Abstract
Meningitis is a potential complication of elective intracranial surgery (EIS). The prevalence of meningitis after EIS varies greatly in the literature. The objective of this study was to estimate the overall pooled prevalence of meningitis following EIS. Four databases (PubMed, Scopus, Web of Science, and Embase) were searched to identify relevant studies. Meta-analyses of proportions were used to combine data. Cochran's Q and I2 statistics were used to assess and quantify heterogeneity. Additionally, several subgroup analyses were conducted to investigate the source of heterogeneity and examine differences in the prevalence based on variables such as geographical regions, income level, and meningitis type. The meta-analysis included 83 studies (30 959 patients) from 26 countries. The overall pooled prevalence of meningitis after EIS was 1.6% (95% CI 1.1–2.1), with high heterogeneity present (I2 = 88%). The pooled prevalence in low- to middle-income countries and high-income countries was 2.7% (95% CI 1.6–4.1) and 1.2% (95% CI 0.8–1.7), respectively. Studies that reported only aseptic meningitis had a pooled prevalence of 3.2% (95% CI 1.3–5.8). The pooled prevalence was 2.8% (95% CI 1.5–4.5) in studies that reported only bacterial meningitis. Similar prevalence rates of meningitis were observed in the subgroups of tumor resection, microvascular decompression, and aneurysm clipping. Meningitis is a rare but not exceptional complication following EIS, with an estimated prevalence of 1.6%.
Similar content being viewed by others
Introduction
Meningitis is a potential complication of elective intracranial surgery (EIS). Post-EIS meningitis can be either bacterial or aseptic [1, 2]. Symptoms of both types of meningitis are similar and include fever, neck stiffness, decreased level of consciousness, and headache [3,4,5,6,7,8]. However, the symptoms of bacterial meningitis tend to be more severe than those of aseptic meningitis [3]. Meningitis without any of these symptoms is very unlikely [3, 4]. A diagnosis of meningitis is made based on clinical symptoms and/or positive cerebrospinal fluid (CSF) bacterial cultures [9, 10].
Postoperative bacterial meningitis can be caused by either Gram-positive or Gram-negative bacteria [3, 6, 11]. It is a serious and potentially life-threatening complication that can lead to severe complications [4]. Therefore, prompt and appropriate medical treatment is essential [4, 12].
Aseptic meningitis refers to inflammation of the meninges without evidence of bacterial infection [3]; it is most likely caused by an inflammatory reaction to the surgical procedure and red blood cell breakdown products [13, 14], although the exact mechanism of postoperative aseptic meningitis is not fully understood. Other possible causes of postoperative meningitis include drug-induced aseptic meningitis [15, 16].
The prevalence of meningitis after EIS varies greatly in the literature, ranging from 0.1 [17] to almost 10% [18] in large-series studies. Moreover, studies reporting on meningitis after EIS are often single-center with small sample sizes, which limits the generalizability of the findings.
The main objective of this meta-analysis was to estimate the overall pooled prevalence of meningitis following EIS. We also hypothesized that the prevalence of post-EIS meningitis would differ based on geographical location and income levels (as in the case of community-acquired meningitis [19, 20]). Additionally, our second hypothesis was that the prevalence of post-EIS meningitis would be similar between bacterial and aseptic meningitis.
Methods
Overview
The meta-analysis was conducted in accordance with the preferred reporting items for systematic reviews (PRISMA) guidelines and recommendations [21].
Search strategy
We performed an electronic search of English-language articles published between January 2000 and September 2022. We searched PubMed, Scopus, Web of Science, and Embase to identify relevant studies. The key terms were associated with common elective intracranial procedures and pathologies, in particular: craniotomy, tumor resection, microvascular decompression (MVD), vascular lesion resection, aneurysm clipping, and meningitis. The search syntax is presented in Additional file 1: Appendix 1. Three researchers (MM, AM, and OS) independently screened all titles and abstracts for their eligibility for inclusion. The full texts of articles that were potentially relevant for the meta-analysis were retrieved for a detailed eligibility assessment according to the selection criteria. Any discrepancies during the selection and extraction processes were resolved by discussion and consensus. Furthermore, we hand-searched the bibliographies of included articles and related reviews to identify additional studies relevant to the meta-analysis.
Selection criteria
We included studies that reported meningitis following intracranial surgery. The exclusion criteria were: (1) emergency surgery; (2) endonasal surgery; (3) surgery for central nervous system infections, temporal lobe epilepsy, congenital malformations, shunt and Ommaya reservoir-placement, and cranioplasty; (4) stereotactic and functional neurosurgery; (5) studies with less than 50 patients; (6) pediatric studies; (7) nationwide and statewide datasets, reviews, case studies, conference abstracts, animal studies, and letters to the editor.
All studies that missed key data on the number of meningitis cases, contained non-extractable data on the number of meningitis cases after surgery, and had potentially overlapping data were identified and excluded.
Data extraction
Three reviewers (MM, AM, and OS) independently extracted data from included articles into a spreadsheet using Microsoft Excel (2010; Microsoft Corporation, Redmond, WA, USA). We recorded: (a) the last name of the first author; (b) the year of publication; (c) the country in which the study was conducted; (d) enrollment dates; (e) the age of the patients; (f) the number of patients; (g) the number of cases of post-operative meningitis; and (h) the reported type of meningitis (aseptic, bacterial, both, or none).
