAF6 that resulted in suppression of NF-κB and consequently tumor growth inhibition in BC. FOXP3 targeted miR-146a via two forkhead-binding motifs which were located in proximal site of the miR-146a promoter. Tumor suppressor function of FOXP3 was partially inhibited by miR-146a/b negative regulators [158]. Circular RNAs (circRNAs) are a type of noncoding RNAs, defined as continuous loops that are closed covalently and derive from mRNA splicing [159]. There was circMYO9B up-regulation in BC tissues. Knockdown of circMYO9B inhibited BC cell progression, invasion, and migration by miR-4316 sponging and FOXP4 up-regulation [160]. CircRPPH1 was significantly up-regulated in BC tissues and cells, which was associated with lymph node involvement and tumor stage. CircRPPH1 promoted BC progression through miR-296-5p sponging and FOXP4 up-regulation. Down-regulation of circRPPH1 inhibited cell proliferation, metastasis, and glycolysis in BC cells [161].
Forkhead box Q1 (FOXQ1) is involved in gastric epithelial differentiation [162]. FOXQ1 has oncogenic role in different types of cancer [163]. FOXQ1 induces tumor angiogenesis, cell proliferation, resistance to chemotherapy, and EMT [163,164,165]. There was miR-937 down-regulation in BC cell lines and tissues that was associated with TNM stage and lymph node involvement. Down-regulation of miR-937 decreased overall survival in BC patients. MiR-937 inhibited tumor development through FOXQ1 targeting [166]. It has been reported that miR-202 was significantly down-regulated in endometrial adenocarcinoma (EAC) tissues in comparison with the normal samples. Down-regulation of miR-202 was linked to overall survival rate. MiR-202 significantly suppressed tumor growth through FOXR2 inhibition in EAC [167]. There was significant circ-CELSR1 up-regulation in PTX-resistant ovarian tumor tissues and cell lines. It increased OC progression by miR-1252 sponging and FOXR2 up-regulation. Suppression of circCELSR1 increased the PTX sensitivity of ovarian tumor cells [168].
Conclusions
Gynecological and breast tumors are one of the leading causes of cancer-related mortality among women. Late diagnosis is one of the main reasons for treatment failure and high mortality in these patients. Therefore, the introduction of early diagnostic markers can be significantly effective in the management and control of patients in the early stages. FOX protein family has critical role in development and progression of these tumors. On the other hand, miRNAs as non-invasive factors play an important role in regulation of FOX function. Therefore, in the present review, we assessed the role of miR/FOX axis during the progression of these tumors. It has been reported that miR/FOX axis has mainly a tumor suppressor role in these tumors. MicroRNAs were mainly involved in progression of these tumors through FOXM, FOXP, and FOXO. The present review paves the way to suggest a non-invasive diagnostic panel marker based on miR/FOX axis in gynecological and breast cancers. However, further clinical studies on the circulating levels of miRNAs are required to introduce them as the efficient non-invasive tumor markers. Although, miR/FOX axis can be also suggested as a therapeutic target in gynecological and breast cancer patients, further animal studies and clinical trials are required to bring miR/FOX axis into the clinics as an efficient therapeutic target in gynecological and breast cancer patients.
Availability of data and materials
Not applicable.
Abbreviations
- AGO:
-
Argonaute
- BC:
-
Breast cancer
- CC:
-
Cervical cancer
- circRNAs:
-
Circular RNAs
- DNMT3B:
-
DNA methyl transferase 3 beta
- EAC:
-
Endometrial adenocarcinoma
- ER:
-
Estrogen receptor
- eEF2K:
-
Eukaryotic elongation factor 2 kinase
- XPO5:
-
Exportin 5
- ECM:
-
Extracellular matrix
- FOX:
-
Fokhead box
- FOXK:
-
Forkhead box class K
- FoxF2:
-
Forkhead box F2
- FOXO:
-
Forkhead box O
- FOXQ1:
-
Forkhead box Q1
- FHD:
-
Forkhead domain
- HDAC:
-
Histone deacetylase
- IRAK:
-
Interleukin-1 receptor-associated kinase
- LIMK1:
-
LIM domain kinase 1
- lncRNAs:
-
Long non-coding RNAs
- miRNAs:
-
MicroRNAs
- OC:
-
Ovarian cancer
- pre-miRNAs:
-
Precursor miRNAs
- pri-miRNAs:
-
Primary miRNAs
- PR:
-
Progesterone receptor
- RISC:
-
RNA-induced silencing complex
- SIRT1:
-
Sirtuin 1
- TGF-β:
-
Transforming growth factor-β
- TNBC:
-
Triple negative breast cancer
- TRAFs:
-
Tumor necrosis factor receptor-related factors
- TWIST-1:
-
Twist-related protein 1
- UTR:
-
Untranslated regions
References
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Louwman MWJ, et al. Uncommon breast tumors in perspective: incidence, treatment and survival in the Netherlands. Int J Cancer. 2007;121(1):127–35.
