Abstract
Objective
Previous studies showed that the combination of bevacizumab and erlotinib (combination therapy) significantly prolonged progression-free survival (PFS) but no overall survival (OS) compared to erlotinib alone (monotherapy) for advanced EGFR-mutant non-small cell lung cancer (NSCLC). Two phase III randomized controlled trials (RCTs) had reported the OS results in 2021. This meta-analysis aimed to include the results of the two RCTs to make a decision.
Materials and methods
We systematically searched relevant databases for RCTs on the use of bevacizumab plus erlotinib in advanced EGFR-mutant NSCLC. The main outcomes of interest were PFS, OS, and the reported hazard ratio (HR). Fixed-effect model was used to estimate pooled HR.
Results
Total 5 RCTs with 935 patients were eligible for this meta-analysis. All studies reached their primary study endpoints including PFS and OS. Compared to monotherapy, combination therapy remarkably prolonged PFS (HR = 0.60, 95% confidence interval CI 0.51–0.70; p < 0.00001); however, OS was similar between the two groups (HR = 0.90, 95% CI 0.76–1.08; p = 0.26). Subgroup analysis demonstrated that in deletion within exon 19 (19del) mutation subgroup, the combination therapy could only prolong PFS (HR = 0.60, 95% CI 0.47–0.76; p < 0.0001) but not OS (HR = 1.00, 95% CI 0.73–1.37; p = 1.00), and also in leucine-to-arginine substitution in exon 21 (L858R) mutation subgroup (HR = 0.59, p < 0.0001 and HR = 0.80, p = 0.18, respectively). For patients with brain metastasis at baseline, the combination therapy achieved a significant better PFS than the monotherapy (HR = 0.60, 95% CI 0.39–0.90; p = 0.01), and a better OS with the difference marginally significant (HR = 0.69, 95% CI 0.46–1.02; p = 0.06).
Conclusions
Combination of bevacizumab and erlotinib can prolong progression-free survival but not overall survival compared to erlotinib alone in advanced EGFR-mutant non-small cell lung cancer patients. The combination therapy not only can prolong progression-free survival but also has a tendency to prolong overall survival for patients with brain metastasis at baseline.
Similar content being viewed by others
Introduction
Epidermal growth factor receptor (EGFR) mutation represents the most frequent driver-gene alteration in non-small cell lung cancer (NSCLC), and 90% of EGFR mutations involving a deletion within exon 19 (19del) or a leucine-to-arginine substitution in exon 21 (L858R) [1, 2]. Erlotinib, a small-molecule EGFR tyrosine kinase inhibitor (TKI), is recommended as a first-line therapy for patients with advanced NSCLC harboring the two common EGFR mutations [3]. However, most NSCLC patients treated with erlotinib develop therapeutic resistance, with the median progression-free survival (PFS) of 9.7–13.1 months [4, 5]. To overcome this acquired resistance and improve the PFS, several studies [6,7,8,9,10] have investigated the combination of bevacizumab and erlotinib for the NSCLC patients with EGFR mutations. Most of these studies (ARTEMIS [9], NEJ026 [8], JO25567 [7], BEVERLY [10]) have displayed better PFS in the combination therapy than erlotinib monotherapy, but no overall survival (OS) benefit in the combination therapy. Whether this combination therapy was more effective than monotherapy? Some meta-analyses tried to answer this question. For example, Chen’s meta-analysis [11] and Landre’s meta-analysis [12] both showed that the combination therapy significantly prolonged PFS but no OS compared to monotherapy. However, the conclusions of these meta-analyses were controversial, because the OS results of both ATEMIS study and NEJ026 study were immature at that time. As the OS results of the two studies were published in 2021, we conducted a meta-analysis to provide more reliable and stable evidence to determine the PFS and OS benefit in the combination therapy for advanced EGFR-mutant NSCLC. A secondary objective was to investigate whether efficacy differed between 19del and L858R mutation or between with brain metastasis and without brain metastasis.
