Abstract
Recently, mesenchymal stem/stromal cells (MSCs) therapy has become an emerging therapeutic modality for the treatment of inflammatory bowel disease (IBD), given their immunoregulatory and pro-survival attributes. MSCs alleviate dysregulated inflammatory responses through the secretion of a myriad of anti-inflammatory mediators, such as interleukin 10 (IL-10), transforming growth factor-β (TGFβ), prostaglandin E2 (PGE2), tumor necrosis factor-stimulated gene-6 (TSG-6), etc. Indeed, MSC treatment of IBD is largely carried out through local microcirculation construction, colonization and repair, and immunomodulation, thus alleviating diseases severity. The clinical therapeutic efficacy relies on to the marked secretion of various secretory molecules from viable MSCs via paracrine mechanisms that are required for gut immuno-microbiota regulation and the proliferation and differentiation of surrounding cells like intestinal epithelial cells (IECs) and intestinal stem cells (ISCs). For example, MSCs can induce IECs proliferation and upregulate the expression of tight junction (TJs)-associated protein, ensuring intestinal barrier integrity. Concerning the encouraging results derived from animal studies, various clinical trials are conducted or ongoing to address the safety and efficacy of MSCs administration in IBD patients. Although the safety and short-term efficacy of MSCs administration have been evinced, the long-term efficacy of MSCs transplantation has not yet been verified. Herein, we have emphasized the illumination of the therapeutic capacity of MSCs therapy, including naïve MSCs, preconditioned MSCs, and also MSCs-derived exosomes, to alleviate IBD severity in experimental models. Also, a brief overview of published clinical trials in IBD patients has been delivered.
Similar content being viewed by others
Introduction
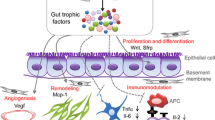
Inflammatory bowel disease (IBD) is a chronic disease of unknown origin characterized by serious inflammation and mucosal destruction in the intestine [1]. There are two chief forms for IBD: Crohn’s disease (CD), which is most communal in the colon and terminal ileum and can target the entire gastrointestinal (GI) tract, and ulcerative colitis (UC), which is a mucosal inflammation comprising the rectum and colon [2]. Nowadays, IBD is typically suggested to be induced through interfaces among environmental [3], genetic [4], infectious [3, 5], and immune factors [6]. Indeed, IBD is initiated and preceded by the coinciding of several genetic and environmental stimuli, which finally disturb the immune–microbiome axis (Fig. 1). Now, shedding light on the various aspects of IBD pathogenesis, with a particular emphasis on immunological pathogenesis to introduce novel and effective therapeutic approaches is of paramount importance.
The IBD pathogenesis. The interactions between environmental factors, genetic susceptibility, and microbial flora may perturb intestinal hemostasis and thus induce the transduction of dysregulated immune responses and underlie resultant tissue damage. Inflammatory bowel diseases (IBD), innate lymphoid cells (ILC), T helper cell (Th), interleukin (IL), transforming growth factor-beta (TGF-β), regulatory B cells (Bregs), regulatory T cells (Tregs), regulatory dendritic cells (rDCs), natural killer T (NKT) cells, tumor necrosis factor α (TNFα)
The existence of large quantities of symbionts near the epithelial surface of the intestine brings about a huge challenge to the host since it must constrain the triggering of dysregulated inflammatory responses to the microorganisms, while supporting its aptitude to trigger strong immune responses for attacking pathogens [6, 7]. The results derived from the genome-wide association studies (GWAS) have evidenced the correlation between the innate and the adaptive immune system in adjusting the sensitive equilibrium of the mucosal immune system [8, 9]. However, accumulating proof implies that a dysregulated immune response against the microorganisms in the intestine may lead eventually to IBD onset and progress in genetically susceptible individuals [10]. T helper cell (Th) type 1 (Th1) and Th17-mediated immune responses with improved levels of interleukin (IL)-1β, tumor necrosis factor-alpha (TNF- α), interferon-gamma (IFN-γ), IL-6, IL-8, IL-12, IL-17, and IL-23 are typically observed in CD patients, while Th2-associated immunological response with enhanced IL-4, IL-5, and IL-13 levels are a common pathological sign in UC patients [10,11,12]. Given the central role of immune response in IBD pathogenesis, scientists are trying to recognize and advance new and more efficient treatments based on the alleviation of dysregulated immune responses in IBD patients, leading to reduced disease severity and improved life qualities.
Growing reports show that mesenchymal stem/stromal cells (MSCs) trigger robust anti-inflammatory and immunoregulatory effects, causing improved tissue repair [13, 14]. MSCs have displayed appreciated therapeutic benefits in a large number of IBD animal studies and some clinical trials on patients suffering from IBD [15, 16]. Recent studies showed that CD-derived diseased mesentery tissue MSCs was found to lose their immunosuppressive capability in the treatment of CD by distinct regulation of pathogenic T-cell responses and/or T-cell infiltration into the colon [17]. However, exogenous MSCs could be an ideal source for IBD therapy. MSCs can engraft the intestinal mucosa, regulate inflammation and thus repair injured tissues. Transplanted MSCs, in fact, restore gut microbiome alteration and promote efficient eradication of pathogenic bacteria. MSCs-mediated immunomodulation is exerted through secretion of the multiple anti-inflammatory mediators, such as TNF-stimulated gene 6 (TSG-6), prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), and transforming growth factor-beta (TGF-β) [18, 19]. MSCs prohibit the Th1/17-mediated inflammatory responses, thus attenuating the levels of the inflammatory molecules and offering a suitable milieu for tissue recovery [20, 21]. They also potentiate the release of the inhibitory factor IL-10 by targeting macrophage polarization [22]. Apart from their capability to moderate immune response, MSCs augment intestinal epithelial cell (IECs) survival and proliferation and simultaneously upregulate the expression of tight junction (TJs)-related molecules in such IECs [23, 24]. These effects, in turn, maintain the intestinal integrity and support barrier function [25]. Noteworthy, current studies have shown that using the pre-conditioned MSCs [26, 27] or MSCs-derived exosome [28, 29], as an innovative cell-free approach, can be more favored strategy compared to naïve MSCs cells therapy. Modified MSCs or MSCs-derived exosomes could circumvent MSCs' low migration and engraftment to colon tissue, making them a rational and effective therapeutic strategy [30]. This review provides an overview for elucidating the therapeutic effects of MSCs therapy in IBD, with a special focus on recent in vivo studies.
Immunological pathogenesis of IBD
The IBD is an autoimmune condition of the GI tract characterized by loss of tolerance to self-antigens and intestinal flora, preceded by over-activated and destructive immune responses. Although the detailed etiopathogenesis of IBD has remained elusive, genetic predisposition, dysbiosis, environmental factors, and aberrant immune responses have all been implicated [31]. The main stimuli of persistent inflammation and tissue destruction in IBD patients are excessive immune cell infiltration in colonic lesions and their products, which upregulate pro-inflammatory cytokines and chemokines expression levels [32].
Several immunopathological studies on IBD have demonstrated that IL-17-producing cells, Th17, play a central role in IBD development, while its suppression in patients with acute colitis may reduce inflammation and thus mitigate disease severity [33, 34]. The IL-17 and signal transducer and activator of transcription 3 (STAT3) are upregulated in inflamed colon tissue as compared to healthy counterparts [35]. Mechanistically, IL-17 activates STAT3, which provokes chronic inflammatory immune responses; hence IL-17 inhibition mediated by phosphorylated STAT3 can potentially reverse inflammation and IBD progression [36]. Besides, the bidirectional interactions of Tregs and Th17 modulate Th17-mediated immune responses. In fact, Th17 and Tregs interactions and a balance between them is a crucial prognostic indicator in the immunopathogenesis of IBD [37]. In light of this, experimental colitis models have indicated that Foxp3+ Tregs have anti-inflammatory functions in the intestine by suppressing Th17 responses and thus reducing Th17/Treg ratio [38, 39].
Other cytokines with significant implications in IBD pathogenesis are the IL-1 family members. IL-1β expression and function are mostly associated with innate immune cells, such as macrophages and monocytes. However, in UC patients, it is expressed in colonic mucosa and enhances inflammation [40]. Similarly, another IL-1 family member, IL-18, is abundantly expressed in the mucosa of CD patients, enhancing Th1 response while interfering with immunoregulatory cytokine release from mucosal T cells, such as IL-10 [41]. Despite contradictory findings on the IL-10 level in IBD patients, it is well documented that IL-10 down-regulation is associated with detrimental effects and disease progression [42]. Moreover, IL-33 and its cognate receptor, ST2, are upregulated in UC patients and can positively regulate IL-5 and IL-13 expression, leading to enhanced Th-2 response and tissue protection [43, 44]. Similarly, TNF-α could enhance the levels of IL-1β, IL-6, and IL-33 in IBD patients, thus its level negatively correlates with the clinical outcome of these patients [45, 46]. Also, TGF-β could dampen inflammation by suppressing IL-33, promote epithelial compensation and fibrosis and maintain intestinal homeostasis and mucosal tolerance [47]. Furthermore, IL-6 and its soluble receptor is overexpressed in UC and CD patients and promotes inflammation via STAT3 activation and substantially contributes to the development of colorectal cancer (CRC) in UC patients [48, 49].
A growing body of evidence indicates that chemokines are critical not only for systemic inflammation, but also for homeostasis and immune regulation [50, 51]. Multiple chemokines are released by a variety of immune cells infiltrated in IBD lesions, including macrophages and neutrophils. These cells serve key roles in the development and progression of IBD [52]. In this context, upregulated levels of chemokine ligand (CCL) 2, CCL4, CCL7, and C-X-C motif chemokine ligand 10 (CXCL10) in IBD tissues outlines their significance in immune infiltration and disease severity [53]. These discoveries have paved the path for the development of therapeutic strategies to target various chemokines in IBD patients.