Statistical analysis
The primary outcome of interest was the proportion of meningitis cases after EIS. The meta-analysis was conducted to calculate the overall pooled prevalence. We used the DerSimonian and Laird random-effects model [22] since we assumed high between-study heterogeneity. A Freeman–Tukey double arcsine transformation was used to stabilize the variance [23]. To evaluate the heterogeneity, we used Cochran’s Q test and the I2 statistic, which represents the percentage of total variation across studies. A Cochran's Q P-value of 0.10 indicates significant heterogeneity. The I2 statistic value of greater than 50% suggests substantial heterogeneity [24]. To assess how much the effect size varies, we calculated the prediction interval for the primary outcome and subgroup analyses.
In order to test our hypothesis, we performed a predefined subgroup analysis by income level according to the World Bank income classification (low- and middle-income countries [LMICs] and high-income countries [HICs]), continent, and WHO regions (African Region (AFR), Region of the Americas (AMR), South-East Asia Region (SEAR), European Region (EUR), Eastern Mediterranean Region (EMR), and Western Pacific Region (WPR)) under the WHO classification (https://www.who.int/countries), country, and type of meningitis. We also did a post hoc analysis by type of procedure. A subgroup analysis and univariate meta-regression by publication year, sample size, quality of included studies, and each covariate included in the subgroup analysis were further investigated for association with the pooled estimates and as potential sources of heterogeneity. The subgroup analyses were performed when data from at least two studies were available. A leave-one-out sensitivity analysis was performed to assess the robustness of the results. Publication bias was visualized with a funnel plot and evaluated using Egger's regression test. A value of P less than 0.05 was considered significant. The differences between two subgroups were considered insignificant if their confidence intervals (CI) overlapped. Results are presented in a forest plot with a 95% CI and 95% prediction intervals. All analyses were done in RStudio (version 1.3.1093).
Level of evidence
Study quality was assessed using modified Oxford Centre for Evidence-Based Medicine recommendations [25]. Studies were rated from 1 (low risk of bias or high quality) to 5 (high risk of bias or low quality). Randomized clinical trials were rated 1; nonrandomized clinical trials and prospective comparative studies were rated 2; case–control studies and retrospective cohort studies were rated 3; case series and cross-sectional studies were rated 4; and case reports were rated 5.
Results
Search results
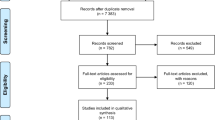
Database searching yielded 11 048 results, of which 4514 were duplicates. All titles and abstracts of 6534 articles were screened for potentially relevant data. Of these, 220 articles were qualified for full-text evaluation. Additionally, four articles were identified during the hand-searching of bibliographies of relevant articles and included in the meta-analysis. Finally, 83 articles [17, 18, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69, 69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106] from 26 different countries were included in the meta-analysis. The process of study identification is shown in Fig. 1.
Flowchart showing search strategy. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/
Thirty-one of the included studies were conducted in Asia, 26 in Europe, 22 in North America, 2 in South America, and 2 in Africa. The most common neurosurgical procedures described in the included articles were tumor resection, followed by microvascular decompression and aneurysm clipping. Using modified Oxford Centre for Evidence-Based Medicine recommendations, we have identified 74 studies (89.2%) as level 4 (case series and cross-sectional studies) and 9 studies (10.18%) as level 2 (nonrandomized clinical trials and prospective comparative studies). The characteristics of the included studies are presented in Additional file 2: Appendix 2.
Overall prevalence of meningitis after EIS
Among the 30 959 patients (from 83 studies) included in the meta-analysis, 616 experienced meningitis. The prevalence of meningitis after EIS reported in the included studies ranged from 0.0% to 15.3%. The overall pooled estimate was 1.6% (95% CI 1.1–2.1). The 95% prediction interval was 0.0–7.4. The results of the pooled analysis are presented in Table 1.
Meningitis prevalence after EIS by geographic location
The highest pooled prevalence of meningitis after EIS was in EMR (3.9%), followed by WPR (2.4%). The AMR had the lowest pooled prevalence of meningitis after EIS (0.9%). The results of the pooled analysis by WHO region are presented in Table 1 and Fig. 2. In the subgroup analysis by continent, the highest prevalence of meningitis after EIS was in Asia (2.5%), followed by South America (1.5%). The results for estimates by continent are shown in Table 1 and Additional file 3: Appendix 3. Estimates by country are shown in Additional file 4: Appendix 4.
Meningitis prevalence after EIS by income
We identified 24 papers from LMICs and 59 from HICs reporting meningitis after EIS. The pooled prevalence was 2.7% and 1.2% in the LMICs and HICs, respectively. The results for estimates by income level are shown in Table 1 and Additional file 5: Appendix 5.
Prevalence of meningitis after EIS by type of meningitis
Most studies did not report data on meningitis types, and nine studies reported no cases of meningitis after EIS. Eleven studies reported data on aseptic meningitis. The pooled prevalence in the subgroup was 3.2%. Bacterial meningitis was reported in nine studies. The pooled prevalence was 2.8%. Data on both aseptic meningitis and bacterial meningitis were reported in seven studies, with an estimated pooled prevalence of 2.9%. Table 2 and Additional file 6: Appendix 6 show the results of the pooled analysis by type of meningitis.