Rosen PP. Rosen’s breast pathology. Philadelphia: Lippincott Williams & Wilkins; 2001.
Tavassoli FA. Pathology and genetics of tumours of the breast and female genital organs. Geneva: World Health Organization Classification of Tumours; 2003.
Minami K, et al. Lysophosphatidic acid receptor-2 (LPA2)-mediated signaling enhances chemoresistance in melanoma cells treated with anticancer drugs. Mol Cell Biochem. 2020;469(1–2):89–95.
Moghbeli M, et al. ErbB1 and ErbB3 co-over expression as a prognostic factor in gastric cancer. Biol Res. 2019;52(1):2.
Li X, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–9.
Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–92.
Buckley AM, et al. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol. 2020;17(5):298–313.
Torres-Collado AX, Knott J, Jazirehi AR. Reversal of resistance in targeted therapy of metastatic melanoma: lessons learned from vemurafenib (BRAF(V600E)-specific inhibitor). Cancers. 2018;10(6):157.
Wu T, Gu X, Cui H. Emerging roles of SKP2 in cancer drug resistance. Cells. 2021;10(5):1147.
Maeda H, Khatami M. Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med. 2018;7(1):11.
DeSantis C, et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62.
Spurdle AB, et al. Genome-wide association study identifies a common variant associated with risk of endometrial cancer. Nat Genet. 2011;43(5):451–4.
Randall ME, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24(1):36–44.
Oronsky B, et al. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol. 2017;34(6):103.
Nezhat FR, et al. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262–7.
Rosen B, et al. The impacts of neoadjuvant chemotherapy and of debulking surgery on survival from advanced ovarian cancer. Gynecol Oncol. 2014;134(3):462–7.
Lokadasan R, et al. Targeted agents in epithelial ovarian cancer: review on emerging therapies and future developments. Ecancermedicalscience. 2016;10:626.
Kumar L, Gupta S. Integrating chemotherapy in the management of cervical cancer: a critical appraisal. Oncology. 2016;91(Suppl 1):8–17.
Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7(11):847–59.
Dai S, et al. Structural basis for DNA recognition by FOXG1 and the characterization of disease-causing FOXG1 mutations. J Mol Biol. 2020;432(23):6146–56.
Lai E, et al. Hepatocyte nuclear factor 3/fork head or “winged helix” proteins: a family of transcription factors of diverse biologic function. Proc Natl Acad Sci. 1993;90(22):10421–3.
Benayoun BA, Caburet S, Veitia RA. Forkhead transcription factors: key players in health and disease. Trends Genet. 2011;27(6):224–32.
Clark KL, et al. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364(6436):412–20.
Obsil T, Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene. 2008;27(16):2263–75.
Cirillo LA, Zaret KS. Specific interactions of the wing domains of FOXA1 transcription factor with DNA. J Mol Biol. 2007;366(3):720–4.
Romanelli MG, Lorenzi P, Morandi C. Nuclear localization domains in human thyroid transcription factor 2. Biochim Biophys Acta (BBA) Mol Cell Res. 2003;1643(1–3):55–64.
Hancock WW, Özkaynak E. Three distinct domains contribute to nuclear transport of murine Foxp3. PLoS ONE. 2009;4(11):e7890.
Wang L, et al. Forkhead box protein C1 promotes cell proliferation and invasion in human cervical cancer. Mol Med Rep. 2018;17(3):4392–8.
Gu F, et al. Effects of forkhead box protein A1 on cell proliferation regulating and EMT of cervical carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(21):7189–96.
Sahoo SS, et al. FOXA2 suppresses endometrial carcinogenesis and epithelial-mesenchymal transition by regulating enhancer activity. J Clin Invest. 2022;132(12):e157574.
Li C, et al. Forkhead box protein C2 (FOXC2) promotes the resistance of human ovarian cancer cells to cisplatin in vitro and in vivo. Cell Physiol Biochem. 2016;39(1):242–52.
