Abstract
Purpose
Diabetes is the leading cause of kidney disease. Up to 40% of the population with diabetes experience diabetic kidney disease (DKD). The correlation of DKD with insulin resistance (IR) indices has been shown in previous studies. In this study, the objective was to evaluate surrogate IR indices, including the Triglyceride-Glucose (TyG) index, Visceral Adiposity Index (VAI), Lipid Accumulation Product (LAP), and Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) to find the most valuable index for the correlation between albuminuria and IR in the type 2 diabetes (T2D) population. Albuminuria is defined as urine albumin excretion of > 30 mg/day.
Methods
In this cross-sectional study, 2934 participants were enrolled and evaluated for urinary albumin excretion, and albuminuria was detected in 526 of the entries. The logistic regression models and Receiver Operating Characteristic (ROC) curve analysis were performed to assess the relationship of TyG index, VAI, LAP, and HOMA-IR's with albuminuria in patients with T2D.
Results
The TyG index had the highest association (OR 1.67) with the presence of albuminuria in patients with T2D, followed by HOMA-IR (OR 1.127), VAI (OR 1.028), and LAP (OR 1.004). These four indices remained independent after adjustment for multiple confounders. Based on the ROC curve, TyG revealed the best area under the curve (AUC) for revealing albuminuria with sufficient accuracy (AUC: 0.62) in comparison with other measured indices. The calculated TyG index cut-off point for the presence of albuminuria was 9.39.
Conclusion
Among the indices, TyG index had the most significant correlation with albuminuria in patients with T2D.
Similar content being viewed by others
Introduction
Diabetes is the most common metabolic disorder with numerous micro- and macrovascular complications, including, diabetic nephropathy [1, 2]. Albuminuria, defined as the presence of albumin in the urine (albumin excretion rate > 30 mg/d), has been highly correlated to kidney damage and is detectable in the early stages of Chronic Kidney Disease (CKD) [3]. CKD is a global health issue associated with a high rate of complications. It is approximated that more than 850 million people suffer from kidney diseases around the world, the majority of whom showed evidence of CKD [4]. Although several pathogenic mechanisms are associated with the onset and progression of CKD, diabetes mellitus is the leading cause of the disease, and approximately 40% of patients with T2D experience diabetic kidney disease [5, 6]. Nearly half of patients (51%) who receive dialysis therapy suffer from diabetes mellitus as the primary origin of their kidney failure [7, 8].
Studies have indicated that IR and related mechanisms are pathogenic factors for renal failure [9]. IR is a major pathogenic mechanism in T2D but also can be found in patients with Type 1 Diabetes (T1D) [10]. IR is detectable in the early stages of CKD. In addition, as patients' renal function deteriorates, the IR level increases [11].
Although the gold standard method for evaluation of IR is hyperinsulinemic-euglycemic clamp (insulin clamp), this technique is costly and rather sophisticated to perform in clinic [12, 13]. Another indicator used for the assessment of the IR level is the HOMA-IR [14]. However, in the case of patients with T2D, using the HOMA-IR method for the measurement of IR is not reliable enough since a considerable proportion of patients with T2D receive insulin therapy [15, 16].
Therefore, several attempts have been made to discover other indices that could be more accessible for assessing IR [17]. Furthermore, in addition to HOMA-IR, the TyG, VAI, and LAP index have been identified as the surrogate indices for IR [18,19,20]. Several studies have shown an association between these indices and IR levels [21,22,23,24,25].
To the best of our knowledge, there has been no relevant literature that compares the relationship between these four indices and albuminuria in patients with T2D. Thus, the present study was aimed to evaluate this relationship and to identify the cut-off point for the most valuable index to reveal albuminuria in patients with T2D.
Materials and methods
This is a cross-sectional study of an ongoing cohort which was carried out in the outpatients Diabetes Clinic of Vali-Asr Hospital (affiliated with Tehran University of Medical Sciences), a large tertiary referral center. All eligible patients with T2D who attended the outpatient clinic between 2011 and 2021 were enrolled consecutively in the study.
The ethical committee of the Tehran University of Medical Sciences (TUMS) approved the study with the registered number of IR.TUMS.IKHC.REC.1400.085.
T2D was diagnosed according to the 2022 American Diabetes Association guidelines [26], and albuminuria was characterized by persistent albuminuria (> 30 mg/d) that was confirmed on two routine check-ups 3–6 months apart.