Meningitis prevalence after EIS by procedure type
Fifty-five studies reported on meningitis after tumor resection. Meningitis after microvascular decompression and aneurysm clipping were reported in 21 and 5 studies, respectively. The pooled prevalence of meningitis after tumor resection, MVD, and aneurysm clipping was 1.9%, 1.2%, and 0.4%, respectively. Table 2 and Additional file 7: Appendix 7 show the results of a subgroup analysis based on the type of procedure.
Between-study heterogeneity and publication bias
We found no significant publication bias in the included studies on the prevalence of meningitis after EIS, as shown by the funnel plot (Fig. 3) and the Egger’s linear regression test of funnel plot asymmetry (P = 0.2228). An influential analysis showed that the study by Huang et al. [18] contributed the most to the overall prevalence of meningitis after EIS and between-study heterogeneity. After omitting this study, the overall prevalence and heterogeneity decreased to 1.45% and I2 = 83.5%, respectively (Additional file 8: Appendix 8). The results of univariate meta-regression showed that neither publication year (P = 0.82; R2 = 0%) nor sample size (P = 0.40; R2 = 0%) were associated with the prevalence of meningitis after EIS; these covariates could not explain heterogeneity either. The results of univariate meta-regression for covariates included in a subgroup analysis were as follows: WHO region (P = 0.22; R2 = 0%), continent (P = 0.11; R2 = 0.73%), country (P = 0.15; R2 = 14.29%), income (P = 0.007; R2 = 13.19%), surgery type (P = 0.62; R2 = 0%), and type of meningitis (P = 0.0004; R2 = 6.11%).
Discussion
This systematic review and meta-analysis analyzed data from 83 studies representing 26 countries and including 30 959 patients. The objective was to estimate the prevalence of meningitis following EIS. The results showed an overall prevalence of 1.6%. The prevalence of post-EIS meningitis was found to be slightly higher in the subgroup of countries with low to middle-income levels when compared to high-income countries. We did not find evidence of a significant difference in the prevalence of post-EIS meningitis between different WHO regions and continents. Furthermore, there were no significant differences in the prevalence of post-EIS meningitis between bacterial and aseptic meningitis. The included studies were highly heterogeneous, and only part of the heterogeneity was explained by the meta-regression, subgroup, and sensitivity analyses.
The highest prevalence of postoperative meningitis was reported by Xiang et al. [95] (15.3%) and Wang et al. [92] (14.1%). The authors reported aseptic meningitis after MVD (for trigeminal neuralgia) and jugular foramen tumor surgery, respectively. In both studies, the authors did not provide a possible explanation for the high prevalence of postoperative meningitis. Some of the risk factors reported in the literature include CSF leakage, perioperative steroid use, older age, external ventricular or lumbar drainage, repeat operations, surgery duration, and increased intraoperative blood loss [11, 36, 107,108,109]. The estimated prevalence of post-EIS meningitis in this study is comparable to the overall rate of nosocomial meningitis reported by Korinek et al. [110]. In their study, over 21% of procedures were emergent. Although they did not find emergency procedures to be a risk factor for postoperative meningitis, other studies have reported it [107, 109].
In the study of vestibular schwannomas by Huang et al. [18], just a tiny portion (3.48%) of the cerebrospinal fluid (CSF) samples collected from suspected meningitis patients tested positive for bacterial meningitis, indicating that the majority of cases were likely aseptic. This study's findings showed similar rates of aseptic and bacterial meningitis, however, the limited reporting of CSF culture results in most studies made it challenging to conduct a comprehensive analysis of the prevalence of each type of meningitis. The causes of aseptic meningitis might sometimes be misunderstood. The term "aseptic" refers to any meningitis with negative CSF bacterial cultures. And it might be caused by either infectious (viral, parasitic, or fungal) or non-infectious causes [10]. Reaction to heme breakdown products released during surgery appears to be the most likely cause of post-neurosurgical aseptic meningitis [6]. This could explain the high proportion of aseptic meningitis reported in several large-series studies of tumor resection [18, 83] since these procedures are often associated with substantial blood loss [111]. The duration of the operation and the size of the tumor are risk factors for meningitis after tumor surgery [49, 107], and both might be associated with increased blood loss.
This study has some limitations. First, database searching was limited by language restrictions. This might have resulted in omitting potentially relevant non-English studies, introducing language bias. Second, database searching was limited to studies published after 2000. This was done to estimate the relatively current status of the prevalence of meningitis after EIS. Third, the exclusion criteria applied limit the results’ generalizability to all elective intracranial procedures. Therefore, its important to consider the methods used in this study when interpreting the presented results, as they do not cover certain elective intracranial procedures (e.g., shunt placement or functional neurosurgery). Fourth, the majority of the studies included were retrospective case series, which have inherent limitations. These studies often report the mean or median age rather than an age range. Hence, it is possible that some of the studies included patients who were under 18 years old. Fifth, due to the large number of screened results, there is a possibility that some studies might have been omitted. However, the impact of potentially omitted studies should be minimized by the relatively large number of included studies. Sixth, the small number of included studies was from EMR, SEAR, AFR, South America, and Africa; thus, this might have affected the estimates for these subgroups as well as overall estimates. Finally, we could not control for many possible sources of heterogeneity (risk factors for postoperative meningitis, patient characteristics, scrub techniques, antibiotic prophylaxis, and patients' pre- and post-operative management) due to the lack of data.