O’Regan RM, Nahta R. Targeting forkhead box M1 transcription factor in breast cancer. Biochem Pharmacol. 2018;154:407–13.
Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8.
Maharati A, et al. MicroRNAs as the critical regulators of tyrosine kinase inhibitors resistance in lung tumor cells. Cell Commun Signal. 2022;20(1):27.
Lee Y, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23(20):4051–60.
Lee Y, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–9.
Lund E, et al. Nuclear export of microRNA precursors. Science. 2004;303(5654):95–8.
Yi R, et al. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17(24):3011–6.
Weng Y-T, Chang Y-M, Chern Y. The impact of dysregulated microRNA biogenesis machinery and microRNA sorting on neurodegenerative diseases. Int J Mol Sci. 2023;24(4):3443.
Moghbeli M. MicroRNAs as the critical regulators of Cisplatin resistance in ovarian cancer cells. J Ovarian Res. 2021;14(1):127.
Zangouei AS, Alimardani M, Moghbeli M. MicroRNAs as the critical regulators of Doxorubicin resistance in breast tumor cells. Cancer Cell Int. 2021;21(1):213.
Zangouei AS, et al. Non coding RNAs as the critical factors in chemo resistance of bladder tumor cells. Diagn Pathol. 2020;15(1):136.
Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics: a comprehensive review. EMBO Mol Med. 2012;4(3):143–59.
Moghbeli M, et al. Molecular mechanisms of the microRNA-132 during tumor progressions. Cancer Cell Int. 2021;21(1):439.
Chen X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006.
Mitchell PS, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–8.
Michael A, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16(1):34–8.
Moghbeli M. Molecular interactions of miR-338 during tumor progression and metastasis. Cell Mol Biol Lett. 2021;26(1):13.
Hou T, et al. MicroRNA-196a promotes cervical cancer proliferation through the regulation of FOXO1 and p27Kip1. Br J Cancer. 2014;110(5):1260–8.
Lin H, et al. Unregulated miR-96 induces cell proliferation in human breast cancer by downregulating transcriptional factor FOXO3a. PLoS ONE. 2010;5(12):e15797.
Jägle S, et al. SNAIL1-mediated downregulation of FOXA proteins facilitates the inactivation of transcriptional enhancer elements at key epithelial genes in colorectal cancer cells. PLoS Genet. 2017;13(11):e1007109.
Friedman J, Kaestner KH. The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci. 2006;63:2317–28.
Shen S-Q, et al. miR-204 regulates the biological behavior of breast cancer MCF-7 cells by directly targeting FOXA1. Oncol Rep. 2017;38(1):368–76.
Salem M, et al. miR-590-3p promotes ovarian cancer growth and metastasis via a novel FOXA2–versican pathway. Can Res. 2018;78(15):4175–90.
Cui YM, et al. FOXC2 promotes colorectal cancer metastasis by directly targeting MET. Oncogene. 2015;34(33):4379–90.
Song L, et al. FOXC2 positively regulates YAP signaling and promotes the glycolysis of nasopharyngeal carcinoma. Exp Cell Res. 2017;357(1):17–24.
Wang T, et al. Emerging roles and mechanisms of FOXC2 in cancer. Clin Chim Acta. 2018;479:84–93.
Amin NM, Shi H, Liu J. The FoxF/FoxC factor LET-381 directly regulates both cell fate specification and cell differentiation in C. elegans mesoderm development. Development. 2010;137(9):1451–60.
Fatima A, et al. Foxc1 and Foxc2 deletion causes abnormal lymphangiogenesis and correlates with ERK hyperactivation. J Clin Investig. 2016;126(7):2437–51.
Norrmén C, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol. 2009;185(3):439–57.
Xu Y-Y, et al. MicroRNA-495 downregulates FOXC1 expression to suppress cell growth and migration in endometrial cancer. Tumor Biol. 2016;37(1):239–51.
Sherman JH, et al. Foxd4 is essential for establishing neural cell fate and for neuronal differentiation. Genesis. 2017;55(6):e23031.
Bond KH, et al. FOXD1 regulates cell division in clear cell renal cell carcinoma. BMC Cancer. 2021;21(1):312.
Quintero-Ronderos P, Laissue P. The multisystemic functions of FOXD1 in development and disease. J Mol Med. 2018;96(8):725–39.