Materials and methods
Study design
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Search strategy
The PubMed, EMBASE, and Cochrane Trials databases were rigorously reviewed for randomized controlled trial (RCT) up to June 2022 focusing on the combination of erlotinib and bevacizumab in NSCLC. The complete search terms included: (Carcinoma, Non-Small-Cell Lung [MeSH term] OR Carcinoma, Non Small Cell Lung [Text Word]) AND (Erlotinib Hydrochloride [MeSH term] OR Hydrochloride, Erlotinib [Text Word]) AND (Bevacizumab [MeSH term] OR Avastin [Text Word]). We also manually searched the reference lists for further eligible articles, and the corresponding abstracts at the annual American Society of Clinical Oncology (ASCO) and European Society of Medical Oncology (ESMO) meetings.
Inclusion criteria
To ensure the quality and reliability of this meta-analysis, only high-quality RCTs that met the following criteria were included: (1) Population: patients with histologically or cytologically confirmed advanced EGFR-mutant NSCLC. (2) Intervention: erlotinib plus bevacizumab. (3) Comparison: erlotinib as a single agent. (4) Outcome: OS, PFS, objective response rate (ORR), and adverse events (AEs).
Data extraction
Data extraction was performed by two reviewers independently, and disagreement over eligibility of a study was resolved by consensus. The reviewers extracted the key information as following: first author’s name, year of publication, clinical trial information, study design, intervention details, number of patients, pathologic features, EGFR mutation status, and relevant outcomes. Considering that some results (ORR and AEs) remain unchanged until now and they have been proved in previous meta-analyses [11,12,13] in 2020, the main outcomes of interest in this meta-analysis were PFS, OS, and the available reported hazard ratio (HR) for the outcomes.
Quality assessment of included studies
The Cochrane Collaboration’s risk of bias tool [14] was adopted to assess risk of bias for each RCT by two investigators. Seven items were used to evaluate heterogeneity in each trial: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. The quality of each RCT was categorized as high, low, or unclear. Discrepancies were resolved by discussion with a third investigator.
Statistical analysis
Review Manager (version 5.3, the Cochrane Collaboration) was used for data analysis. For the time-to-event variables, the HR and 95% confidence interval CI were extracted from the original RCT. Heterogeneity across studies was assessed with a forest plot and the inconsistency statistic (I2). If the heterogeneity was moderate or severe (I2 ≥ 50%), a random-effect model would be applied; otherwise, the fixed-effect model would be applied. Subgroup analysis was conducted according to EGFR mutation subtype (19del and L858R) and brain metastasis at inclusion (with and without brain metastasis). Results were reported with HR and 95% CI for PFS and OS. A two-sided p < 0.05 was considered statistically significant. Graphical funnel plot was generated to visually inspect for publication bias.
Results
Search results
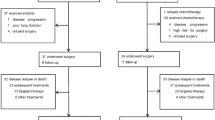
A total of 773 eligible studies were identified by searching the databases. The data from the BEVERLY study presented at the 2021 ESMO Congress were also included online. There were 294 duplicate records were removed and the remaining 480 studies were reviewed for title and abstract. Subsequently, 29 potentially eligible studies were assessed by full-text review. Finally, 5 RCTs were selected according to the inclusion criteria (Fig. 1).
Characteristics and quality assessment of included studies
The characteristics of these 5 RCTs (3 phase III trials and 2 phase II trials) are shown in Table 1, and all the RCTs were judged to have a low risk of bias using the Cochrane Collaboration criteria. Of the 5 RCTs, 3 were performed in Asia, 1 in Italy, and 1 in the United States. A total of 935 patients were enrolled, 553 (59.1%) were women, 467 (49.9%) received combination therapy, 499 were 19del mutation and 430 were L858R mutation. The combination therapy was the erlotinib (150 mg/d) plus bevacizumab (15 mg/kg, Q3w), whereas the monotherapy was administered erlotinib alone.
Main outcomes analysis
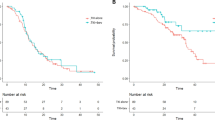
The combination therapy achieved a significant PFS benefit (HR = 0.60, 95% CI 0.51–0.70; p < 0.00001) compared to monotherapy, but failed to be translated into OS benefit (HR = 0.90, 95% CI 0.76–1.08; p = 0.26) in all patient population (Fig. 2).