Despite these limits, this is the first systematic review and meta-analysis to estimate a pooled prevalence of meningitis after EIS. Subgroup and sensitivity analyses, as well as meta-regression, were performed to examine the various factors likely to affect our results and account for between-study variance. Finally, since the presence of high heterogeneity may be of concern, we provided prediction intervals, as recommended by Migliavaca et al. [112].
Conclusions
Meningitis is a rare but not exceptional complication of elective intracranial surgery, with an overall estimated pooled prevalence of 1.6% and a prediction interval of 0–7.4%. Improving the quality of observational studies reporting on meningitis, in particular in terms of reporting meningitis type and CSF culture results, might be beneficial for future evaluation of this issue.
Availability of data and materials
Data collected for the study and the code used to perform the meta-analyses, meta-regression, and sensitivity analyses can be made available upon reasonable request to the corresponding author.
References
Ross D, Rosegay H, Pons V. Differentiation of aseptic and bacterial meningitis in postoperative neurosurgical patients. J Neurosurg. 1988;69:669–74.
Brown J, de L R Bayston, PD Lees, IK Pople, EM. The management of neurosurgical patients with postoperative bacterial or aseptic meningitis or external ventricular drain-associated ventriculitis. Br J Neurosurg. 2000;14:7–12.
Forgacs P, Geyer CA, Freidberg SR. Characterization of chemical meningitis after neurological surgery. Clin Infect Dis. 2001;32:179–85.
van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849–59.
Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467–92.
Zarrouk V, Vassor I, Bert F, Bouccara D, Kalamarides M, Bendersky N, et al. Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis. 2007;44:1555–9.
van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362:146–54.
Finlayson AI, Penfield W. Acute postoperative aseptic leptomeningitis: review of cases and discussion of pathogenesis. Arch Neurol Psychiatry. 1941;46:250–76.
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;39:1267–84.
Kaur H, Betances EM, Perera TB. Aseptic Meningitis. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK557412/. Accessed 25 Apr 2022.
Kourbeti IS, Vakis AF, Ziakas P, Karabetsos D, Potolidis E, Christou S, et al. Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg. 2015;122:1113–9.
Hoffman O, Weber RJ. Pathophysiology and treatment of bacterial meningitis. Ther Adv Neurol Disord. 2009;2:1–7.
Lin T-Y, Chen W-J, Hsieh M-K, Lu M-L, Tsai T-T, Lai P-L, et al. Postoperative meningitis after spinal surgery: a review of 21 cases from 20,178 patients. BMC Infect Dis. 2014;14:220.
Blomstedt GC. Infections in neurosurgery: a retrospective study of 1143 patients and 1517 operations. Acta Neurochir (Wien). 1985;78:81–90.
Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406.
Morris A, Low DE. Nosocomial bacterial meningitis, including central nervous system shunt infections. Infect Dis Clin North Am. 1999;13:735–50.
Ben Ammar M, Piccirillo E, Topsakal V, Taibah A, Sanna M. Surgical results and technical refinements in translabyrinthine excision of vestibular schwannomas: the Gruppo Otologico experience. Neurosurgery. 2012;70:1481–91 (discussion 1491).
Huang X, Xu M, Xu J, Zhou L, Zhong P, Chen M, et al. Complications and management of large intracranial vestibular schwannomas via the retrosigmoid approach. World Neurosurg. 2017;99:326–35.
Brouwer MC, van de Beek D. Epidemiology of community-acquired bacterial meningitis. Curr Opin Infect Dis. 2018;31:78.
McGill F, Heyderman RS, Panagiotou S, Tunkel AR, Solomon T. Acute bacterial meningitis in adults. Lancet. 2016;388:3036–47.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–11.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009) — Centre for Evidence-Based Medicine (CEBM), University of Oxford. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009. Accessed 2 Oct 2022.
Arab A, Hawsawi A, Bafaquh M, Orz Y, AlYamany M, Alobaid A. Medial extension of medial sphenoid wing meningioma from the anterior clinoid line: does it truly affect the surgical outcome? J Neurol Surg Part B Skull Base. 2021;82:624–30.
Arístegui Ruiz MÁ, González-Orús Álvarez-Morujo RJ, Oviedo CM, Ruiz-Juretschke F, García Leal R, Scola YB. Surgical treatment of vestibular schwannoma. Review of 420 cases. Acta Otorrinolaringol Esp. 2016;67:201–11.
Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15:2887.
Bartek J, Gulati S, Unsgård G, Weber C, Förander P, Solheim O, et al. Standardized reporting of adverse events after microvascular decompression of cranial nerves; a population-based single-institution consecutive series. Acta Neurochir (Wien). 2016;158:1775–81.
Betka J, Zvěřina E, Balogová Z, Profant O, Skřivan J, Kraus J, et al. Complications of microsurgery of vestibular schwannoma. BioMed Res Int. 2014;2014: 315952.