Yan JH, et al. FOXD3 suppresses tumor growth and angiogenesis in non-small cell lung cancer. Biochem Biophys Res Commun. 2015;466(1):111–6.
Chu TL, et al. FoxD3 deficiency promotes breast cancer progression by induction of epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2014;446(2):580–4.
Li D, et al. FOXD3 is a novel tumor suppressor that affects growth, invasion, metastasis and angiogenesis of neuroblastoma. Oncotarget. 2013;4(11):2021–44.
Rahmani Z, Mojarrad M, Moghbeli M. Long non-coding RNAs as the critical factors during tumor progressions among Iranian population: an overview. Cell Biosci. 2020;10:6.
Hamidi AA, et al. Long non-coding RNAs as the critical regulators of epithelial mesenchymal transition in colorectal tumor cells: an overview. Cancer Cell Int. 2022;22(1):71.
Zhang D, Zhang Y, Sun X. LINC01133 promotes the progression of cervical cancer via regulating miR-30a-5p/FOXD1. Asia Pac J Clin Oncol. 2021;17(3):253–63.
Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med. 2007;85(6):555–68.
Zhou Y, et al. Maternal exposure to Nanoparticulate titanium dioxide causes inhibition of hippocampal development involving dysfunction of the rho/NMDAR signaling pathway in offspring. J Biomed Nanotechnol. 2019;15(4):839–47.
Yang X, et al. FOXD3-AS1/miR-128-3p/LIMK1 axis regulates cervical cancer progression. Oncol Rep. 2021;45(5):1–12.
Zhang X, et al. MicroRNA-182 promotes proliferation and metastasis by targeting FOXF2 in triple-negative breast cancer. Oncol Lett. 2017;14(4):4805–11.
Wang A, et al. LncRNA ADAMTS9-AS2 regulates ovarian cancer progression by targeting miR-182-5p/FOXF2 signaling pathway. Int J Biol Macromol. 2018;120:1705–13.
Oshimori N, Fuchs E. The harmonies played by TGF-β in stem cell biology. Cell Stem Cell. 2012;11(6):751–64.
Sun N, Taguchi A, Hanash S. Switching roles of TGF-β in cancer development: implications for therapeutic target and biomarker studies. J Clin Med. 2016;5(12):109.
Neel J-C, Humbert L, Lebrun J-J. The dual role of TGFβ in human cancer: from tumor suppression to cancer metastasis. Int Sch Res Not. 2012;2012:1–28.
Leight JL, et al. Matrix rigidity regulates a switch between TGF-β1–induced apoptosis and epithelial–mesenchymal transition. Mol Biol Cell. 2012;23(5):781–91.
Hou P-S, et al. Transcription and beyond: delineating FOXG1 function in cortical development and disorders. Front Cell Neurosci. 2020;14:35.
Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10(4):233–40.
Chan D, et al. Overexpression of FOXG1 contributes to TGF-β resistance through inhibition of p21WAF1/CIP1 expression in ovarian cancer. Br J Cancer. 2009;101(8):1433–43.
Adesina AM, et al. FOXG1 dysregulation is a frequent event in medulloblastoma. J Neurooncol. 2007;85(2):111–22.
Zeng F, et al. MiR-200b promotes the cell proliferation and metastasis of cervical cancer by inhibiting FOXG1. Biomed Pharmacother. 2016;79:294–301.
Liu Y, et al. FOXK transcription factors: regulation and critical role in cancer. Cancer Lett. 2019;458:1–12.
Gao F, Tian J. FOXK1, regulated by miR-365-3p, promotes cell growth and EMT indicates unfavorable prognosis in breast cancer. Onco Targets Ther. 2020;13:623.
Bella L, et al. FOXM1: a key oncofoetal transcription factor in health and disease. Semin Cancer Biol. 2014;29:32–9.
Li L, et al. Prognostic value of FOXM1 in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8(19):32298–308.
Raychaudhuri P, Park HJ. FoxM1: a master regulator of tumor metastasis. Cancer Res. 2011;71(13):4329–33.
Schmadeka R, Harmon BE, Singh M. Triple-negative breast carcinoma: current and emerging concepts. Am J Clin Pathol. 2014;141(4):462–77.
Cadoo K, Fornier M, Morris P. Biological subtypes of breast cancer: current concepts and implications for recurrence patterns. Q J Nucl Med Mol Imaging. 2013;57(4):312–21.