Interaction between MSCs and immune cells
Given their unique and robust immunoregulatory competencies, MSCs moderate immune responses during tissue repair and offer a suitable milieu for tissue regeneration [54]. Based on the molecular and cellular analysis, a diversity of soluble factors in association with cell contact-mediated process in response to the immune cells or other stimuli’s involves in MSCs-induced immunomodulation [55, 56]. MSCs principally regulate the adaptive and innate immune responses through the influencing T cells activation and differentiation and dendritic cells (DCs) maturation. For instance, MSCs inhibit DCs type 1 (DC1) activation to downregulate TNF-α secretion while promoting IL-10-secreting DC2 performance in rodent models of IBD [57]. MSCs also affect DCs to substantially produce galectin 3 (Gal-3) in rodents with colitis [58]. The Gal-3 is a lectin that participates in immunosuppression and adjust various functions such as cellular homeostasis [59].
Also, they downregulate B-cell as well as NK cells activation and conversely promote the growth of T regulatory (Treg) cells by both secretions of soluble factors and cell-to-cell contact [60, 61]. MSCs also inhibit the activation of pro-inflammatory macrophages (M1Mφ), TH1, and TH17, while supporting TH2 and anti-inflammatory macrophages (M2Mφ) largely by secreting IL-10 and TGF-β [62].
The inhibitory effects of MSCs on immune cells are depicted in Fig. 2.
The effects of the MSCs on immune cells functions may vary depending on the MSCs sources [63] and also target tissue [64]. For instance, immunoregulatory potential of adipose tissue (AT)-derived MSCs is stronger than bone marrow (BM)-derived MSCs on peripheral blood mononuclear cell (PBMC) proliferation. [65]. Also, in terms of production of IL-2, IL-4, IL-13, and GM-CSF, chorionic plate-derived MSCs (CP-MSCs) have superiority over BM-MSCs and AT-MSCs [66]. Another study also signified that the inhibitory effects of the BM-MSCs are higher than placenta‐derived MSCs (PD‐MSCs) on T cell activation [67]. In sum, there is universal agreement concerning the immunomodulatory effects of MSCs; however, further study to elucidate the detailed biological mechanisms behind these effects is required.
The rationality of MSCs therapy in IBD
Anti-inflammatory effects
The CD is usually described as a Th1/17-associated disorder since the main inflammatory cytokines in this condition are the Th1/17-related molecules like IL-12, IL-17, IFN-γ, and TNF-α. On the other hand, UC is typically described as a Th2-associated disease due to the upregulated intestinal expression of the Th2-related cytokines (e.g., IL-5, IL-13, and IL-4) [68, 69]. In light of this, the immunological basis of the IBD implies that MSCs-based cell therapies can be a rational therapeutic modality to alleviate IBD pathological signs due to their capacity to moderate inflammatory responses.
Accumulating evidence indicates that MSCs modify immunological response through various mechanisms, such as suppression of T cell-mediated inflammatory responses, targeting dendritic cells (DCs) maturation and phenotype shift and also promoting anti-inflammatory M2 macrophages polarization [70]. In light of this, MSCs administration (1 × 106 cells) stimulated DCs differentiation into regulatory DCs (rDCs) with potent immunoregulatory properties and enhanced colon length, body weight, and overall survival (OS) rate in DSS-induced colitis mice [71]. The rDCs are also implicated in inducing and maintaining the homeostasis of Tregs. MSCs transplantation also decreased expression of IL-6, TNF-α, and IFN-γ and concurrently increased the levels of IL-10, TGF-β, and forkhead box protein P3 (Foxp3), a crucial regulator of Tregs, in colon tissues [71]. Also, MSCs could decrease CD4 + T cell proliferation, inhibit Th1/Th17 cells activation, while inducing Th2 cells function [72]. These events finally diminish pro-inflammatory IL-17 and IFN-γ, and improve TGF-β, IL-10 levels in colon tissue of MSCs-treated IBD models [73]. As well, MSCs could improve the Tregs population and decrease IFN-γ secretion by natural killer (NK) cells in inflamed tissue [74]. Regardless of the pro-inflammatory cytokines, systemic administration of MSCs could affect T-box expressed in T cells (T-bet) and retinoid-related orphan receptor gamma(t) (RORγt) expression in T cells [21]. T-bet and RORγt, as a central regulator of Th1 and Th17 cells, respectively, act as causative factors in IBD progress. Thus, their inhibition by MSCs-secreted mediators could lead to the suppression of Th1/Th17-related pathological events in IBD patients.
Irrespective of cited mediators, PGE2, hepatocyte growth factor (HGF), IDO, and also nitric oxide (NO) involve in MSCs-mediated immunoregulation [14, 75,76,77]. Park and coworkers (2018) exhibited that AT-derived MSCs administration could alleviate inflammatory response in DSS-induced mice with chronic colitis by promoting M2 macrophage polarization as a result of PGE2 secretion [78]. In vitro, AT-MSCs-secreted PGE2 attenuated the proliferation of THP-1 cells, a human leukemia monocytic cell line, and diminished the secretion of inflammatory cytokines IL-1β and IL-18 by macrophages [78]. AT-MSC-secreted PGE2 also attenuated inflammation by enhancing Foxp3 + Tregs numbers in DSS-induced mice colitis [79]. In the DSS-induced colitis model, recent results also exhibited that (umbilical cord blood) UCB-derived MSCs pre-conditioned with IL-1β and IFN-γ could diminish Th1 cell differentiation and stimulate Tregs differentiation in colon tissue, which mainly caused by PGE2 and IDO delivery to target tissue [80]. Also, there is clear evidence that HGF and TSG-6 released by MSCs could attenuate colitis [81]. Li et al. [82] revealed that dental pulp stem cells overexpressing HGF (HGF-DPSCs) decreased intestinal mucosa damage in part through the transdifferentiating into an intestinal stem cell (ISC)-like cells, stimulating ISC-like cell growth, inhibition of the intense immunological responses, and plummeting oxidative stress in DSS-induced ulcerative colitis. Besides, TSG6, a 30-kDa protein, which is secreted by activated macrophages and MSCs, inhibits the association of toll-like receptor 4 (TLR4) with myeloid differentiation primary response 88 (Myd88), thereby down-regulating nuclear factor (NF)-κB activation [83, 84]. In addition, TSG6 stimulates a macrophage phenotypic shift from M1 to M2, having important role in alleviating DSS-induced colitis [85], whereas TSG6−/− MSCs did not suppress the mucosal inflammatory response in colitis' mice [86]. Recently, bioinformatics analyses showed that UC includes communication between macrophages and enterocytes via ligand–receptor pairs, and AT-MSCs may alleviate this condition by communicating with macrophages to block inflammation [87]. Besides, Liu et al. [88] declared that MSCs could improve DSS-induced colitis in part by adjusting the Tregs–immunoglobulin A (IgA) response, increasing the secretion of IgA, and enabling the restoration of intestinal microbiota. These findings offer a potent therapeutic mechanism for MSCs in the IBD treatment.
Supporting intestinal barrier
The intestinal barrier makes a separation between the body and the contents of the intestine. It consists of various sections, including a mucus layer comprising antibacterial peptides lining the luminal surface of the epithelium, the epithelial cell monolayer, junctional proteins, intraepithelial lymphocytes (IELs), and also a subepithelial layer of extracellular matrix (ECM) and mesenchymal cells like myofibroblasts and fibroblasts [89, 90]. The integrity of the intestinal barrier depends on several contributors, such as strong innate immune responses, epithelial paracellular permeability, epithelial cell integrity, and the secretion of mucus [91]. The intestinal barrier provides a shield versus potentially damaging molecules and also pathogenic bacteria, thus supporting intestine immune homeostasis [92]. Intestinal epithelial cells (IECs) play a critical role in maintaining the barrier's integrity given their anatomical and functional location. On the luminal side, IECs release and adjust the contents of the mucus layer, whereas they interrelate and cross-talk with the underlying cells on the basolateral side [93, 94]. In health, IECs underlie a persistent intestine barrier due to the action of tight junction (TJ) proteins [95]. The TJ typically is formed by transmembrane proteins like claudins and occludin accompanied by cytoplasmic proteins like zonula occludens (ZO)-1 [96]. In normal conditions, the expression of TJ proteins contributes to the adjusting of colonic permeability during a regulated process in the intestine [97]. Notwithstanding, these proteins are susceptible to colitis and reduced expression and function of epithelial occludin have been found in IECs of UC patients [98, 99]. Indeed, impairment in the function of intestinal epithelial barrier is a prominent property of IBD.
Recent reports have exhibited that BM-MSCs transplantation may promote the proliferation and inhibit the apoptosis of IECs, thus favoring cellular homeostasis and intestinal integrity in vivo [100]. Also, MSC-derived conditioned media (CM) was capable of promoting injured fetal intestinal epithelial cells (FIEs) proliferation in vitro potently via IL-6, HGF, and vascular endothelial growth factor (VEGF) delivery, largely contributing to IECs integrity and simultaneously suppressing apoptotic pathways [101]. As well, activation of phosphatidylinositol-3-kinase (PI3K)/AKT pathways in IECs and resultant IECs proliferation were observed upon MSCs-derived CM injection by intravenous route in DSS and TNBS-induced colitis [102]. PI3K/Akt pathway controls IECs proliferation via modification of the availability of functional cyclin D1 protein [103]. In addition, Yang et al. [18] revealed that induced pluripotent stem cells (iPSC)-derived MSCs could boost IECs proliferation to ameliorate mucosal healing in a mice colitis model by TSG-6 secretion. In vitro, TSG-6 could promote Akt phosphorylation in mice colonoids (primary cultures derived from intestinal crypts), reflecting the key role of Akt activation in the TSG-6-mediated proliferation of IECs [18]. Similarly, administration of iPSC-MSCs promoted IECs proliferation, raised the Lgr5 + ISCs frequencies, and potentiated intestinal angiogenesis in colitic rodents [16]. Positive regulation of Lgr5 + ISCs proliferation and differentiation, as shown by exogenous PGE2 injection, may augment intestinal integrity and promote mucosal healing in IBD patients [104].