Boublata L, Belahreche M, Ouchtati R, Shabhay Z, Boutiah L, Kabache M, et al. Facial nerve function and quality of resection in large and giant vestibular schwannomas surgery operated by retrosigmoid transmeatal approach in semi-sitting position with intraoperative facial nerve monitoring. World Neurosurg. 2017;103:231–40.
Bowers CA, Gurgel RK, Brimley C, Hawryluk GWJ, Taggart M, Braden S, et al. Surgical treatment of vestibular schwannoma: does age matter? World Neurosurg. 2016;96:58–65.
Bozhkov Y, Shawarba J, Feulner J, Winter F, Rampp S, Hoppe U, et al. Prediction of hearing preservation in vestibular schwannoma surgery according to tumor size and anatomic extension. Otolaryngol Neck Surg. 2022;166:530–6.
Breun M, Nickl R, Perez J, Hagen R, Löhr M, Vince G, et al. Vestibular schwannoma resection in a consecutive series of 502 cases via the retrosigmoid approach: technical aspects, complications, and functional outcome. World Neurosurg. 2019;129:e114–27.
Cardoso AC, Fernandes YB, Ramina R, Borges G. Acoustic neuroma (vestibular schwannoma): surgical results on 240 patients operated on dorsal decubitus position. Arq Neuropsiquiatr. 2007;65:605–9.
Chen S, Cui A, Yu K, Huang C, Zhu M, Chen M. Risk factors associated with meningitis after neurosurgery: a retrospective cohort Study in a Chinese Hospital. World Neurosurg. 2018;111:e546–63.
Choi SW, Ahn JS, Park JC, Kwon DH, Kwun BD, Kim CJ. Surgical treatment of unruptured intracranial middle cerebral artery aneurysms: angiographic and clinical outcomes in 143 aneurysms. J Cerebrovasc Endovasc Neurosurg. 2012;14:289–94.
Ciurea AV, Iencean SM, Rizea RE, Brehar FM. Olfactory groove meningiomas: a retrospective study on 59 surgical cases. Neurosurg Rev. 2012;35:195–202.
Coburger J, Merkel A, Scherer M, Schwartz F, Gessler F, Roder C, et al. Low-grade glioma surgery in intraoperative magnetic resonance imaging: results of a multicenter retrospective assessment of the German Study Group for intraoperative magnetic resonance imaging. Neurosurgery. 2016;78:775–86.
Cueva RA, Mastrodimos B. Approach design and closure techniques to minimize cerebrospinal fluid leak after cerebellopontine angle tumor surgery. Otol Neurotol. 2005;26:1176–81.
D’Amico RS, Englander ZK, Canoll P, Bruce JN. Extent of resection in glioma-a review of the cutting edge. World Neurosurg. 2017;103:538–49.
Elkady A, Soliman MAR, Ali AM. Clinical outcomes of infratentorial meningioma surgery in a developing country. World Neurosurg. 2020;137:e373–82.
Ening G, Osterheld F, Capper D, Schmieder K, Brenke C. Risk factors for glioblastoma therapy associated complications. Clin Neurol Neurosurg. 2015;134:55–9.
Fukuoka T, Nishimura Y, Hara M, Nomura K, Ryu H, Yoshikawa S, et al. Flat posterior cranial fossa affects outcomes of microvascular decompression for trigeminal neuralgia. World Neurosurg. 2018;111:e519–26.
Gjuric M, Rudic M. What is the best tumor size to achieve optimal functional results in vestibular schwannoma surgery? Skull Base. 2008;18:317–25.
Godefroy WP, van der Mey AGL, de Bruine FT, Hoekstra ER, Malessy MJA. Surgery for large vestibular schwannoma: residual tumor and outcome. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2009;30:629–34.
Haque R, Wojtasiewicz TJ, Gigante PR, Attiah MA, Huang B, Isaacson SR, et al. Efficacy of facial nerve-sparing approach in patients with vestibular schwannomas. J Neurosurg. 2011;115:917–23.
Hitchon PW, Holland M, Noeller J, Smith MC, Moritani T, Jerath N, et al. Options in treating trigeminal neuralgia: experience with 195 patients. Clin Neurol Neurosurg. 2016;149:166–70.
Huang B, Ren Y, Wang C, Lan Z, Hui X, Liu W, et al. Risk factors for postoperative meningitis after microsurgery for vestibular schwannoma. PLoS ONE. 2019;14: e0226896.
Jiang X, Wu M, Fu X, Niu C, He F, Sun K, et al. Microvascular decompression for hemifacial spasm associated with vertebral artery: biomedical glue−coated Teflon sling transposition technique. World Neurosurg. 2018;120:e342–8.
Jin Y, Zhao C, Su S, Zhang X, Qiu Y, Jiang J. Residual hemifacial spasm after microvascular decompression: prognostic factors with emphasis on preoperative psychological state. Neurosurg Rev. 2015;38:567–72.
Konglund A, Helseth R, Lund-Johansen M, Helseth E, Meling TR. Surgery for high-grade gliomas in the aging. Acta Neurol Scand. 2013;128:185–93.