Carey LA, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–34.
Schüller U, et al. Forkhead transcription factor FoxM1 regulates mitotic entry and prevents spindle defects in cerebellar granule neuron precursors. Mol Cell Biol. 2007;27(23):8259–70.
Wang I-C, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25(24):10875–94.
Wierstra I. The transcription factor FOXM1 (Forkhead box M1): proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv Cancer Res. 2013;118:97–398.
Wang X, et al. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci. 2002;99(26):16881–6.
Wang X, et al. Increased hepatic Forkhead Box M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration through diminished p27Kip1 protein levels and increased Cdc25B expression. J Biol Chem. 2002;277(46):44310–6.
Wang Q, et al. Targeted interfering DEP domain containing 1 protein induces apoptosis in A549 lung adenocarcinoma cells through the NF-κB signaling pathway. Onco Targets Ther. 2017;10:4443.
Zhang L, et al. DEPDC1, negatively regulated by miR-26b, facilitates cell proliferation via the up-regulation of FOXM1 expression in TNBC. Cancer Lett. 2019;442:242–51.
Kenney JW, et al. Eukaryotic elongation factor 2 kinase, an unusual enzyme with multiple roles. Adv Biol Regul. 2014;55:15–27.
Wang X, Xie J, Proud CG. Eukaryotic elongation factor 2 kinase (eEF2K) in cancer. Cancers. 2017;9(12):162.
Moore CE, et al. Elongation factor 2 kinase promotes cell survival by inhibiting protein synthesis without inducing autophagy. Cell Signal. 2016;28(4):284–93.
Bayraktar R, et al. Dual suppressive effect of miR-34a on the FOXM1/eEF2-kinase axis regulates triple-negative breast cancer growth and invasion. Clin Cancer Res. 2018;24(17):4225–41.
Shi M, Cui J, Xie K. Signaling of miRNAs-FOXM1 in cancer and potential targeted therapy. Curr Drug Targets. 2013;14(10):1192–202.
Yuan F, Wang W. MicroRNA-802 suppresses breast cancer proliferation through downregulation of FoxM1. Mol Med Rep. 2015;12(3):4647–51.
Li X-R, et al. miR-342-3p suppresses proliferation, migration and invasion by targeting FOXM1 in human cervical cancer. FEBS Lett. 2014;588(17):3298–307.
Liang L, Zheng YW, Wang YL. miR-4429 Regulates the proliferation, migration, invasion, and epithelial-mesenchymal transition of cervical cancer by targeting FOXM1. Cancer Manag Res. 2020;12:5301.
Li M, et al. Circular PVT1 regulates cell proliferation and invasion via miR-149-5p/FOXM1 axis in ovarian cancer. J Cancer. 2021;12(2):611.
Hong H, et al. The novel circCLK3/miR-320a/FoxM1 axis promotes cervical cancer progression. Cell Death Dis. 2019;10(12):1–19.
Gao F, et al. LncRNA SBF2-AS1 promotes the progression of cervical cancer by regulating miR-361-5p/FOXM1 axis. Artif Cells Nanomed Biotechnol. 2019;47(1):776–82.
Dong G, et al. FBXL19-AS1 promotes cell proliferation and inhibits cell apoptosis via miR-876-5p/FOXM1 axis in breast cancer. Acta Biochim Biophys Sin. 2019;51(11):1106–13.
Tan X, et al. miR-671-5p inhibits epithelial-to-mesenchymal transition by downregulating FOXM1 expression in breast cancer. Oncotarget. 2016;7(1):293.
He S, et al. MiR-216b inhibits cell proliferation by targeting FOXM1 in cervical cancer cells and is associated with better prognosis. BMC Cancer. 2017;17(1):1–12.
Hu Q, et al. miR-197 is downregulated in cervical carcinogenesis and suppresses cell proliferation and invasion through targeting forkhead box M1. Oncol Lett. 2018;15(6):10063–9.
Shi C, Zhang Z. MicroRNA-320 suppresses cervical cancer cell viability, migration and invasion via directly targeting FOXM1. Oncol Lett. 2017;14(3):3809–16.
Xia N, et al. MiR-374b reduces cell proliferation and cell invasion of cervical cancer through regulating FOXM1. Eur Rev Med Pharmacol Sci. 2019;23(2):513–21.