Applied exclusion criteria were non-diabetic renal diseases, feverish infection or history of infectious disease in the last six months, any chronic disease (e.g., cardiac failure, liver failure, malignancy, any kind of insulin therapy, patients with T1D, other special types of diabetes, benign prostatic hyperplasia, severe uncontrolled hypertension, severe hyperglycemia), and recent excessive exercise.
Demographic factors and clinical data of participants, including age, sex, height, weight, waist circumference, blood pressure (systolic and diastolic), duration of diabetes, and medications for diabetes and dyslipidemia were recorded.
The body weight and height of enrolled patients were measured to the nearest 0.1 kg and 0.001 m, respectively. Waist circumference was calculated at the central point between the lower level of the lowest palpable rib and the highest of the iliac crest to the nearest 0.001 m. Blood pressure was evaluated after a ten-minute rest. The measurement was repeated after 15 min, and the average was recorded as the blood pressure value.
Blood samples were taken after ten or more hours of fasting at 8:00 am. Fasting blood sugar (using hexokinase enzyme method) and fasting insulin (using radioimmunoassay techniques (Immunotech, Prague, Czech Republic)) were measured.
Participants' serum creatinine level was evaluated by the Jaffe technique (Pars Azmun, Karaj, Iran). Accuracy of random samples were evaluated by the central reference laboratory (Tehran, Iran), which the results and kits approval was met. Hemoglobin A1c (HbA1c) was measured by high performance liquid chromatography (A1C, DS5 Pink kit; Drew, Marseille, France). Fasting plasma glucose (FPG) and 2-h postprandial (2hPP) glucose measurements were conducted using enzymatic colorimetric methods by the glucose oxidase test. The estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration Equation [27]. HOMA-IR, a reliable surrogate of peripheral insulin resistance, was then calculated as FPG (mg/dL) multiplied by insulin (IU), divided by 405. Serum lipids concentration including total cholesterols, triglycerides (TG), high-density lipoprotein cholesterol (HDLC), and low-density lipoprotein cholesterol (LDL-C), were measured using enzymatic methods (Parsazmun, Karaj, Iran). In TG below 400 mg/dL, the Friedewald formula was applied for the calculation of Plasma Low-Density Lipoprotein Cholesterol (LDL-C) (mg/dL) [28]. This formula is an estimation of LDL-C level based on TC, TG, and HDL-C levels as described below:
Otherwise, the enzymatic method was used. Also, two hours later (10:00 am), 2-h postprandial glucose (2 h-PPG) was measured through the hexokinase enzyme method.
The Estimated glomerular filtration rate (eGFR) was calculated in participants by the Cockcroft-Gault formula [29] as described below.
Creatinine clearance (ml/min): [(140 − age) × body weight] /[plasma creatinine × 72] (× 0.85 if female).
The definition of TyG, VAI, LAP, and HOMA-IR indices are:
The TyG index was defined as the Ln [fasting TG (mg/dL) * fasting glucose (mg/dL)/2] for both genders.
The VAI index was calculated through sex-specific formulas; males (waist circumference (cm)/(39.68 + (1.88*BMI)) *(TG/1.03) *(1.31/HDL-C); females: (waist circumference (cm)/(36.58 + (1.89*BMI)) * (TG/0.81) *(1.52/HDL-C), where both TG and HDL-C levels are reported in mmol/L.
The LAP index was determined as (waist circumference(cm) – 65) * (TG (mmol/L)) in males and (waist circumference(cm) − 58) * (TG (mmol/L)) in females.
The HOMA-IR index was calculated by: fasting glucose in mmol/l*fasting insulin in μU/ml/22.5
Statistical analysis
SPSS version 24 was used for statistical analysis. The normality of the data was evaluated using Kolmogorov–Smirnov tests. Continuous variables with normal distribution are provided as mean ± standard deviation and categorical variables are presented as frequency and percentage (%).
The TyG, HOMA-IR, LAP, and VAI indices, were compared among patients with and without albuminuria by univariate analyses, using t-tests to compare continuous variables and chi-square for categorical ones between the groups.
ROC curve analysis was performed and the Youden index was used to calculate the optimized cut-offs value of TyG, HOMA-IR, LAP, and VAI indices.
The Logistic regression models were used to measure the relationship between the indices and albuminuria among T2D patients in univariate and multivariate logistic regression analyses. A univariate logistic regression analysis was conducted on each index. Two multivariable models were constructed for each index. Model 1 was adjusted for sex, age, blood pressure; other models (model 2) were adjusted for the duration of diabetes, blood LDL-C level, retinopathy, lipid-lowering agents use (divided into three categories), and waist circumference (was not adjusted in models which are included the LAP and VAI indices due to their formulas) in addition to model 1's confounders. Finally, model 2 was adjusted for HOMA-IR to demonstrate whether these indices remained independent (model 3). P-value less than 0.05 was considered statistically significant during analysis.