Kunert P, Dziedzic T, Czernicki T, Nowak A, Marchel A. Surgery for sporadic vestibular schwannoma. Part II. Complications (not related to facial and auditory nerves). Neurol Neurochir Pol. 2016;50:90–7.
Lawrence JD, Tuchek C, Cohen-Gadol AA, Sekula RF. Utility of the intensive care unit in patients undergoing microvascular decompression: a multiinstitution comparative analysis. J Neurosurg. 2016;126:1967–73.
Lazard DS, Tosello M, Bozorg-Grayeli A, Vitte E, Bouccara D, Kalamarides M, et al. Early complications and symptoms of cerebellopontine angle tumor surgery: a prospective analysis. Eur Arch Otorhinolaryngol. 2011;268:1575–82.
Lee MH, Jee TK, Lee JA, Park K. Postoperative complications of microvascular decompression for hemifacial spasm: lessons from experience of 2040 cases. Neurosurg Rev. 2016;39:151–8 (discussion 158).
Leonetti JP, Anderson D, Marzo S, Moynihan G. Prevention and management of cerebrospinal fluid fistula after transtemporal skull base surgery. Skull Base. 2001;11:87–92.
Li D, Tang J, Ren C, Wu Z, Zhang L-W, Zhang J-T. Surgical management of medium and large petroclival meningiomas: a single institution’s experience of 199 cases with long-term follow-up. Acta Neurochir (Wien). 2016;158:409–25.
Lipschitz N, Kohlberg GD, Tawfik KO, Walters ZA, Breen JT, Zuccarello M, et al. Cerebrospinal fluid leak rate after vestibular schwannoma surgery via middle cranial fossa approach. J Neurol Surg Part B Skull Base. 2019;80:437–40.
Magill ST, Nguyen MP, Aghi MK, Theodosopoulos PV, Villanueva-Meyer JE, McDermott MW. Postoperative diffusion-weighted imaging and neurological outcome after convexity meningioma resection. J Neurosurg. 2021;135:1008–15.
Makarenko S, Carreras EM, Akagami R. Craniotomy for perisellar meningiomas: comparison of simple (appropriate for endoscopic approach) versus complex anatomy and surgical outcomes. J Neurosurg. 2017;126:1191–200.
Mangus BD, Rivas A, Yoo MJ, Alvarez J, Wanna GB, Haynes DS, et al. Management of cerebrospinal fluid leaks after vestibular schwannoma surgery. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2011;32:1525–9.
Margalit N, Shahar T, Barkay G, Gonen L, Nossek E, Rozovski U, et al. Tuberculum sellae meningiomas: surgical technique, visual outcome, and prognostic factors in 51 cases. J Neurol Surg Part B Skull Base. 2013;74:247–58.
Memari F, Hassannia F, Abtahi SHR. Surgical outcomes of cerebellopontine angle tumors in 50 cases. Iran J Otorhinolaryngol. 2015;27:29–34.
Mori K, Wada K, Otani N, Tomiyama A, Toyooka T, Tomura S, et al. Long-term neurological and radiological results of consecutive 63 unruptured anterior communicating artery aneurysms clipped via lateral supraorbital keyhole minicraniotomy. Oper Neurosurg Hagerstown Md. 2018;14:95–103.
Nanda A, Maiti TK, Bir SC, Konar SK, Guthikonda B. Olfactory groove meningiomas: comparison of extent of frontal lobe changes after lateral and bifrontal approaches. World Neurosurg. 2016;94:211–21.
Noorani I, Lodge A, Durnford A, Vajramani G, Sparrow O. Comparison of first-time microvascular decompression with percutaneous surgery for trigeminal neuralgia: long-term outcomes and prognostic factors. Acta Neurochir (Wien). 2021;163:1623–34.
Nussbaum ES, Touchette JC, Madison MT, Goddard JK, Lassig JP, Meyers ME, et al. Procedural complications in patients undergoing microsurgical treatment of unruptured intracranial aneurysms: a single-center experience with 1923 aneurysms. Acta Neurochir (Wien). 2022;164:525–35.
Obaid S, Nikolaidis I, Alzahrani M, Moumdjian R, Saliba I. Morbidity rate of the retrosigmoid versus translabyrinthine approach for vestibular schwannoma resection. J Audiol Otol. 2018;22:236–43.
Oesman C, Mooij JJA. Long-term follow-up of microvascular decompression for trigeminal neuralgia. Skull Base Off J North Am Skull Base Soc Al. 2011;21:313–22.
Ölander C, Gudjonsson O, Kinnefors A, Laurell G, Edfeldt L. Complications in translabyrinthine surgery of vestibular schwannoma. Acta Otolaryngol (Stockh). 2018;138:639–45.
Pallini R, Fernandez E, Lauretti L, Doglietto F, D’Alessandris QG, Montano N, et al. Olfactory groove meningioma: report of 99 cases surgically treated at the Catholic University School of Medicine. Rome World Neurosurg. 2015;83(219–231):e1-3.
Patel RS, Yousem DM, Maldjian JA, Zager EL. Incidence and clinical significance of frontal sinus or orbital entry during pterional (frontotemporal) craniotomy. AJNR Am J Neuroradiol. 2000;21:1327–30.