Subgroup analyses stratified by the EGFR mutation subtype and brain metastasis at inclusion were conducted. The PFS of both 19del mutation subtype (HR = 0.60, p < 0.0001) and L858R mutation subtype (HR = 0.59, p < 0.0001) seemed to be improved by the combination therapy, but OS of the two subtypes were still not prolonged (HR = 1.00, p = 1.00 and HR = 0.80, p = 0.18, respectively) (Fig. 3). For patients with brain metastasis at inclusion, the combination therapy achieved a significant better PFS than the monotherapy (HR = 0.60, 95% CI 0.39–0.90; p = 0.01), and a better OS with the difference marginally significant (HR = 0.69, 95% CI 0.46–1.02; p = 0.06) (Fig. 4).
Discussion
Erlotinib, a first-generation reversible TKI, targets the EGFR signaling pathway. Bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody, targets the VEGF signaling pathway. Additional clinical benefits may be conferred by the dual blockade of the EGFR and VEGF pathways that critical to tumor growth, metastasis and angiogenesis [15, 16]. Therefore, quite a few clinical studies [6,7,8,9,10] had attempted this combination therapy: erlotinib plus bevacizumab. Although some clinical studies had achieved prolongation of PFS, there was no corresponding prolongation of OS. This meta-analysis is motivated by uncertainty about the value of combination therapy compared with monotherapy.
According to the results of this meta-analysis, the combination therapy significantly prolonged PFS (Fig. 2A), but did not prolong OS (Fig. 2B) compared to monotherapy for advanced EGFR-mutant NSCLC patients. Although final survival outcomes from study ATEMIS and NEJ026 were included in this meta-analysis, there was still no prolongation of OS, consistent with the findings of three previous meta-analyses [11,12,13]. Based on the advantages of dual blockade of EGFR and VEGF pathways, the prolongation of PFS by combination therapy is currently indisputable, while there are other relevant reasons for the failure to prolong OS. For instance, OS might have been influenced by the subsequent treatments used after disease progression, especially the use of third-generation TKI osimertinib. In the study ATEMIS, more patients from the monotherapy group received osimertinib as subsequent treatment than in the combination therapy group (29.2% vs 17.2%), and this ratio was 28.9% vs 23.3% in another study [6]. In addition, the number of patients receiving subsequent anticancer treatment was also greater in the monotherapy group in the ATEMIS study (50.0% vs 33.8%). Thirdly, Grade ≥ 3 adverse events occurred more frequently in the combination therapy group than in the monotherapy group [6, 9]. Therefore, the combination therapy might be compromised because of the above problems.
In general, the L858R mutation subgroup derives less benefit from first-line EGFR TKIs than the 19del mutation subgroup, which has been repeatedly demonstrated in previous clinical trials and real-world studies [17,18,19]. The prognosis of patients with L858R mutation urgently needs to be improved in the era of TKI targeted therapy [20]. Would adding a VEGF inhibitor in combination with the TKI change the subgroup’s outcome? Therefore, subgroup analysis was conducted in this meta-analysis. In 19del mutation subgroup, the HR for PFS was 0.60 with a 95% confidence interval of 0.47 to 0.76 (p < 0.0001), and the HR for OS was 1.00 with a 95% confidence interval of 0.73 to 1.37 (p = 1.00) (Fig. 3A, B). In L858R mutation subgroup, the HR for PFS was 0.59 with a 95% confidence interval of 0.46 to 0.77 (p < 0.0001), and the HR for OS was 0.80 with a 95% confidence interval of 0.58 to 1.11 (p = 0.18) (Fig. 3C, D). The subgroup analysis showed that the combination therapy only prolonged PFS, but not OS, regardless of L858R or 19del mutation, which was consistent with the results of overall population. When comparing the HR value alone, both PFS and OS of L858R mutation were improved more significantly than those with 19del mutation. This suggests that the combination therapy can reverse the poor prognosis of L858R mutation subgroup, and the combination therapy may be one of the treatment options for patients with L858R mutation [21, 22].