Results
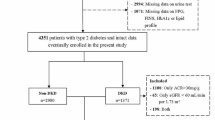
A total of 2934 participants with T2D were included in this study. Patients were divided into two groups depending on the presence of albuminuria. In 526 participants albuminuria was detected, and 2408 T2D patients did not meet the albuminuria criteria.
The mean age of participants was 56.46 years, of whom 52.9% were women. Albuminuria was more common in men (52.9%). In addition, 54.2% of patients without albuminuria were women.
Those with albuminuria had higher diastolic blood pressure and longer duration of diabetes (P- value < 0.001).
Also, more proportion of patients with albuminuria (140 patients: 26.7%) had cardiovascular diseases compared to another group (530 patients: 22.0%). Similarly, the proportion of patients with retinopathy in the group with albuminuria (128 patients: 24.4%) was higher than the other (222 patients: 9.2%). In addition, patients with albuminuria had higher FBS (182.39 mg/dL), HbA1c (8.32%), and Triglyceride (207.34 mg/dL) levels.
The total cholesterol and LDL-C were higher in patients with T2D and albuminuria compared to those without albuminuria (192.23 ± 50.97 VS. 179.38 ± 44.59, and 111.64 ± 69.36 VS. 101.56 ± 34.36 mg/dL, respectively (both P-values < 0.001)).
Moreover, TyG, HOMA-IR, LAP, and VAI indices were higher in patients with albuminuria (all P-values < 0.001).
However, age, non-HDL-C, HDL-C, eGFR, and the HbA1c did not show a significant difference (all P-values > 0.05) between the two groups (Table 1).
The multivariable logistic regression analysis revealed that TyG, HOMA-IR, LAP, and VAI were positively correlated with the incidence of albuminuria among patients with T2D, and they remained independent after adjustments for multiple confounders in all models. Furthermore, these models showed that the TyG index and albuminuria had the strongest association (OR 1.674 95% CI (1.376–2.037; P-value < 0.001), followed by HOMA-IR (OR 1.127; 95% CI 1.072–1.185; P-value < 0.001), VAI (OR 1.028; 95% CI 1.009–1.047; P-value < 0.003) and LAP (OR 1.004; 95% CI 1.002–1.006; P-value < 0.001). The regression analysis demonstrated that with each unit increase of the TyG index, the risk of developing albuminuria increased by 1.67 times, and this relation was independent of HOMA-IR (Table 2).
The ROC curve was utilized to evaluate the significance of these indices as an indicator for albuminuria, and it was determined that the TyG index plot had the largest AUC with sufficient accuracy (0.62 (range, 0.59–0.65)). On the other hand, the results showed that VAI had the smallest AUC 0.55 (range, 0.52–0.58), and the calculated AUC for HOMA-IR and LAP were 0.59 (range, 0.57–0.62) and 0.57 (range, 0.54–0.60), respectively (Fig. 1).
The best-calculated TyG cut-off for albuminuria evaluation was 9.39 with 0.64 and 0.55 sensitivity and specificity, respectively (Table 3).
Discussion
This study evaluated the potential association between TyG, LAP, VAI, HOMA-IR indices, and albuminuria in patients with T2D. This study’s main aim was to investigate which index was the most valuable for revealing albuminuria in T2D patients. In this cross-sectional study, we found that TyG, LAP, VAI, and HOMA-IR indices all had strong positive associations with albuminuria. This relationship remained significant after adjustments for multiple confounders. In addition, all of these models indicated that the TyG index was the best indicator of albuminuria independent of HOMA-IR. Also, the TyG index showed the greatest AUC in comparison with other indices.
There is a paucity of evidence in the literature regarding these indices and their relationship with albuminuria in patients with type 2 diabetes. Recent studies have shown that IR level is correlated with nephropathy and declining renal function [30, 31]. Due to lack of a low-cost and available technique for estimation of insulin sensitivity, TyG, LAP, VAI, and HOMA-IR indices were introduced as high-correlated indices with IR levels. Various studies have evaluated the relation of these indices with IR-dependent diseases, including diabetes, metabolic syndrome, cardiovascular diseases, and microvascular complications [32,33,34].