Dai S, et al. Long non-coding RNA WT1-AS inhibits cell aggressiveness via miR-203a-5p/FOXN2 axis and is associated with prognosis in cervical cancer. Eur Rev Med Pharmacol Sci. 2019;23(2):486–95.
Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(15):2479–87.
Kousteni S. FoxO1: a molecule for all seasons. J Bone Miner Res. 2011;26(5):912–7.
Navaei ZN, et al. PI3K/AKT signaling pathway as a critical regulator of Cisplatin response in tumor cells. Oncol Res. 2021;29(4):235–50.
Xu H, et al. Inhibition of microRNA-181a may suppress proliferation and invasion and promote apoptosis of cervical cancer cells through the PTEN/Akt/FOXO1 pathway. J Physiol Biochem. 2016;72(4):721–32.
Xu Y, et al. Down-regulation of microRNA-135b inhibited growth of cervical cancer cells by targeting FOXO1. Int J Clin Exp Pathol. 2015;8(9):10294.
Yang L, et al. miR-96 enhances the proliferation of cervical cancer cells by targeting FOXO1. Pathol Res Pract. 2020;216(4):152854.
Peralta-Arrieta I, et al. DNMT3B modulates the expression of cancer-related genes and downregulates the expression of the gene VAV3 via methylation. Am J Cancer Res. 2017;7(1):77.
Rahman MM, et al. DNA methyltransferases 1, 3a, and 3b overexpression and clinical significance in gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2010;41(8):1069–78.
Li W, et al. miR-29c plays a suppressive role in breast cancer by targeting the TIMP3/STAT1/FOXO1 pathway. Clin Epigenetics. 2018;10(1):1–14.
Liu D-Z, et al. MicroRNA-9 promotes the proliferation, migration, and invasion of breast cancer cells via down-regulating FOXO1. Clin Transl Oncol. 2017;19(9):1133–40.
Oh BY, et al. Twist1-induced epithelial-mesenchymal transition according to microsatellite instability status in colon cancer cells. Oncotarget. 2016;7(35):57066.
Croset M, et al. TWIST1 expression in breast cancer cells facilitates bone metastasis formation. J Bone Miner Res. 2014;29(8):1886–99.
Jin L, et al. FOXO3a inhibits the EMT and metastasis of breast cancer by regulating TWIST-1 mediated miR-10b/CADM2 axis. Transl Oncol. 2021;14(7):101096.
Chen J, Chen H, Pan L. SIRT1 and gynecological malignancies. Oncol Rep. 2021;45(4):1–11.
Lin Z, Fang D. The roles of SIRT1 in cancer. Genes Cancer. 2013;4(3–4):97–104.
Alves-Fernandes DK, Jasiulionis MG. The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int J Mol Sci. 2019;20(13):3153.
Xia X, et al. MicroRNA-506-3p inhibits proliferation and promotes apoptosis in ovarian cancer cell via targeting SIRT1/AKT/FOXO3a signaling pathway. Neoplasma. 2020;67(2):344–53.
Sang Y, et al. circRNA_0025202 regulates tamoxifen sensitivity and tumor progression via regulating the miR-182-5p/FOXO3a axis in breast cancer. Mol Ther. 2019;27(9):1638–52.
Zhu D, et al. Overexpression of miR-148a inhibits viability and invasion of ovarian cancer OVCAR3 cells by targeting FOXO3. Oncol Lett. 2019;18(1):402–10.
Li J, et al. microRNA-150 promotes cervical cancer cell growth and survival by targeting FOXO4. BMC Mol Biol. 2015;16(1):1–9.
Kim J-H, et al. Molecular networks of FOXP family: dual biologic functions, interplay with other molecules and clinical implications in cancer progression. Mol Cancer. 2019;18(1):1–19.
Carvalho MI, et al. Intratumoral FoxP3 expression is associated with angiogenesis and prognosis in malignant canine mammary tumors. Vet Immunol Immunopathol. 2016;178:1–9.
Chiu YC, et al. Foxp2 regulates neuronal differentiation and neuronal subtype specification. Dev Neurobiol. 2014;74(7):723–38.
Tanida I, et al. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1(2):84–91.
Aparicio I, et al. The autophagy-related protein LC3 is processed in stallion spermatozoa during short-and long-term storage and the related stressful conditions. Animal. 2016;10(7):1182–91.
Hu Z, et al. miR-29c-3p inhibits autophagy and cisplatin resistance in ovarian cancer by regulating FOXP1/ATG14 pathway. Cell Cycle. 2020;19(2):193–206.