Presence of brain metastasis is a well-known adverse prognostic factor and common site of disease progression in EGFR-mutant NSCLC. Previous study showed that the anti-VEGF drug bevacizumab was effective in treating brain metastasis [23], and also could significantly reduce the incidence of brain metastasis [24]. In two retrospective studies [25, 26], bevacizumab combined with TKI prolonged the OS in patients with brain metastasis compared to TKI monotherapy. Of all the 5 RCT studies, only 2 studies [8, 9] conducted further subgroup analysis for patients with brain metastasis, and thus only the 2 studies were included. The subgroup analysis suggested that the combination therapy not only provided significant improvement in PFS (HR = 0.60, 95% CI 0.39–0.90; p = 0.01), but also had a positive trend to prolong OS (HR = 0.69, 95% CI: 0.46–1.02; p = 0.06) in patients with brain metastasis (Fig. 4A, B). The statistical P-value did not reach significant significance, which may be explained by the following reasons. First of all, only two RCTs were included. Secondly, the treatment-related adverse effects associated with the combination therapy [6, 8, 9]. However, it had given us good enlightenment and pointed out the direction of future research. It is the only way to include more large-scale RCT studies.
It is worth mentioning that osimertinib has replaced erlotinib as the preferred first-line treatment for advanced EGFR-mutant NSCLC patients and second-line treatment for patients with EGFR T790M mutation. Can osimertinib combined with bevacizumab further prolong PFS and OS in patients with EGFR-mutant NSCLC? Several studies had presented the results. BOOSTER [27] and WJOG8715L [28] were two studies of second-line treatment, and neither study showed an extension of PFS and OS. WJOG9717L [29] was a study of first-line treatment, and it also showed that PFS was not prolonged in the combination group, and the OS data were immature. Despite only three phase II studies, the prospects for combination therapy are not promising.
Conclusion
This meta-analysis reveals that the combination of erlotinib and bevacizumab can prolong progression-free survival but not overall survival compared to erlotinib alone in advanced EGFR-mutant non-small cell lung cancer. For patients with brain metastasis, the combination therapy not only can prolong progression-free survival, but also has a tendency to prolong overall survival. This combination therapy model deserves to be considered in the future, especially for patients with brain metastasis.
Availability of data and materials
All data generated or analyzed during this study are included in the published RCT articles (and its supplementary information files).
References
Hsu WH, Yang JC, Mok TS, Loong HH. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. 2018;29:i3–9.
Castellanos E, Feld E, Horn L. Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12:612–23.
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19:254–66.
Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol. 2018;14:1117–32.
Yang Z, Hackshaw A, Feng Q, Fu X, Zhang Y, Mao C, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140:2805–19.
Stinchcombe TE, Janne PA, Wang X, Bertino EM, Weiss J, Bazhenova L, et al. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1448–55.
Yamamoto N, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, et al. Erlotinib plus bevacizumab vs erlotinib monotherapy as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer: survival follow-up results of the randomized JO25567 study. Lung Cancer. 2021;151:20–4.
Kawashima Y, Fukuhara T, Saito H, Furuya N, Watanabe K, Sugawara S, et al. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir Med. 2022;10:72–82.
Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY, Cui JW, et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): a multicenter phase 3 study. Cancer Cell. 2021;39(1279–91):e3.
Gridelli C, Rossi A, Ciardiello F, De Marinis F, Crino L, Morabito A, et al. BEVERLY: rationale and design of a randomized open-label phase III trial comparing bevacizumab plus erlotinib versus erlotinib alone as first-line treatment of patients with EGFR-mutated advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer. 2016;17:461–5.
Chen F, Chen N, Yu Y, Cui J. Efficacy and safety of epidermal growth factor receptor (EGFR) inhibitors plus antiangiogenic agents as first-line treatments for patients with advanced EGFR-mutated non-small cell lung cancer: a meta-analysis. Front Oncol. 2020;10:904.
Landre T, Des Guetz G, Chouahnia K, Duchemann B, Assie JB, Chouaid C. First-line angiogenesis inhibitor plus erlotinib versus erlotinib alone for advanced non-small-cell lung cancer harboring an EGFR mutation. J Cancer Res Clin Oncol. 2020;146:3333–9.
Deng Z, Qin Y, Liu Y, Zhang Y, Lu Y. Role of antiangiogenic agents combined with EGFR tyrosine kinase inhibitors in treatment-naive lung cancer: a meta-analysis. Clin Lung Cancer. 2021;22:e70–83.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Larsen AK, Ouaret D, El Ouadrani K, Petitprez A. Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther. 2011;131:80–90.
Langer C, Soria JC. The role of anti-epidermal growth factor receptor and anti-vascular endothelial growth factor therapies in the treatment of non-small-cell lung cancer. Clin Lung Cancer. 2010;11:82–90.