HOMA-IR is a model which has been used widely in studies and clinical practice to estimate IR [35]. VAI is a measurable index for estimating visceral adipose distribution, which is associated with vascular diseases [19, 36]. LAP is an index to assess central lipid distribution and lipotoxicity [37, 38]. LAP and VAI consist of anthropometric measures (such as waist circumference) and plasma laboratory data (such as triglyceride or HDL-C) in their formula. TyG index is another marker that has been proposed as a reliable index for evaluating the IR level, and studies emphasized its ability to evaluate microvascular damage [39].
Tang Chen et al. [40] found that the TyG index is more valuable than VAI and LAP for predicting CKD in the general population. Moreover, the mentioned study on the general Chinese population showed that LAP and VAI were not independent of albuminuria; however, in our study these associations were significant in all models.
Additionally, Dai D. et al. evaluated the relation of VAI and LAP and chronic kidney disease. They concluded LAP and VAI were related to kidney disease in a randomized rural population in China [41]. Nonetheless, in the present study, which only included patients with T2D, VAI and LAP were related to albuminuria.
Zhao S. et al. demonstrated an association between the TyG index and albuminuria among the elderly population (more than 65 years old) [42]. In parallel, our study revealed that the TyG index is associated with albuminuria among patients with T2D.
After examining LAP, VAI, and TyG indices, Fiorentino et al. concluded that the LAP index had the most correlation with IR and subclinical vascular damages. In Fiorentino’s study, subjects had been chosen from a population with different degrees of insulin sensitivity, including normal glucose tolerance, impaired glucose tolerance, impaired fasting glucose, and patients with T2D [43]. However, in this study, we evaluated patients with diabetes and IR, particularly.
Furthermore, Ying Pan et al. showed that there was a significant association between TyG index and albuminuria in hospitalized patients [44]. However, in our study we evaluated this relationship in an outpatient setting.
Conclusion
Of the calculated indices of insulin resistance in this study, we concluded that the TyG index had the most significant correlation with albuminuria in patients with T2D. Due to the simplicity and practicality of calculating the TyG index, its utilization would be a favorable method for evaluating albuminuria. Nevertheless, extensive and longitudinal research is needed to confirm these relationships.
Study strengths and limitations
This study compared the association of TyG, LAP, VAI, and HOMA-IR indices and albuminuria in the T2D population for the first time and indicated that all mentioned indices have a significant relationship with the presence of albuminuria. In addition, our study was conducted on a large cohort of individuals with T2D who were enrolled over a decade.
However, some shortcomings need to be interpreted in this study. First, this was a cross-sectional study that could not determine causality. Another drawback of this study was the inability to compare these indices with the hyperinsulinemic-euglycemic clamp test which is the gold standard method for detecting insulin sensitivity and glucose utilization.
Availability of data and materials
The dataset used in this study is available upon request from the corresponding author.
References
Iqbal N. The burden of type 2 diabetes: strategies to prevent or delay onset. Vasc Health Risk Manag. 2007;3(4):511–20.
Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88(11):1322–35.
Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313(8):837–46.
Murton M, et al. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: a systematic review. Adv Ther. 2021;38(1):180–200.
Lovre D, et al. Managing diabetes and cardiovascular risk in chronic kidney disease patients. Endocrinol Metab Clin North Am. 2018;47(1):237–57.
Oshima M, et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat Rev Nephrol. 2021;17(11):740–50.
Ohta M, et al. Comparison of the prevalence of chronic kidney disease in Japanese patients with Type 1 and Type 2 diabetes. Diabet Med. 2010;27(9):1017–23.
Lok CE, et al. The growing volume of diabetes-related dialysis: a population based study. Nephrol Dial Transplant. 2004;19(12):3098–103.
De Cosmo S, et al. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant. 2013;28(1):29–36.
Kaul K, Apostolopoulou M, Roden M. Insulin resistance in type 1 diabetes mellitus. Metabolism. 2015;64(12):1629–39.
Leyking S, Fliser D. Insulin resistance in CKD. Am Soc Nephrol. 2014. https://doi.org/10.2215/CJN.01290214.
Tam CS, et al. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35(7):1605–10.
Matthews D, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Matthews DR, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Otten J, Ahrén B, Olsson T. Surrogate measures of insulin sensitivity vs the hyperinsulinaemic–euglycaemic clamp: a meta-analysis. Diabetologia. 2014;57:1781–8.
Bonora E, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63.