In addition to targeting IECs and ISCs biological process, treatment of colitic rats with AT-MSCs-derived CM enhanced the expression of mucin 2 (Muc2), the major colonic mucin which its deficiency leads to the perturbation of colon tissue hemostasis [105]. Muc2−/− mice have bacteria in direct communication with the IECs and far down in the crypts, sustaining inflammation and cancer [106]. AT-MSCs-derived CM also upregulated the expression of TJ-related proteins, claudin-1, and ZO-1 [105]. Both claudin-1 and ZO-1 are known for their barrier-forming abilities and serve urgent roles in protecting intestinal function and integrity [107]. Recent results have shown that miR-181 containing MSC-derived exosome [108] and also superoxide dismutase 3 (SOD3) overexpressing MSCs [109] can promote claudin-1 and ZO-1 expression and consequently upgrade intestinal barrier function, conferring the unique competencies of MSCs-based cell therapies to support intestinal barrier in IBD patients.
Preclinical studies based on MSCs therapy in IBD
Naive MSCs
A growing body of proof suggests MSCs as a promising therapeutic tool for IBD treatment mainly due to their immunomodulatory and anti-inflammatory attributes. In addition, they stimulate colon epithelial integrity and repair by increasing the proliferation of IECs in part by enhancing circulating insulin-like growth factor-1 (IGF-1) in colitis rodents [110]. In this section, we have focused on the transplantation of naïve MSCs in IBD preclinical models to elucidate the affiliated mechanism behind the observed in vivo desired effects (Table 1).
Recent studies display that intraperitoneally injected MSCs (2 × 106) could diminish colitis development and also decrease serum levels of IL-1β, IL-12, and IL-6 in vivo [58]. The anti-inflammatory effects might arise from the inhibition of the inflammatory phenotype of DCs in colon tissue by MSCs-secreted Gal-3 [58]. In addition to the negative regulation of Th1, galectin-3 inhibits DCs-mediated Th17 polarization in response to the dectin-1 agonist curdlan and lipopolysaccharide (LPS), which acts as an inducer of TLR2/4 pathways and thereby promotes inflammation [111]. Notably, Gal-3−/− DCs produce more prominent levels of the Th17-secreted IL-23 than Gal-3+ / + DCs, highlighting its significant role in MSCs-mediated anti-inflammatory effects in colitis [111]. Likewise, Jo et al. [71] presented that rDCs stimulated by MSCs decreased the severity of colitis in mice by lowering the levels of inflammatory mediators, while favoring IL-10, TGF-β, and Foxp3 levels in lesion sites. The TGFβ and IL-10 accompanied with PGE2 are recognized to serve a key role in the induction of rDCs in colon tissue. Thereby, it seems that MSCs favor rDCs differentiation in a paracrine manner [112]. Irrespective of rDCs, Tregs and responding anti-inflammatory mediators participate in the attenuation of colitis severity following MSCs therapy [113]. For instance, Lu and coworkers [113] showed that systemic administration of gingiva (G)-derived MSCs into a mice colitis model of IBD sustained their OS and relieved disease-associated pathological symptoms. These effects were due to the robust enhancement in Tregs, inhibited release of pro-inflammatory cytokines, and augmented levels of anti-inflammatory cytokines [113]. Importantly, the anti-IL-10R antibody abolished the protective impacts of G-MSCs, thereby indicating the key role of IL-10 in this light [113]. Further, IP administration of UCB-MSCs reduced T cell infiltration into the inflamed colon and, while improving Tregs population in a mouse model of IBD [114]. The treated mice showed lower destruction of the mucosal epithelium and diminished focal crypt lesions and goblet cell loss [114]. These findings offer further proof representative of the significant role of Tregs in protecting against colitis in vivo. Also, systemic injection of AT-MSCs (1 × 106) compromised DSS-induced colitis via two main mechanisms: improving Tregs frequencies and stimulating M2 polarization [115].
Recent study in a TNBS-induced colitis mice model, human UC-MSCs transplantation protected against experimental colitis by promoting CD5 + B cells and IL-10-secreting CD5 + regulatory B cells (Bregs) [116]. It has previously been evinced that deficiency or reduction of Bregs function intensifies intestinal inflammation in mice models and is in association with IBD pathogenesis [117]. In addition to the enhanced Bregs population, human UC-MSCs therapy led to improved Tregs while decreasing Th1/Th17 cell populations in colon tissue of treated mice [116]. Treatment improved OS rate, attenuated symptoms and ameliorated macroscopic and histologic scores in experimental mice [116]. Further analysis demonstrated that MSCs-secreted thrombospondin-1 (THBS1) may boost IL-10 + Bregs and control the development and recurrence of colitis [118]. These results confer the pivotal role of Tregs, rDC, and also Bregs in adjusting IBD progress and clarify its importance for further investigations to provide a new therapeutic avenue.
Currently, up-regulation of endoplasmic reticulum (ER) stress-related proteins was exhibited after systemic infusion of UC-MSCs in an experimental model of colitis [119]. Appropriate ER function, as induced by MSCs therapy, plays a key role in protecting intestinal homeostasis and is required for protein folding, modification, and secretion [120, 121]. The UC-MSCs transplantation reduced the disease activity index (DAI) score, which is composed of the alteration of body weight, diarrhea, hematochezia, and weakened neutrophil infiltration [119]. Also, UC-MSCs injection diminished the destructive effects of MMP2 and MMP9 activation [119]. Thus, down-regulation MMPs can be an efficient therapeutic plan to restrain IBD severity [119]. Reduced levels of MMP-2 are also paralleled by a lessened infiltration of immune cells and pro-inflammatory cytokines levels [119]. Thus, the key function of MMPs and ER stress in the induction of colitis makes them and ideal targets for IBD treatment. Interestingly, MSCs also could sustain IECs proliferation by activating the Wnt signaling pathway and inhibiting apoptosis-inducing proteins [122]. This effect, in turn, leads to amelioration of epithelial integrity and then inhibits uncontrolled signal transduction between the epithelium and adjacent immune cells [123]. Furthermore, MSCs promote the expression of TJs proteins in IECs, thereby reducing inflammation-stimulated permeability [124].
Some proofs imply that combination therapies with MSCs and other therapeutics may ultimately result in more favored effects than alone MSCs therapy in vivo [125,126,127,128]. For example, human UCB-derived platelet lysate enhanced the immunomodulatory effects of AT-MSCs in the experimental model of IBD, causing a reduction in colitis scores, growth of the inflamed colon region, and inflammatory cytokine levels [126]. Likewise, IP injection of AT-MSCs in combination with oral administration of sulfasalazine, an anti-rheumatic drug with immunomodulatory potential, could alleviate TNBS-induced colitis in vivo through the improving M2/M1 macrophage ratio, attenuation of monocyte chemoattractant protein-1 (MCP-1/CCL2), CXCL9, and improving IL-10, arginase 1 (Arg-1) levels [127]. The down-regulation of MCP-1 and CXCL9 levels in colon tissue declines the migration and infiltration of monocytes/macrophages, finally lowering colon inflammation [129]. Also, sulfasalazine, in combination with AT-MSCs, was found to downregulate the NF-κB signaling pathway, while enhancing the B-cell lymphoma 2 (Bcl-2)/Bcl-2-associated X protein (Bax) ratio in colon tissue of the rats [127]. The achieved results verify the potent anti-inflammatory and pro-survival capacity of the used combination treatment in treated animals. In another study, wogonin, a natural flavonoid, improved the therapeutic effects of MSCs on DSS-induced colitis in part via increasing IL-10 expression [128]. In vitro analysis showed that wogonin could promote IL-10 secretion from MSCs by prompting transcript factor hypoxia-inducible factor 1-alpha (HIF-1α) expression through up-regulation of AKT/glycogen synthase kinase β (GSK3β) signal pathway [128]. Furthermore, in TNBS-induced rat colitis, granulocyte colony-stimulating factor (G-CSF) addition to MSC improved the recruitment of MSCs to the colonic mucosa and then reduced DAI score, MPO function, TNF-α levels, and NF-κB p65 expression more prominently compared with rats receiving MSCs alone [125].
Preconditioned MSCs
As described, decreased MSC in vivo activities post-transplantation were supposed to be the chief cause for their restricted therapeutic influences. Thereby, scientists have sought various strategies to augment the therapeutic influences of MSCs. Among them, pre-conditioning has attracted increasing attention. Pre-conditioning mainly depends on a myriad of methods to enhance the MSC's therapeutic competencies in vivo [130]. Hypoxia, incubation with pharmacological/chemical agents or biomolecules (e.g., trophic factors and cytokines), pre-conditioning with physical factors, and finally genetic engineering is the most crucial of them [131]. Such strategies potentiate MSCs’ proliferative, secretory, migratory, and differentiated abilities, providing more favored outcomes in vivo post-transplantation [132, 133]. For instance, [134] found that hypoxic-preconditioned MSCs decrease colon inflammation more evidently compared to normoxic-MSCs largely by up-regulating iNOS expression in MSCs [134]. The miR-216a-5p secreted from hypoxic-MSCs also demonstrates better therapeutic efficiency in experimental colitis in part by promoting the M2 macrophage phenotype [135]. Likewise, AT-MSCs induced with IFN-γ and kynurenic acid substantially upregulated the expression and secretion of IDO-1, finally alleviating CD pathology-like colitis injury and fibrosis in vivo [136]. In addition, MSCs co-cultured with peripheral blood mononuclear cell (PBMC) have demonstrated an upregulated expression of CXCL9 and CXCL10, leading to stronger T cell suppression. PBMC also could increase VEGF, HGF, FGF and CCL2 expression by MSCs [137]. Likewise, MSC were more metabolically active and generated larger amounts of G-CSF, IL-6 and MCP-1 upon exposure with PBMCs [138]. The overview of published studies based on the application of pre-conditioned MSCs is offered in Table 2.