Picarelli H, de Oliveira ML, Marta GN, Solla DJF, Teixeira MJ, Figueiredo EG. Mortality, morbidity, and prognostic factors in the surgical resection of brain metastases: a contemporary cohort study. J Neurol Surg Part Cent Eur Neurosurg. 2020;81:279–89.
Pollock BE, Stien KJ. Posterior fossa exploration for trigeminal neuralgia patients older than 70 years of age. Neurosurgery. 2011;69:1255–60.
Ribeiro BB, Pereira RD, Vaz R, Carvalho B, Pereira NR. Nonemergent craniotomy surgical site infection: a retrospective cohort study. Porto Biomed J. 2022;7: e152.
Roche P-H, Pellet W, Moriyama T, Thomassin J-M. Translabyrinthine approach for vestibular schwannomas: operative technique. Prog Neurol Surg. 2008;21:73–8.
Roehm PC, Gantz BJ. Management of acoustic neuromas in patients 65 years or older. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2007;28:708–14.
Sameshima T, Fukushima T, McElveen JT, Friedman AH. Critical assessment of operative approaches for hearing preservation in small acoustic neuroma surgery: retrosigmoid vs middle fossa approach. Neurosurgery. 2010;67:640–4.
Samii M, Günther T, Iaconetta G, Muehling M, Vorkapic P, Samii A. Microvascular decompression to treat hemifacial spasm: long-term results for a consecutive series of 143 patients. Neurosurgery. 2002;50:712–8 (discussion 718-719).
Sandell T, Eide PK. Effect of microvascular decompression in trigeminal neuralgia patients with or without constant pain. Neurosurgery. 2008;63:93–9 (discussion 99-100).
Shimizu K, Matsumoto M, Wada A, Sugiyama T, Tanioka D, Okumura H, et al. Supine no-retractor method in microvascular decompression for hemifacial spasm: results of 100 consecutive operations. J Neurol Surg Part B Skull Base. 2015;76:202–7.
Slattery WH, Francis S, House KC. Perioperative morbidity of acoustic neuroma surgery. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2001;22:895–902.
Sluyter S, Graamans K, Tulleken CA, Van Veelen CW. Analysis of the results obtained in 120 patients with large acoustic neuromas surgically treated via the translabyrinthine-transtentorial approach. J Neurosurg. 2001;94:61–6.
Sonoda Y, Shibahara I, Matsuda K, Saito R, Kawataki T, Oda M, et al. Opening the ventricle during surgery diminishes survival among patients with newly diagnosed glioblastoma treated with carmustine wafers: a multi-center retrospective study. J Neurooncol. 2017;134:83–8.
Srinivas D, Veena Kumari HB, Somanna S, Bhagavatula I, Anandappa CB. The incidence of postoperative meningitis in neurosurgery: an institutional experience. Neurol India. 2011;59:195–8.
Stastna D, Mannion R, Axon P, Moffat DA, Donnelly N, Tysome JR, et al. Facial nerve function outcome and risk factors in resection of large cystic vestibular schwannomas. J Neurol Surg Part B Skull Base. 2022;83:e216–24.
Tao X, Wang K, Dong J, Hou Z, Wu Z, Zhang J, et al. Clinical features, surgical management, and prognostic factors of secretory meningiomas: a single-center case series of 149 patients. J Neurooncol. 2018;136:515–22.
Theodros D, Rory Goodwin C, Bender MT, Zhou X, Garzon-Muvdi T, De la Garza-Ramos R, et al. Efficacy of primary microvascular decompression versus subsequent microvascular decompression for trigeminal neuralgia. J Neurosurg. 2017;126:1691–7.
Troude L, Boucekine M, Baucher G, Farah K, Boissonneau S, Fuentes S, et al. Ipsilateral vs contralateral approach in tuberculum sellae meningiomas surgery: a retrospective comparative study. Neurosurg Rev. 2021;44:3581–91.
Turel MK, Thakar S, Rajshekhar V. Quality of life following surgery for large and giant vestibular schwannomas: a prospective study. J Neurosurg. 2015;122:303–11.
Wang X, Yuan J, Liu D, Xie Y, Wu M, Xiao Q, et al. Efficacy of the suboccipital paracondylar-lateral cervical approach: the series of 64 jugular foramen tumors along with follow-up data. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.660487.
Wilkinson E, Roberts D, Cassis A, Schwartz M. Hearing outcomes after middle fossa or retrosigmoid craniotomy for vestibular schwannoma tumors. J Neurol Surg Part B Skull Base. 2016;77:333–40.
Wongsirisuwan M. Short- and Long-term effectiveness of keyhole microvascular decompression for trigeminal neuralgia. J Med Assoc Thai. 2018;101:209–16.
Xiang H, Wu G, Ouyang J, Liu R. Prospective study of neuroendoscopy versus microscopy: 213 cases of microvascular decompression for trigeminal neuralgia performed by one neurosurgeon. World Neurosurg. 2018;111:e335–9.
Xie S, Xiao X-R, Li H, Meng G-L, Zhang J-T, Wu Z, et al. Surgical treatment of pontine cavernous malformations via subtemporal transtentorial and intradural anterior transpetrosal approaches. Neurosurg Rev. 2020;43:1179–89.