Abbasi F, et al. Relationship between several surrogate estimates of insulin resistance and a direct measure of insulin-mediated glucose disposal: comparison of fasting versus post-glucose load measurements. Diabetes Res Clin Pract. 2018;136:108–15.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Amato MC, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes Care. 2006;29(1):151–3.
Ji B, et al. Association between the visceral adiposity index and homeostatic model assessment of insulin resistance in participants with normal waist circumference. Angiology. 2017;68(8):716–21.
Oh JY, Sung YA, Lee HJ. The visceral adiposity index as a predictor of insulin resistance in young women with polycystic ovary syndrome. Obesity. 2013;21(8):1690–4.
Mirmiran P, Bahadoran Z, Azizi F. Lipid accumulation product is associated with insulin resistance, lipid peroxidation, and systemic inflammation in type 2 diabetic patients. Endocrinol Metab. 2014;29(4):443–9.
Ebrahimi M, et al. Lipid accumulation product (LAP) index for the diagnosis of nonalcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Lipids Health Dis. 2023;22(1):41.
Nabipoorashrafi SA, et al. The accuracy of triglyceride-glucose (TyG) index for the screening of metabolic syndrome in adults: A systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2022;32(12):2677–88.
American Diabetes Association Professional Practice Committee. 2.Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(1):S17–38.
Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Liao MT, et al. Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol. 2012;2012: 691369.
Kobayashi S, et al. Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis. 2005;45(2):275–80.
Lambrinoudaki I, et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ. 2018;27(6):716–24.
Raimi TH, et al. Triglyceride-glucose index and related parameters predicted metabolic syndrome in Nigerians. Metab Syndr Relat Disord. 2021;19(2):76–82.
Gu S, et al. Insulin resistance is associated with urinary albumin-creatinine ratio in normal weight individuals with hypertension and diabetes: The REACTION study. J Diabetes. 2020;12(5):406–16.
Gayoso-Diz P, et al. Insulin resistance index (HOMA-IR) levels in a general adult population: curves percentile by gender and age. The EPIRCE study. Diabetes Res Clin Pract. 2011;94(1):146–55.
Bemelmans RH, et al. Increased visceral adipose tissue is associated with increased resting heart rate in patients with manifest vascular disease. Obesity. 2012;20(4):834–41.
Ayundini G, et al. A systematic review on the association between lipid accumulation product index and type 2 diabetes mellitus. J ASEAN Fed Endocr Soc. 2019;34(1):16.
Tongdee P, et al. Lipid accumulation product and index of central lipid distributions for subclinical atherosclerosis in perimenopausal/menopausal women. J Med Assoc Thai. 2016;7(7):S42–8.
Chiu H, et al. Associations between triglyceride-glucose index and micro-and macro-angiopathies in type 2 diabetes mellitus. Nutrients. 2020;12(2):328.
Chen T, et al. Comparison of novel metabolic indices in estimation of chronic kidney diseases in a southern Chinese population. Diabetes Metab Syndr Obes. 2020;13:4919–27.
Dai D, et al. Visceral adiposity index and lipid accumulation product index: two alternate body indices to identify chronic kidney disease among the rural population in Northeast China. Int J Environ Res Public Health. 2016;13(12):1231.
Zhao S, et al. Association between macro-and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18(1):1–8.
Fiorentino TV, et al. Relationships of surrogate indexes of insulin resistance with insulin sensitivity assessed by euglycemic hyperinsulinemic clamp and subclinical vascular damage. BMJ Open Diabetes Res Care. 2019;7(1): e000911.
Pan Y, et al. Association between diabetes complications and the triglyceride-glucose index in hospitalized patients with type 2 diabetes. J Diabetes Res. 2021. https://doi.org/10.1155/2021/8757996.
Acknowledgements
None.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
Data collection and manuscript writing: S.A.N, A.A, and S.A.S. Study design and data analysis: S.A.N. and S.R. Manuscript editing and tables creation: S.A.N and S.A.S. Validation and review: S.R, R.A.B, F.M, and A.Y. Quality control: A.Y, M.N, and A.E. All authors agreed on the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the Declaration of Helsinki principles and granted by the Tehran University of Medical Sciences ethics committee. Informed consent was obtained from all participants included in the study.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nabipoorashrafi, S.A., Adeli, A., Seyedi, S.A. et al. Comparison of insulin resistance indices in predicting albuminuria among patients with type 2 diabetes. Eur J Med Res 28, 166 (2023). https://doi.org/10.1186/s40001-023-01134-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01134-2