Pre-treated (primed) MSCs
Current consequences have displayed that stimulation with TNF-α boosts PGE2 synthesis in MSCs and thus constrain the proliferation and differentiation of T lymphocytes and macrophages in the inflamed colon in IBD preclinical models [139]. Regardless of the immunomodulatory traits, PGE2 promotes intestinal repair [140], stimulates epithelial cell proliferation through activating the EGFR axis, and inhibits apoptosis by up-regulation of Bcl-2 and NF-κB [141]. Given that the colonic mucosa isolated from IBD patients has lower levels of PGE2 than normal mucosa, it seems that low PGE2 levels may trigger the initiation of inflammation in IBD patients [142]. Also, short in vitro pre-treatment with polyinosinic: polycytidylic acid (usually abbreviated poly I: C) could augment the therapeutic efficacy of UC-MSCs in DSS-induced mice model of colitis [143]. Meanwhile, IP administration of 1 × 106 poly I: C pre-treated UC-MSCs reduced the clinical and histopathological severity of colitis in comparison to the naive UC-MSCs administration [143]. UC-MSCs priming with poly I: C also decreased pro-inflammatory cytokines levels, improved IL-10 levels in colonic tissues, mitigated Th1/17 cell proliferation, and improved Treg differentiation in vivo [144]. Improvement in the production of PGE2 by UC-MSCs in response to TLR3 activation by poly I: C is thought to be responsible for the positive effects observed in treated mice [144]. Besides, although IL-1β is known as the most important inflammatory mediator, IL-1β pre-treated MSCs have shown better efficacy in the treatment of DSS-induced colitis [145]. The pre-treatment of MSCs with IL-1β may modify the balance of immune cells in the spleen and the mesenteric lymph nodes (MLNs) by improving COX-2, IL-6, and IL-8 expression accompanied by augmenting M2/M1 macrophage ratio [145]. Notably, IL-1β also up-regulates CXCR4 expression in MSCs, thereby can improve their migration ability to the inflammatory site of the intestine post-transplantation [145]. Irrespective of colon damage, IL-1β stimulation can improve the homing capacity of MSCs by enriching CXCR4 expression in animal models of liver injuries [146]. Besides, the therapeutic merits of IL-1β pre-treated MSCs as a result of improving their anti-inflammatory influences also have been documented in neurodegenerative diseases [147]. Such effects make IL-1β an efficient biomolecule to sustain MSCs migration and favor their therapeutic efficacy in IBD patients. Likewise, systemic injection of MSCs upon exposure with IL-25 decreased infiltrating inflammatory cells frequencies and enhanced Tregs in serum and colonic mucosa of the IBD rat model, leading to inhibited intestinal inflammation and decreased DAI score [148]. The IL-25 has dual immunomodulatory possessions: it improves Th2-associated immune responses and conversely suppresses Th1 and Th17 cell-associated immune responses [149, 150]. In this light, IL-25 pre-treated MSCs can induce the desired effect on Th1- and Th17-mediated pathological conditions like CD [26]. Further, IL-25 improves the potential of MSC to trigger IECs regeneration, and thus MSC therapy with IL-25 could be a new road for IBD therapy [151]. In addition, Yu and colleagues [80] exhibited that IFN-γ increases the efficacy of human UCB-MSCs transplantation by improving PGE2 release and IDO activity in IBD animal models. PGE2 in association with IDO inhibits Th1 cell differentiation and enhances Tregs differentiation, suggesting that a combination of PGE2 and IDO may be effective therapeutic mediators for potentiating the MSCs-induced immunosuppression [80]. Importantly, IFN-γ pre-treated MSCs may stimulate ISCs proliferation and enterocyte differentiation in vivo, thus easing intestinal repair in IBD murine models [152]. As well, TNFα and IFNγ treatment caused rapid consumption of glucose and metabolic skewing toward glycolysis in MSCs, largely increasing the efficacy of MSCs in IBD [153]. Molecular analysis signified that PI3K–AKT signaling axis was rapidly induced and required for the skewing toward glycolysis stimulated by TNFα and IFNγ [153].
Based on the recent finding, hypoxic pre-conditioning can promote the anti-inflammatory and proliferative potential of MSCs in colitis in part by promoting NO production through iNOS activity in MSCs [134]. The NO adjusts various key activities of the GI mucosa, including maintenance of adequate perfusion, adjustment of microvascular and epithelial permeability, and controlling of the immune response [154]. In the inflamed regions, MSCs-secreted NO alleviates oxidative stress [155] and suppresses NF-κB translocation, thereby exerting a protective effect on intestinal cells [156]. Albeit, aberrant expression of NO have a pathogenic role in UC [157], but not CD [158], highlighting the importance of conducting more comprehensive studies on the various aspect of MSCs-secreted NO on IBD progress or therapy.
Genetically modified MSCs
The therapeutic potential of MSCs has been assessed in various reports, particularly concerning their immunomodulatory and pro-regenerative traits. Notwithstanding, limited engraftment and inadequate favorable influences of MSCs illuminates the necessity of designing novel strategies to improve their survival, migration, and beneficial capability. Genetic engineering of MCSs has been developed as a valued tool to trigger the expression of diverse proteins and soluble mediators with an extensive spectrum of utilities like microRNAs, growth factors, enzymes, cytokines, chemokines, and transcription factors [159, 160].
Current reports exhibit that overexpression of various genes such as intercellular adhesion molecule (ICAM) [30], CXCR4 [161], and CXCR2 [162] can improve MSCs homing to the injured area and thus enhance succeeding anti-inflammatory and pro-survival effects in IBD animal models. Meanwhile, Liu et al. displayed that ICAM-1-overexpressing MSCs improved recovery and diminished pathological damages in the IBD mice model more evidently than naïve MSCs [30]. ICAM-1 overexpressing MSCs also reduced Th1 and Th17 subpopulation while enhancing Tregs frequency in the spleen of treated mice, ensuring down-regulated IFN-γ and IL-17A and upregulated Foxp3 levels [30]. Likewise, CXCR‑4 overexpressing MSCs induced better migration and homing potential in IBD murine model and also exerted superior influences on treating colitis compared with naïve MSCs [161]. Also, they enhanced the levels of occludin and VEGF in treated IBD murine, suggesting their capacity to alleviate colitis by supporting intestinal integrity and mucosa repair [161]. As described, a reduction in pivotal TJs proteins like occludin and ZO-1 is observed in both IBD and experimental models of inflammation [163]. Studies in UC patients exhibit that attenuation of occludin expression and enhancement in claudin 1/occludin ratio has a close association with UC severity [163]. Thus, MSCs injection can diminish disease severity in IBD patients by improving the expression of ZO-1 and occludin and consequently repairing the intestinal barrier [29]. In addition, overexpression of long non-coding RNA H19 (H19) may decrease miR-139 and miR-141 expression in MSCs, thereby promoting the functions of their responding targets ICAM-1 and CXCR4, respectively [164]. Mechanistically, upregulation of ICAM-1 and CXCR4 in H19-overexpressing MSCs potentiates their migration and homing post-transplantation [164]. H19 also can trigger IECs proliferation and precede the expression of TJ-related proteins, thus heightening intestinal integrity [165].
Other studies have focused on enriching the anti-oxidant potential of MSCs using genetic engineering. Nuclear factor erythroid 2-related factor (Nrf2) signaling adjusts multiple gene expressions by making interfaces with the anti-oxidant response element (ARE). Up-regulation of the Nrf2/ARE axis dampens numerous pathologic mechanisms correlating with the autoimmune response and also IBD. Recently, Zhou and colleagues [166] showed that systemic administration of Nrf-2-overexpressing hair follicle (HF)-MSCs (1 × 106 cells) had a more prominent therapeutic effect in colitic rats compared with naïve MSCs. Nrf-2-overexpressing HF-MSCs favored intestinal integrity, improved IL-13 and IL-10 expression in colon tissue, and reduced DAI scores in treated rats [166]. Genetically modified HF-MSCs to overexpress Nrf-2 [166] and HGF [82] also could attenuate oxidative stress in colitic rats, as documented by a decline in malondialdehyde (MDA) and myeloperoxidase (MPO), while promoting SOD. Genetically modified MSCs to overexpress HGF also could decrease TNF-α and IFN-γ levels, enhance IL-10 levels, upregulate the expression of TJ-related protein ZO-1, and finally promote the proliferation of IECs in radiation-induced intestinal injury (RIII) [167]. Thereby, promoting HGF expression may be an efficient approach to ameliorate IBD as a result of down-regulation of inflammation and maintaining intestinal integrity.
Genetic modification of MSCs to overexpress HIF-1 [168], IFN-y [169], and IL-35 [170] exhibited great potential to raise MSCs’ anti-inflammatory and pro-regeneration capabilities. Accordingly, HIF-1 overexpressing MSCs could stimulate M2 macrophage polarization in a TNBS-induced mouse colitis model and also improve colon length and intestinal mucosa integrity [168]. Besides, IFN-γ overexpressing MSCs could induce more strong immunosuppressive impacts on the proliferation of T cells than naive MSCs in vitro [169]. The systemic injection of manipulated MSCs to overexpress IFN-γ, in turn, attenuated the severity of colitis, as evidenced by improved body weight, enhanced colon length, reduced DAI score, and amelioration of small intestine tissues structure [169]. The positive effects largely were dependent on the promoted Tregs and Th2 cells frequencies both in mesenteric lymph node and spleen, increased IDO expression accompanied by decreased inflammatory cytokine levels in colon tissue of treated models [169]. Further, IL-35 has currently been described as an immunosuppressive cytokine acting as an inhibitor of chronic inflammatory and autoimmune diseases [171, 172]. Wang et al. [173] exhibited that IL-35 recombinant protein has multiple anti-inflammatory impacts in IBD experimental models, including reducing the infiltrations of macrophages, CD4 + T, and CD8 + T cells and potentiating the infiltration of Treg cells. In this light, Yan et al. [170] evaluated the potent effects of IL-35 overexpressing MSCs in a dextran sodium sulfate (DSS)-induced colitis mice model. IL-35 overexpressing MSCs displayed superiority over naïve MSCs in terms of colon length and Tregs population in colon tissue of treated mice. They concluded that engineered MSCs to overexpress IL-35 could mitigate IBD severity largely via lowering the levels of the pro-inflammatory cytokine [170].