Zhang Y, Sheng J, Kang S, Fang W, Yan Y, Hu Z, et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS ONE. 2014;9:e107161.
Liu Y, Ren Z, Wang J, Zhang S. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is especially beneficial to patients with exon 19 deletion compared with exon 21 L858R mutation in non-small-cell lung cancer: systematic review and meta analysis. Thorac Cancer. 2016;7:406–14.
Chen CL, Wang ST, Liao WC, Chen CH, Tu CY, Chen HJ, et al. When to add anti-angiogenesis drugs to EGFR-mutated metastatic non-small cell lung cancer patients: a real-world study from Taiwan. BMC Cancer. 2022;22:571.
Lee CK, Wu YL, Ding PN, Lord SJ, Inoue A, Zhou C, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol. 2015;33:1958–65.
Li WQ, Cui JW. Non-small cell lung cancer patients with ex19del or exon 21 L858R mutation: distinct mechanisms, different efficacies to treatments. J Cancer Res Clin Oncol. 2020;146:2329–38.
Huang YH, Hsu KH, Chin CS, Tseng JS, Yang TY, Chen KC, et al. The clinical outcomes of different first-line EGFR-TKIs plus bevacizumab in advanced EGFR-mutant lung adenocarcinoma. Cancer Res Treat. 2022;54:434–44.
Masuda C, Sugimoto M, Wakita D, Monnai M, Ishimaru C, Nakamura R, et al. Bevacizumab suppresses the growth of established non-small-cell lung cancer brain metastases in a hematogenous brain metastasis model. Clin Exp Metastasis. 2020;37:199–207.
Fu Y, Hu J, Du N, Jiao S, Li F, Li X, et al. Bevacizumab plus chemotherapy versus chemotherapy alone for preventing brain metastasis derived from advanced lung cancer. J Chemother. 2016;28:218–24.
Chiu TH, Tung PH, Huang CH, Ju JS, Huang AC, Wang CC, et al. The different overall survival between single-agent EGFR-TKI treatment and with bevacizumab in non-small cell lung cancer patients with brain metastasis. Sci Rep. 2022;12:4398.
Jiang T, Zhang Y, Li X, Zhao C, Chen X, Su C, et al. EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs alone in patients with EGFR-mutant NSCLC and multiple brain metastases. Eur J Cancer. 2019;121:98–108.
Soo RA, Han JY, Dafni U, Cho BC, Yeo CM, Nadal E, et al. A randomised phase II study of osimertinib and bevacizumab versus osimertinib alone as second-line targeted treatment in advanced NSCLC with confirmed EGFR and acquired T790M mutations: the European thoracic oncology platform (ETOP 10–16) BOOSTER trial. Ann Oncol. 2022;33:181–92.
Akamatsu H, Toi Y, Hayashi H, Fujimoto D, Tachihara M, Furuya N, et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with EGFR T790M-mutated non-small cell lung cancer previously treated with epidermal growth factor receptor-tyrosine kinase inhibitor: west Japan oncology group 8715L phase 2 randomized clinical trial. JAMA Oncol. 2021;7:386–94.
Kenmotsu H, Wakuda K, Mori K, Kato T, Sugawara S, Kirita K, et al. Randomized phase 2 study of osimertinib plus bevacizumab versus osimertinib for untreated patients with nonsquamous NSCLC harboring EGFR mutations: WJOG9717L study. J Thorac Oncol. 2022;17:1098–108.
Acknowledgements
The authors would like to thank Tian Lu for her assistance in language improvement, and all the patients who participated in these RCTs.
Funding
This study was supported by Qilu Special Project of clinical research fund of Shandong Medical Association (Grant number: YXH2022ZX02031).
Author information
Authors and Affiliations
Contributions
Li RJ and Li WY developed the concept, researched the data, and wrote the manuscript equally. Zhang F created the figures and the tables. Li SS searched the literature. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval consent to participate
Not applicable. This was a meta-analysis and all patients enrolled were from published RCT studies.
Competing interests
All authors declared no competing interests to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, R., Li, W., Zhang, F. et al. Bevacizumab plus erlotinib versus erlotinib alone for advanced EGFR-mutant non-small cell lung cancer: a meta-analysis of randomized clinical trials. Eur J Med Res 28, 302 (2023). https://doi.org/10.1186/s40001-023-01272-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01272-7