Xu M, Xu J, Huang X, Chen D, Chen M, Zhong P. Small extended bifrontal approach for midline anterior skull base meningiomas: our experience with 54 consecutive patients. World Neurosurg. 2019;125:e35-43.
Yamashiro S, Nishi T, Koga K, Goto T, Kaji M, Muta D, et al. Improvement of quality of life in patients surgically treated for asymptomatic unruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. 2007;78:497–500.
Yanagawa T, Hatayama T, Harada Y, Sato E, Yamashita K, Tanaka M, et al. Preoperative risk assessment for predicting the opening of mastoid air cells in lateral suboccipital craniotomy for microvascular decompression. Clin Neurol Neurosurg. 2020;189: 105624.
Yang D, Wang Z, Jiang D, Chen H. The efficacy and safety of microvascular decompression for idiopathic trigeminal neuralgia in patients older than 65 years. J Craniofac Surg. 2014;25:1393–6.
Zeng YJ, Zhang H, Yu S, Zhang W, Sun XC. Efficacy and safety of microvascular decompression and gamma knife surgery treatments for patients with primary trigeminal neuralgia: a prospective study. World Neurosurg. 2018;116:e113–7.
Zhang X, Fei Z, Chen Y-J, Fu L-A, Zhang J-N, Liu W-P, et al. Facial nerve function after excision of large acoustic neuromas via the suboccipital retrosigmoid approach. J Clin Neurosci. 2005;12:405–8.
Zhang Z, Nguyen Y, De Seta D, Russo FY, Rey A, Kalamarides M, et al. Surgical treatment of sporadic vestibular schwannoma in a series of 1006 patients. Acta Otorhinolaryngol Ital Organo Uff Della Soc Ital Otorinolaringol E Chir Cerv-facc. 2016;36:408–14.
Zhang Z, Wang Z, Huang Q, Yang J, Wu H. Removal of large or giant sporadic vestibular schwannomas via translabyrinthine approach: a report of 115 cases. ORL J Oto-Rhino-Laryngol Its Relat Spec. 2012;74:271–7.
Zhao X, Hao S, Wang M, Han C, Xing D, Wang C. Management of veins during microvascular decompression for idiopathic trigeminal neuralgia. Br J Neurosurg. 2018;32:484–8.
Zhao X, Wang Z, Ji Y, Wang C, Yu R, Ding X, et al. Long-term facial nerve function evaluation following surgery for large acoustic neuromas via retrosigmoid transmeatal approach. Acta Neurochir. 2010;152:1647–52.
Chen C-H, Chang C-Y, Lin L-J, Chen WL, Chang Y-J, Wang S-H, et al. Risk factors associated with postcraniotomy meningitis: a retrospective study. Medicine. 2016;95: e4329.
Chen C, Zhang B, Yu S, Sun F, Ruan Q, Zhang W, et al. The incidence and risk factors of meningitis after major craniotomy in china: a retrospective cohort study. PLoS ONE. 2014;9: e101961.
Kourbeti IS, Jacobs AV, Koslow M, Karabetsos D, Holzman RS. Risk factors associated with postcraniotomy meningitis. Neurosurgery. 2007;60:317–25 (discussion 325-326).
Korinek A-M, Baugnon T, Golmard J-L, van Effenterre R, Coriat P, Puybasset L. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2006;59:126–33 (discussion 126-133).
Rajagopalan V, Chouhan RS, Pandia MP, Lamsal R, Rath GP. Effect of Intraoperative blood loss on perioperative complications and neurological outcome in adult patients undergoing elective brain tumor surgery. J Neurosci Rural Pract. 2019;10:631–40.
Migliavaca CB, Stein C, Colpani V, Barker TH, Ziegelmann PK, Munn Z, et al. Meta-analysis of prevalence: I2 statistic and how to deal with heterogeneity. Res Synth Methods. 2022;13:363–7.
Acknowledgements
Not applicable.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by MG, MM, AM, OS. Statistical analyses were performed by RC. The first draft of the manuscript was written by RC. Writing—reviewing and editing: MK and BC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Human and animal ethics
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Appendix 1.
Literature search strategy.
Additional file 2: Appendix 2.
Characteristics of included studies.
Additional file 3:
Appendix 3. Forest plot of the prevalence of meningitis after elective intracranial surgery (EIS) by continents.
Additional file 4: Appendix 4.
Subgroup analysis of meningitis after elective intracranial surgery (EIS) by country.
Additional file 5: Appendix 5.
Forest plot of the prevalence of meningitis after elective intracranial surgery (EIS) by income.
Additional file 6: Appendix 6.
Forest plot of the prevalence of meningitis after elective intracranial surgery (EIS) by type of meningitis.
Additional file 7: Appendix 7.
Forest plot of the prevalence of meningitis after elective intracranial surgery (EIS) by type of surgery.
Additional file 8: Appendix 8.
An influential analysis (Random effects model).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chojak, R., Koźba-Gosztyła, M., Gaik, M. et al. Meningitis after elective intracranial surgery: a systematic review and meta-analysis of prevalence. Eur J Med Res 28, 184 (2023). https://doi.org/10.1186/s40001-023-01141-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01141-3