MSCs-derived exosome
Exosome superiority over parental MSCs
Exosomes are a most eminent subtype of extracellular vesicles (EVs) with a diameter in the variety of 30–100 nm. They are secreted by human cells, such as stem cells, and target biological processes in recipient cells [174]. Exosomes include various biomolecules like proteins, lipids, messenger RNA (mRNA), and microRNAs (miRNAs) as cargo. Their contents also could be varied depending on the stem cell environment. They are produced and released in a highly adjusted process: construction of endocytic vesicles by invagination of the plasma membrane, formation of multivesicular bodies (MVBs) following endosomal membranes' inward budding, and lastly, integration of MVBs with the plasma membrane and secretion of the vesicular contents called exosome [175]. Intestine tissue repair following MSCs treatment is predominantly ensured by MSCs-induced paracrine effects, reflecting the prominence of MSCs-derived exosome application rather than parental MSCs therapy. Certainly, MSCs-derived exosomes influence multiple biological processes in target cells as same as parental MSCs, while attenuating concerns regarding the direct application of parental cells, such as cell aging and potential tumor formation [176]. They exhibit better stability in circulation, biocompatibility, low immunogenicity and toxicity, and also strong targeting ability than parental cells [177], hence delivering a more favored therapeutic effect. However, more preclinical and clinical design investigations are needed to compare the efficacy of stem cells and exosomes.
Application of MSCs-derived exosome in IBD
Like MSCs, the positive effects of MSCs-derived exosome therapy in IBD conditions rely on two main mechanisms: inhibition of inflammation and supporting intestinal barrier [178, 179]. Exosome therapy could mitigate weight loss and colon shortening by activating the anti-inflammatory reactions and hindrance of the inflammatory axis in vivo [178]. As well, MSC-derived exosome (200 μg) offer a better outcome than parental MSCs transplantation at a dose of 1 × 106 cells. These studies indicate the anti-inflammatory impacts of exosomes in IBD animal models [178]. Further, other studies signified that MSCs-derived exosomes could reduce the IBD severity in animal models by up-regulation of IL-10 while attenuating TNF-α, IL-1β, IL-6, iNOS, and, more importantly, IL-7 levels in colon tissues and spleens of treated animals [180,181,182]. Alteration in the cytokine expression profiles upon exosome treatment potentiates M2 macrophage polarization and also improves Tregs differentiation [183]. IL-7 acts as a master regulator of T-cell differentiation and stimulates the expression of cell adhesion molecules (CAMs) and MCP-1, leading to increased immune cell infiltration into colon tissue [184]. As a result, targeting IL-7 expression may bring about the impaired migration and infiltration of immune cells to colon tissue, thus promoting anti-inflammatory effects. Also, OE-MSCs-derived exosomes inhibited the differentiation of Th1/17 cells but supported Treg cells differentiation in IBD experimental models by improving IL-10 and TGF-β levels [73]. Because of the positive effects of the IL-10 and TGF-β on intestinal hemostasis, improvement of their levels by MSCs treatment could alleviate IBD symptoms [185]. Of course, overexpression of TGF-β in the colons of mice may trigger colonic fibrosis [186]. Thus, a controlled level of TGF-β is required for tissue hemostasis. Current reports also note that miR-125a and miR-125b enriched exosomes downregulate Th17 cell differentiation through inhibition of STAT3 expression, which is required for early Th17 cell development [187]. Respecting to previous reports, the reduced miR-125a and miR-125b levels and conversely improved STAT3 levels have a positive association with disease severity in IBD patients [188], and thus exosome treatment may cause positive outcomes in IBD patients. Apart from its contribution to the Th17 development, the roles of SAT3 in the regulation of IEC's fate during colitis have been evinced [189].
Administration of the exosomes carrying miR-378a-5p also resulted in down-regulation of NLRP3 inflammasomes, impairment of cell pyroptosis, and thus increased cell survival in DSS-induced colitis in mice [190]. Likewise, BM-MSCs-derived exosomal miR-539-5p could inhibit pyroptosis by NLRP3/caspase-1 signaling to bypass IBD progression in mice models [191]. In addition, Xu et al. [192] suggested that MSCs-derived exosome could ameliorate colitis through the suppression of casp11/4-induced macrophage pyroptosis. They found that exosome carrying miR-203a-3p.2 could suppress casp4-induced macrophage pyroptosis in an inflammatory environment. As described, NLRP3 participates in the pathogenesis of IBD by substantial activation of inflammation in IECs and continued activation of macrophages [190]. As a result, targeting its expression and activation by therapeutic modalities such as MSCs treatment [78, 193] or NLRP3-specific inflammasome inhibitors [194, 195] offer an anti-inflammatory milieu and finally protect against IBD. Molecular analysis also has exhibited that exosomal miR-181a can affect the intestinal microbiota, immune responses, and intestinal barrier integrity in colitic rodents [108]. As a diminished level of miR-181a was found to be involved in the enhanced susceptibility to IBD development [196], improvement of its level in the inflamed colon by therapeutic modalities (e.g., exosome treatment) may give rise to better therapeutic outcomes. Meanwhile, Gu et al. [108] showed that miR-181a containing MSCs-derived exosome improved claudin-1, ZO-1, and NF-κB inhibitor (IκB) levels in colon tissue of exosome-treated mice, in addition to the reduction of pro-inflammatory cytokine levels. Indeed, although reduced levels of miR-181a and the TJs proteins such as ZO-1 and occludin are detected in IBD patients [197], exosome treatment improved their expression and thus ameliorated intestinal integrity and impaired inflammatory response. Also, metallothionein-2 (MT-2) in exosomes seems to be required for the suppression of inflammatory responses, enabling IBD treatment in preclinical models [198]. MTs, a negative regulator of NF-kB, adjust the inflammation and homeostasis of heavy metals and ameliorate oxidative stress [199]. Interestingly, MT−/− mice show severe inflammatory responses which induce tissue damage [200]. Liu et al. found that systemic injection of MT-2-containing MSCs-derived exosome improved survival and stool consistency, reduced rectal bleeding and colon shortening, increased M2 macrophage activity, and attenuated MPO activity, ensuing colon tissue repair in colitic mice [198].
Irrespective of the suggested mechanisms, exosomes can attenuate DSS-induced colitis in mice by controlling of ubiquitin modification level [28]. Ubiquitination serves a significant role in the adjustment of multiple biological activities (e.g., regulation of inflammation) [201]. Increasing evidence showed that E3 ubiquitin ligases like ring finger protein (RNF) 183, RNF 20, A20, Pellino 3, tripartite motif-containing 62 (TRIM62), and Itch contribute to the development of IBD [202]. For instance, RNF183 up-regulates the NF-κB pathway by promoting the ubiquitination and subsequent degradation of IκBα [202]. Recent reports revealed that exosome therapy profoundly augmented the proliferating ability of colon mucosa epithelial cells and concomitantly reduced the expression of ubiquitin and its related molecules, NEDD8 activating enzyme E1 (NAe1), ubiquitin-conjugating enzyme E2M (UBE2M), and ubiquitin-like modifier activating enzyme 3 (Uba3), in the colon tissues and spleens of DSS-induced IBD mice [28]. These results confer that targeting ubiquitination can make possible the down-regulation of expression of pro-inflammatory cytokine and chemokine and thus may be a valued target to modify the inflammatory axis in IBD. In addition to ubiquitination, neddylation, a recently described post-translational modification, contributes to the development of IBD mediated by potentiating DCs maturation [203, 204]. However, down-regulation of the neddylation attenuates mucosal inflammation by reducing cytokine production, inhibiting the expression of the costimulatory molecules, and thereby hindrance of T cell stimulation [204]. Accordingly, a recent study showed that miR-326 carrying MSCs-derived exosome might enable inhibition of the neddylation process and thereby relieves DSS-induced IBD [205]. Recent reports also have clarified that MSC-derived exosome could improve the gut microbiota composition by substantially supporting the structure of OTUs and colitis-induced reduction in α-diversity, enhancing the frequency of 'healthy' bacteria, attenuating disease-associated bacteria and detrimental functions, and promoting other vital cellular functions [206]. In addition, MSCs-derived exosome could convey miR-378a-3p to downregulate the GATA-binding protein 2 (GATA2) expression, which downregulates aquaporin-4 (AQP4) to block the peroxisome proliferator-activated receptor α (PPAR-α) signaling pathway, finally inhibiting the incidence of IBD [207]. Further, Zhang et al. (2022) demonstrated that exosome can improve intestinal lymphatic drainage, suppress lymphangiogenesis, and macrophages infiltration by the miR-302d-3p/VEGFR3/AKT axis to alleviate IBD [208]. On the other hand, hypoxic MSCs-derived exosomes reduced UC injury by restricting IES reactive oxygen species accumulation and DNA damage mainly by upregulation of HIF-1α expression and function [209].
A summary of studies based on the therapeutic merits of MSCs-derived exosomes is provided in Table 3.
Clinical trials
The promising results from animal studies have encouraged researchers to design and conduct a variety of clinical trials. Meanwhile, both autologous and allogeneic MSCs transplantation has been accomplished given their immune-suppressive and regenerative competencies with remarkable safety and acceptable efficacy [210]. With respect to published reports, both systemic and local delivery could engender reasonable success in IBD patients [211,212,213].
Autologous
Recent trials in 5 patients with refractory Crohn’s fistulas exhibited that intracolonic administration of autologous AT-MSCs (35 × 106 cells/patient) had no stern side effects while causing the complete cessation of drainage in 3 of them during six weeks of follow-up [211]. Another phase 1 trial in 12 patients with CD (NCT03803917) exhibited that AT-MSCs intracolonic administration was safe and enabled complete fistula healing in 57% of patients [214]. The major adverse effect was postprocedure proctalgia enduring a few days. Also, two patients experienced small abscesses, 1 had urinary retention, and 1 had minor bleeding during liposuction [214]. Likewise, the safety and feasibility of AT-MSCs injection (1–2 × 107 cells/patient) was demonstrated in a phase 1 trial in CD patients [215]. As well, autologous BM-MSCs (1–2 × 106 cells/kg) by intravenous route did not provoke serious side effects in 10 patients with refractory Crohn's fistulas [216]. Fortunately, the intervention reduced DAI during 6 weeks post-treatment in 3 of them. In contrast, 3 of them required surgery because of disease worsening [216]. Further, Dhere et al. [217] reports evidenced modest safety and feasibility of systemic injection of autologous BM-MSCs (1 × 107 cells/kg) in 12 patients with CD.