Li H, et al. MiR-374b-5p-FOXP1 feedback loop regulates cell migration, epithelial-mesenchymal transition and chemosensitivity in ovarian cancer. Biochem Biophys Res Commun. 2018;505(2):554–60.
Cheng L, et al. MiR-449b-5p regulates cell proliferation, migration and radioresistance in cervical cancer by interacting with the transcription suppressor FOXP1. Eur J Pharmacol. 2019;856:172399.
Qin C, et al. LncRNA TSLNC8 inhibits proliferation of breast cancer cell through the miR-214-3p/FOXP2 axis. Eur Rev Med Pharmacol Sci. 2019;23(19):8440–8.
Zhang L, et al. SOX2 regulates lncRNA CCAT1/MicroRNA-185-3p/FOXP3 axis to affect the proliferation and self-renewal of cervical cancer stem cells. Nanoscale Res Lett. 2021;16(1):1–12.
Zhang Q, et al. FoxP3-miR-150-5p/3p suppresses ovarian tumorigenesis via an IGF1R/IRS1 pathway feedback loop. Cell Death Dis. 2021;12(3):1–16.
Rothe M, et al. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78(4):681–92.
Li J, et al. The relationship between TRAF6 and tumors. Cancer Cell Int. 2020;20(1):1–12.
Li N, et al. Targeting interleukin-1 receptor-associated kinase 1 for human hepatocellular carcinoma. J Exp Clin Cancer Res. 2016;35(1):1–10.
Zhu J, Mohan C. Toll-like receptor signaling pathways—therapeutic opportunities. Mediators Inflamm. 2010;2010:1–7.
Rhyasen GW, et al. Targeting IRAK1 as a therapeutic approach for myelodysplastic syndrome. Cancer Cell. 2013;24(1):90–104.
Wee ZN, et al. IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel. Nat Commun. 2015;6(1):1–16.
Zhang X, et al. Expression of IRAK1 in lung cancer tissues and its clinicopathological significance: a microarray study. Int J Clin Exp Pathol. 2014;7(11):8096.
Liu R, et al. FOXP3 controls an miR-146/NF-κB negative feedback loop that inhibits apoptosis in breast cancer cells. Can Res. 2015;75(8):1703–13.
Wilusz JE, Sharp PA. A circuitous route to noncoding RNA. Science. 2013;340(6131):440–1.
Wang N, et al. Circular RNA circMYO9B facilitates breast cancer cell proliferation and invasiveness via upregulating FOXP4 expression by sponging miR-4316. Arch Biochem Biophys. 2018;653:63–70.
Yang L, et al. CircRPPH1 serves as a sponge for miR-296-5p to enhance progression of breast cancer by regulating FOXP4 expression. Am J Transl Res. 2021;13(7):7556.
Li Y, et al. Forkhead box Q1: a key player in the pathogenesis of tumors. Int J Oncol. 2016;49(1):51–8.
Tang H, et al. Forkhead box Q1 is critical to angiogenesis and macrophage recruitment of colorectal cancer. Front Oncol. 2020;10:2561.
Feuerborn A, et al. The forkhead factor FoxQ1 influences epithelial differentiation. J Cell Physiol. 2011;226(3):710–9.
Qiao Y, et al. FOXQ1 regulates epithelial-mesenchymal transition in human cancers. Can Res. 2011;71(8):3076–86.
Han X, et al. MicroRNA-937 inhibits the malignant phenotypes of breast cancer by directly targeting and downregulating forkhead box Q1. Onco Targets Ther. 2019;12:4813.
Deng X, et al. miR-202 suppresses cell proliferation by targeting FOXR2 in endometrial adenocarcinoma. Dis Markers. 2017;2017:1–8.
Zhang S, et al. circCELSR1 (hsa_circ_0063809) contributes to paclitaxel resistance of ovarian cancer cells by regulating FOXR2 expression via miR-1252. Mol Ther Nucleic Acids. 2020;19:718–30.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NT, ML, ASZ, and IA were involved in search strategy, drafting, and graphical illustrations. MM supervised the project and revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Taghehchian, N., Lotfi, M., Zangouei, A.S. et al. MicroRNAs as the critical regulators of Forkhead box protein family during gynecological and breast tumor progression and metastasis. Eur J Med Res 28, 330 (2023). https://doi.org/10.1186/s40001-023-01329-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01329-7