Allogeneic
A trial in 82 patients (41 patients in either control or intervention group) displayed that systemic injection of allogeneic 1 × 106 UC-MSCs/kg can attenuate DAI, Harvey–Bradshaw index (HBI), and corticosteroid dosage without serious side effects [218]. Moreover, local administration of allogeneic 3 × 107 MSCs/patients improved the healing of perianal fistulas [219]. Also, intracolonic injection of 3–9 × 107 allogeneic BM-MSCs/patient resulted in smaller fistula tracts after 4 years with no long-term side effects in CD patients [220]. In a phase I/IIa clinical trial, local administration of allogeneic AT-MSCs (20 × 106 cells/patient) into 24 patients with CD caused a decrease in the number of draining fistulas (69.2% of the patients), complete closure of the treated fistula (56.3% of the patients) and complete closure of all existing fistula tracts (30% of patients) [221]. Achieved results implied the safety, feasibility, and also efficacy of allogeneic AT-MSCs administration in CD patients [221]. In addition, Forbes and coworkers [222] accomplished a phase 2 trial (NCT01090817) and displayed that allogeneic MSCs transplantation (2 × 106 cells/kg weekly for 4 weeks) decreased DAI and CD endoscopic index of severity (CDEIS) scores in patients with luminal CD refractory to biologic therapy. Interestingly, a phase 3 randomized, double-blind controlled trial was conducted at 49 hospitals in eight countries from July 6, 2012, to July 27, 2015, to evaluate the safety and efficacy of local administration of allogeneic, expanded, AT-MSCs (Cx601) as a capable novel therapeutic tool to treat IBD [223]. Intralesional injection of 120 × 106 Cx601 cells/patients in 107 CD patients showed an acceptable safety profile and significant efficacy in CD patients who did not respond to conventional or biological treatments [223]. Recently, MSCs therapy was well tolerated and results exhibited that clinical remission post-treatment with MSCs may be sustained for up to 104 weeks in patients with perianal fistulizing CD [224].
Conclusion and future direction
MSC therapy has attracted increasing attention in the context of IBD therapy, given its low immunogenicity along with pro-survival and anti-inflammatory competencies. These influential inherent possessions have ensured the success of MSCs treatment in IBD animal models. A myriad of clinical studies also has investigated the safety and efficacy of MSCs therapy in IBD patients (Table 4, Fig. 3). Among various cell sources, BM-MSCs have been the most widely used cells, followed by UC- and AT-derived MSCs. Among cell administration routes, intravenous as well as intracolonic routes have been the most common routes. In most studies, the dose injected was about 1–2 × 106 cells/kg. Although the short-term safety and feasibility of MSCs transplantation have been verified, some drawbacks must be circumvented to use in the clinic. In this light, long-term side effect of MSC therapy hurdle their application. Also, the poor migration and engraftment of transplanted MSCs, particularly when injected by the intravenous route, is another potential fence. Hence, designing novel strategies to promote their engraftments, such as cell priming using safe ingredients or genetic modification of MSCs, is suggested. Further, the MSC investigations must also note the patient selection, disease activity, and disease stage based on therapeutic efficacy. In addition, as MSCs-derived exosomes show better engraftment, exosome therapy has been proposed as an alternative treatment for parental MSCs. The main downside of exosome therapy is low yield, which hurdles its clinical application. Importantly, this drawback can be chiefly tackled by substituting the traditional 2D culture system with a 3D system. For instance, Kim et al. have found that 3D spheroid culture of BM-MSCs brings about more exosomes than 2D culture and also the non-adherent round cell morphology itself may be a causative factor [225]. These results offer dependable data to evolve an optimal process for the mass generation of exosomes. In addition, hollow fiber 3D culture system may offer prolonged manufacture of MSC-exosome with better immunoregulatory competencies in vivo [226]. In sum, we suggest that pre-conditioned MSCs-derived exosomes can be a more appropriate therapeutic option compared with other modalities to attenuate disease severity in IBD patients.
Clinical trials based on MSCs therapy in IBD conditions registered on https://clinicaltrials.gov (June 2022). The schematic exhibits the clinical studies in the light of cell source (A), cell type (B), administration route (C), condition (D), study phase (E), and study location (F). Inflammatory bowel diseases (IBD), mesenchymal stem/stromal cell (MSC), bone marrow (BM), umbilical cord (UC), adipose tissue (AT), Wharton's jelly (WJ), Crohn's disease (CD), ulcerative colitis (UC), not applicable (NA)
Availability of data and materials
Not applicable.
Abbreviations
- MSCs:
-
Mesenchymal stem/stromal cells
- AT:
-
Adipose tissue
- BM:
-
Bone marrow
- UC:
-
Umbilical cord
- miRNAs:
-
MicroRNAs
- TNFα:
-
Tumor necrosis factor α
- TGF-β:
-
Transforming growth factor-beta
- TSG6:
-
TNFα-stimulated gene-6
- IECs:
-
Intestinal epithelial cells
- PGE2:
-
Prostaglandin E2
- Tregs:
-
Regulatory T cells
- Th:
-
T helper
- IL:
-
Interleukin
- TJs:
-
Tight junction
- TNBS:
-
2,4,6-Trinitrobenzene sulfonic acid
- DSS:
-
Dextran sulfate sodium
References
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785-94. e4.
Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019. https://doi.org/10.1016/j.mayocp.2018.09.013.
Frolkis A, Dieleman LA, Barkema HW, Panaccione R, Ghosh S, Fedorak RN, Madsen K, et al. Environment and the inflammatory bowel diseases. Can J Gastroenterol. 2013;27(3):e18–24.
McGovern DP, Kugathasan S, Cho JH. Genetics of inflammatory bowel diseases. Gastroenterology. 2015;149(5):1163-76. E2.
Dalal SR, Chang EB. The microbial basis of inflammatory bowel diseases. J Clin Investig. 2014;124(10):4190–6.
Iliev ID, Cadwell K. Effects of intestinal fungi and viruses on immune responses and inflammatory bowel diseases. Gastroenterology. 2021;160(4):1050–66.
Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–23.
Rivas MA, Beaudoin M, Gardet A, Stevens C, Sharma Y, Zhang CK, Boucher G, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43(11):1066–73.
Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13(1):3–10.
Siegmund B, Zeitz M. Innate and adaptive immunity in inflammatory bowel disease. World J Gastroenterol. 2011;17(27):3178–83.
Verdier J, Begue B, Cerf-Bensussan N, Ruemmele F. Compartmentalized expression of Th1 and Th17 cytokines in pediatric inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18(7):1260–6.
Su J, Chen T, Ji X-Y, Liu C, Yadav PK, Wu R, Yang P, et al. IL-25 downregulates Th1/Th17 immune response in an IL-10–dependent manner in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(4):720–8.
Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS, Pathak Y, et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther. 2021;12(1):1–30.
Tavakoli S, Ghaderi Jafarbeigloo HR, Shariati A, Jahangiryan A, Jadidi F, Jadidi Kouhbanani MA, Hassanzadeh A, et al. Mesenchymal stromal cells; a new horizon in regenerative medicine. J Cell Physiol. 2020;235(12):9185–210.
Tsuchiya A, Kojima Y, Ikarashi S, Seino S, Watanabe Y, Kawata Y, Terai S. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflamm Regen. 2017;37(1):1–15.
Soontararak S, Chow L, Johnson V, Coy J, Wheat W, Regan D, Dow S. Mesenchymal stem cells (MSC) derived from induced pluripotent stem cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl Med. 2018;7(6):456–67.
Dadgar N, Altemus J, Li Y, Lightner AL. Effect of Crohn’s disease mesenteric mesenchymal stem cells and their extracellular vesicles on T-cell immunosuppressive capacity. J Cell Mol Med. 2022;26(19):4924–39.
Yang H, Feng R, Fu Q, Xu S, Hao X, Qiu Y, Feng T, et al. Human induced pluripotent stem cell-derived mesenchymal stem cells promote healing via TNF-α-stimulated gene-6 in inflammatory bowel disease models. Cell Death Dis. 2019;10(10):1–16.
Song W-J, Li Q, Ryu M-O, Nam A, An J-H, Jung YC, Ahn J-O, et al. Canine adipose tissue-derived mesenchymal stem cells pre-treated with TNF-alpha enhance immunomodulatory effects in inflammatory bowel disease in mice. Res Vet Sci. 2019;125:176–84.
Zhou C, Wu X-R, Liu H-S, Liu X-H, Liu G-H, Zheng X-B, Hu T, et al. Immunomodulatory effect of urine-derived stem cells on inflammatory bowel diseases via downregulating Th1/Th17 immune responses in a PGE2-dependent manner. J Crohns Colitis. 2020;14(5):654–68.
Chen QQ, Yan L, Wang CZ, Wang WH, Shi H, Su BB, Zeng QH, et al. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol. 2013;19(29):4702.
Song J-y, Kang HJ, Hong JS, Kim CJ, Shim J-Y, Lee CW, Choi J. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci Rep. 2017;7(1):1–11.
Duan L, Huang H, Zhao X, Zhou M, Chen S, Wang C, Han Z, et al. Extracellular vesicles derived from human placental mesenchymal stem cells alleviate experimental colitis in mice by inhibiting inflammation and oxidative stress. Int J Mol Med. 2020;46(4):1551–61.
Shamoon M, Martin NM, O’Brien CL. Recent advances in gut microbiota mediated therapeutic targets in inflammatory bowel diseases: emerging modalities for future pharmacological implications. Pharmacol Res. 2019;148: 104344.
Sun T, Gao G-Z, Li R-F, Li X, Li D-W, Wu S-S, Yeo AE, et al. Bone marrow-derived mesenchymal stem cell transplantation ameliorates oxidative stress and restores intestinal mucosal permeability in chemically induced colitis in mice. Am J Transl Res. 2015;7(5):891.
Fu Y, Ni J, Chen J, Ma G, Zhao M, Zhu S, Shi T, et al. Dual-functionalized MSCs that express CX3CR1 and IL-25 exhibit enhanced therapeutic effects on inflammatory bowel disease. Mol Ther. 2020;28(4):1214–28.
Zhang X, Wang S, Ding X, Guo J, Tian Z. Potential methods for improving the efficacy of mesenchymal stem cells in the treatment of inflammatory bowel diseases. Scand J Immunol. 2020;92(3): e12897.
Wu Y, Qiu W, Xu X, Kang J, Wang J, Wen Y, Tang X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am J Transl Res. 2018;10(7):2026.
Yang S, Liang X, Song J, Li C, Liu A, Luo Y, Ma H, et al. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12(1):1–20.
Li X, Wang Q, Ding L, Wang Y-X, Zhao Z-D, Mao N, Wu C-T, et al. Intercellular adhesion molecule-1 enhances the therapeutic effects of MSCs in a dextran sulfate sodium-induced colitis models by promoting MSCs homing to murine colons and spleens. Stem Cell Res Ther. 2019;10(1):1–11.
Sartor RB. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn’s disease. Gastroenterol Clin North Am. 1995;24(3):475–507.
Williams MA, O’Callaghan A, Corr SC. IL-33 and IL-18 in inflammatory bowel disease etiology and microbial interactions. Front Immunol. 2019;10:1091.
Zhao J, Lu Q, Liu Y, Shi Z, Hu L, Zeng Z, Tu Y, et al. Th17 cells in inflammatory bowel disease: cytokines, plasticity, and therapies. J Immunol Res. 2021;2021:14.
Raza A, Yousaf W, Giannella R, Shata MT. Th17 cells: interactions with predisposing factors in the immunopathogenesis of inflammatory bowel disease. Expert Rev Clin Immunol. 2012;8(2):161–8.
Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C, Pouly S, et al. RORγ-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136(1):257–67.
Camporeale A, Poli V. IL-6, IL-17 and STAT3: a holy trinity in auto-immunity? Front Biosci. 2012. https://doi.org/10.2741/4054.
Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58(8):1152–67.
Li Z, Arijs I, De Hertogh G, Vermeire S, Noman M, Bullens D, Coorevits L, et al. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm Bowel Dis. 2010;16(8):1299–310.
Vitale A, Strisciuglio C, Vitale S, Santopaolo M, Bruzzese D, Micillo T, Scarpato E, et al. Increased frequency of regulatory T cells in pediatric inflammatory bowel disease at diagnosis: a compensative role? Pediatr Res. 2020;87(5):853–61.
Rindflesch TC, Blake CL, Cairelli MJ, Fiszman M, Zeiss CJ, Kilicoglu H. Investigating the role of interleukin-1 beta and glutamate in inflammatory bowel disease and epilepsy using discovery browsing. J Biomed Semantics. 2018;9(1):1–14.
Bank S, Julsgaard M, Abed OK, Burisch J, Broder Brodersen J, Pedersen NK, Gouliaev A, et al. Polymorphisms in the NF kB, TNF-alpha, IL-1beta, and IL-18 pathways are associated with response to anti-TNF therapy in Danish patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49(7):890–903.
Maerten P, Shen C, Colpaert S, Liu Z, Bullens D, Van Assche G, Penninckx F, et al. Involvement of interleukin 18 in Crohn’s disease: evidence from in vitro analysis of human gut inflammatory cells and from experimental colitis models. Clin Exp Immunol. 2004;135(2):310–7.
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90.
Imai J, Kitamoto S, Sugihara K, Nagao-Kitamoto H, Hayashi A, Morhardt TL, Kuffa P, et al. Flagellin-mediated activation of IL-33-ST2 signaling by a pathobiont promotes intestinal fibrosis. Mucosal Immunol. 2019;12(3):632–43.
De Jong M, Smits L, van Ruijven B, den Broeder N, Russel M, Römkens T, West R, et al. Increased discontinuation rates of anti-TNF therapy in elderly inflammatory bowel disease patients. J Crohns Colitis. 2020;14(7):888–95.
Kawamoto A, Nagata S, Anzai S, Takahashi J, Kawai M, Hama M, Nogawa D, et al. P010 Synergy of Notch signalling and TNF-α in the inflamed intestinal epithelia of IBD patients leads to up-regulation of UBD, a ubiquitin-like protein. J Crohns Colitis. 2019;13:495.
Marafini I, Zorzi F, Codazza S, Pallone F, Monteleone G. TGF-beta signaling manipulation as potential therapy for IBD. Curr Drug Targets. 2013;14(12):1400–4.
Carey R, Jurickova I, Ballard E, Bonkowski E, Han X, Xu H, Denson LA. Activation of an IL-6: STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(4):446–57.
Li Y, Jia Y, Cui T, Zhang J. IL-6/STAT3 signaling pathway regulates the proliferation and damage of intestinal epithelial cells in patients with ulcerative colitis via H3K27ac. Exp Ther Med. 2021;22(2):1–9.
Martinez-Fierro ML, Garza-Veloz I, Rocha-Pizaña MR, Cardenas-Vargas E, Cid-Baez MA, Trejo-Vazquez F, Flores-Morales V, et al. Serum cytokine, chemokine, and growth factor profiles and their modulation in inflammatory bowel disease. Medicine. 2019;98(38): e17208.
Lee DS, Lee KL, Jeong JB, Shin S, Kim SH, Kim JW. Expression of chemokine CCL28 in ulcerative colitis patients. Gut Liver. 2021;15(1):70.
Boshagh MA, Foroutan P, Moloudi MR, Fakhari S, Malakouti P, Nikkhoo B, Jalili A. ELR positive CXCL chemokines are highly expressed in an animal model of ulcerative colitis. J Inflamm Res. 2019;12:167.
Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16(1):26–42.
Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886.
Sobacchi C, Palagano E, Villa A, Menale C. Soluble factors on stage to direct mesenchymal stem cells fate. Front Bioeng Biotechnol. 2017;5:32.
Joel MDM, Yuan J, Wang J, Yan Y, Qian H, Zhang X, Xu W, et al. MSC: immunoregulatory effects, roles on neutrophils and evolving clinical potentials. Am J Transl Res. 2019;11(6):3890–904.
Ocansey DKW, Qiu W, Wang J, Yan Y, Qian H, Zhang X, Xu W, et al. The achievements and challenges of mesenchymal stem cell-based therapy in inflammatory bowel disease and its associated colorectal cancer. Stem Cells Int. 2020;2020:18.
Nikolic A, Markovic BS, Gazdic M, Harrell CR, Fellabaum C, Jovicic N, Djonov V, et al. Intraperitoneal administration of mesenchymal stem cells ameliorates acute dextran sulfate sodium-induced colitis by suppressing dendritic cells. Biomed Pharmacother. 2018;100:426–32.
Farhad M, Rolig AS, Redmond WL. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology. 2018;7(6): e1434467.
Gao F, Chiu S, Motan D, Zhang Z, Chen L, Ji H, Tse H, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7(1): e2062.
Cheung TS, Galleu A, von Bonin M, Bornhäuser M, Dazzi F. Apoptotic mesenchymal stromal cells induce prostaglandin E2 in monocytes: implications for the monitoring of mesenchymal stromal cell activity. Haematologica. 2019;104(10): e438.
Zhao X, Zhao Y, Sun X, Xing Y, Wang X, Yang Q. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front Bioeng Biotechnol. 2020;8: 575057.
Eljarrah A, Gergues M, Pobiarzyn PW, Sandiford OA, Rameshwar P. Therapeutic potential of mesenchymal stem cells in immune-mediated diseases. Adv Exp Med Biol. 2019. https://doi.org/10.1007/978-3-030-31206-0_5.
Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26(9):617–31.
Cheng HY, Ghetu N, Wallace C, Wei F, Liao S. The impact of mesenchymal stem cell source on proliferation, differentiation, immunomodulation and therapeutic efficacy. J Stem Cell Res Ther. 2014;4(10):1–8.
Lee JM, Jung J, Lee H-J, Jeong SJ, Cho KJ, Hwang S-G, Kim GJ. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012;13(2):219–24.
Fazekasova H, Lechler R, Langford K, Lombardi G. Placenta-derived MSCs are partially immunogenic and less immunomodulatory than bone marrow-derived MSCs. J Tissue Eng Regen Med. 2011;5(9):684–94.
He X-W, He X-S, Lian L, Wu X-J, Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci. 2012;57(12):3136–44.
Kim HS, Shin TH, Lee BC, Yu KR, Seo Y, Lee S, Seo MS, et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013;145(6):1392-403. e8.
Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57(7):1759–67.
Jo H, Eom YW, Kim H-S, Park HJ, Kim HM, Cho M-Y. Regulatory dendritic cells induced by mesenchymal stem cells ameliorate dextran sodium sulfate-induced chronic colitis in mice. Gut Liver. 2018;12(6):664.
Li F, Guo X, Chen S-Y. Function and therapeutic potential of mesenchymal stem cells in atherosclerosis. Front Cardiovasc Med. 2017;4:32.
Tian J, Zhu Q, Zhang Y, Bian Q, Hong Y, Shen Z, Xu H, et al. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate experimental colitis via modulating Th1/Th17 and treg cell responses. Front Immunol. 2020;11:598322.
Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, Noël D, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4(3):65.
Salah RB, Snoussi M, Louati N, Donia C, Frikha F, Hela M, Zouhir B. The lymphoproliferative auto-immune syndrome: a rare cause of peripheral cytopenia. Electron J Gen Med. 2018;15(5):em78.
Umit EG, Baysal M, Bas V, Goze H, Asoglu V, Kirkizlar O, Demir AM. Value of extracellular high mobility group box 1 (HMGB1) in the clinical context of immune thrombocytopenia. J Clin Exp Investig. 2019;10(2):em724.
Meisel R, Brockers S, Heseler K, Degistirici Ö, Bülle H, Woite C, Stuhlsatz S, et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2, 3-dioxygenase. Leukemia. 2011;25(4):648–54.
Park HJ, Kim J, Saima FT, Rhee K-J, Hwang S, Kim MY, Baik SK, et al. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem Biophys Res Commun. 2018;498(4):988–95.
Ju-Hyun A, Woo-Jin S, Qiang L, Sang-Min K, Ji-In Y, Min-Ok R, Nam AR, et al. Prostaglandin E 2 secreted from feline adipose tissue-derived mesenchymal stem cells alleviate DSS-induced colitis by increasing regulatory T cells in mice. BMC Vet Res. 2018;14(1):1–13.
Yu Y, Yoo SM, Park HH, Baek SY, Kim YJ, Lee S, Kim YL, et al. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2, 3-dioxygenase activity in dextran sulfate sodium-induced colitis. J Tissue Eng Regen Med. 2019;13(10):1792–804.
Chen PM, Liu KJ, Hsu PJ, Wei CF, Bai CH, Ho LJ, Sytwu HK, et al. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J Leukoc Biol. 2014;96(2):295–303.
Li N, Zhang Y, Nepal N, Li G, Yang N, Chen H, Lin Q, et al. Dental pulp stem cells overexpressing hepatocyte growth factor facilitate the repair of DSS-induced ulcerative colitis. Stem Cell Res Ther. 2021;12(1):1–13.
Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118(2):330–8.
Mittal M, Tiruppathi C, Nepal S, Zhao Y-Y, Grzych D, Soni D, Prockop DJ, et al. TNFα-stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc Natl Acad Sci. 2016;113(50):E8151–8.
Sala E, Genua M, Petti L, Anselmo A, Arena V, Cibella J, Zanotti L, et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology. 2015;149(1):163-76. e20.
Song W-J, Li Q, Ryu M-O, Ahn J-O, Bhang DH, Jung YC, Youn H-Y. TSG-6 released from intraperitoneally injected canine adipose tissue-derived mesenchymal stem cells ameliorate inflammatory bowel disease by inducing M2 macrophage switch in mice. Stem Cell Res Ther. 2018;9(1):1–12.
Zhang N, Chen Y, Huang C, Wei M, Li T, Lv Y, Song Q, et al. Adipose-derived mesenchymal stem cells may reduce intestinal epithelial damage in ulcerative colitis by communicating with macrophages and blocking inflammatory pathways: an analysis in silico. Aging (Albany NY). 2022;14(6):2665–77.
Liu A, Wang X, Liang X, Wang W, Li C, Qian J, Zhang X. Human umbilical cord mesenchymal stem cells regulate immunoglobulin a secretion and remodel the diversification of intestinal microbiota to improve colitis. Front Cell Infect Microbiol. 2022;12: 960208.
Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5(6):685–94.
Camilleri Á, Madsen K, Spiller R, Van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24(6):503–12.
König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7(10): e196.
Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107(11):686–96.
Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–53.
Al-Ghadban S, Kaissi S, Homaidan FR, Naim HY, El-Sabban ME. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci Rep. 2016;6(1):1–13.
Patterson AM, Watson AJ. Deciphering the complex signaling systems that regulate intestinal epithelial cell death processes and shedding. Front Immunol. 2017;8:841.
Paradis T, Bègue H, Basmaciyan L, Dalle F, Bon F. Tight junctions as a key for pathogens invasion in intestinal epithelial cells. Int J Mol Sci. 2021;22(5):2506.
Lin Y, Lin L, Wang Q, Jin Y, Zhang Y, Cao Y, Zheng C. Transplantation of human umbilical mesenchymal stem cells attenuates dextran sulfate sodium-induced colitis in mice. Clin Exp Pharmacol Physiol. 2015;42(1):76–86.
Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, et al. Epithelial tight junctions in intestinal inflammation. Ann NY Acad Sci. 2009;1165:294.
Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC, Ciclitira PJ, et al. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22(11):3117.
Semont A, Mouiseddine M, Francois A, Demarquay C, Mathieu N, Chapel A, Sache A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17(6):952–61.
Weil BR, Markel TA, Herrmann JL, Abarbanell AM, Meldrum DR. Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery. 2009;146(2):190–7.
Watanabe S, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nasuno M, Yamashita K, et al. Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors. J Gastroenterol. 2014;49(2):270–82.
Sheng H, Shao J, Townsend CM, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52(10):1472–8.
Lee C, An M, Joung JG, Park WY, Chang DK, Kim YH, Hong SN. TNFα induces LGR5+ stem cell dysfunction in patients with Crohn’s disease. Cell Mol Gastroenterol Hepatol. 2021. https://doi.org/10.1016/j.jcmgh.2021.10.010.
Hashemi SM, Hassan ZM, Hossein-Khannazer N, Pourfathollah AA, Soudi S. Investigating the route of administration and efficacy of adipose tissue-derived mesenchymal stem cells and conditioned medium in type 1 diabetic mice. Inflammopharmacology. 2019. https://doi.org/10.1007/s10787-019-00661-x.
Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105(39):15064–9.
Jahovic N, Gedik N, Ercan F, şirvanci S, Yüksel M, şener G, Alican I. Effects of statins on experimental colitis in normocholesterolemic rats. Scand J Gastroenterol. 2006;41(8):954–62.
Gu L, Ren F, Fang X, Yuan L, Liu G, Wang S. Exosomal MicroRNA-181a derived from mesenchymal stem cells improves gut microbiota composition, barrier function, and inflammatory status in an experimental colitis model. Front Med. 2021;8:898.
Tak L-J, Kim H-Y, Ham W-K, Agrahari G, Seo Y, Yang JW, An E-J, et al. Superoxide dismutase 3-transduced mesenchymal stem cells preserve epithelial tight junction barrier in murine colitis and attenuate inflammatory damage in epithelial organoids. Int J Mol Sci. 2021;22(12):6431.
Xu J, Wang X, Chen J, Chen S, Li Z, Liu H, Bai Y, et al. Embryonic stem cell-derived mesenchymal stem cells promote colon epithelial integrity and regeneration by elevating circulating IGF-1 in colitis mice. Theranostics. 2020;10(26):12204.
Lee AF, Chen H-Y, Wan L, Wu S-Y, Yu J-S, Huang AC, Miaw S-C, et al. Galectin-3 modulates Th17 responses by regulating dendritic cell cytokines. Am J Pathol. 2013;183(4):1209–22.
Popov A, Schultze JL. IDO-expressing regulatory dendritic cells in cancer and chronic infection. J Mol Med. 2008;86(2):145–60.
Lu Y, Xu Y, Zhang S, Gao J, Gan X, Zheng J, Lu L, et al. Human gingiva-derived mesenchymal stem cells alleviate inflammatory bowel disease via IL-10 signalling-dependent modulation of immune cells. Scand J Immunol. 2019;90(3): e12751.
Li Y, Ma K, Zhang L, Xu H, Zhang N. Human umbilical cord blood derived-mesenchymal stem cells alleviate dextran sulfate sodium-induced colitis by increasing regulatory T cells in mice. Front Cell Dev Biol. 2020;8:1412.
Kawata Y, Tsuchiya A, Seino S, Watanabe Y, Kojima Y, Ikarashi S, Tominaga K, et al. Early injection of human adipose tissue-derived mesenchymal stem cell after inflammation ameliorates dextran sulfate sodium-induced colitis in mice through the induction of M2 macrophages and regulatory T cells. Cell Tissue Res. 2019;376(2):257–71.
Chao K, Zhang S, Qiu Y, Chen X, Zhang X, Cai C, Peng Y, et al. Human umbilical cord-derived mesenchymal stem cells protect against experimental colitis via CD5+ B regulatory cells. Stem Cell Res Ther. 2016;7(1):1–12.
Oka A, Ishihara S, Mishima Y, Tada Y, Kusunoki R, Fukuba N, Yuki T, et al. Role of regulatory B cells in chronic intestinal inflammation: association with pathogenesis of Crohn’s disease. Inflamm Bowel Dis. 2014;20(2):315–28.
Liu J, Lai X, Bao Y, Xie W, Li Z, Chen J, Li G, et al. Intraperitoneally delivered mesenchymal stem cells alleviate experimental colitis through THBS1-mediated induction of IL-10-competent regulatory B cells. Front Immunol. 2022;13: 853894.
Banerjee A, Bizzaro D, Burra P, Di Liddo R, Pathak S, Arcidiacono D, Cappon A, et al. Umbilical cord mesenchymal stem cells modulate dextran sulfate sodium induced acute colitis in immunodeficient mice. Stem Cell Res Ther. 2015;6(1):1–14.
Kaser A, Blumberg RS. Endoplasmic reticulum stress and intestinal inflammation. Mucosal Immunol. 2010;3(1):11–6.
Kaser A, Blumberg RS. Endoplasmic reticulum stress in the intestinal epithelium and inflammatory bowel disease. Semin Immunol. 2009. https://doi.org/10.1016/j.smim.2009.01.001.
Gao J-G, Yu M-S, Zhang M-M, Gu X-W, Ren Y, Zhou X-X, Chen D, et al. Adipose-derived mesenchymal stem cells alleviate TNBS-induced colitis in rats by influencing intestinal epithelial cell regeneration, Wnt signaling, and T cell immunity. World J Gastroenterol. 2020;26(26):3750.
Martini E, Krug SM, Siegmund B, Neurath MF, Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017;4(1):33–46.
Stavely R, Sakkal S, Stojanovska V, Nurgali K. Mesenchymal stem cells for the treatment of inflammatory bowel disease: from experimental models to clinical application. Inflamm Regen. 2014;34(4):184–97.
Tang Y, Chen Y, Wang X, Song G, Li Y, Shi L. Combinatorial intervention with mesenchymal stem cells and granulocyte colony-stimulating factor in a rat model of ulcerative colitis. Dig Dis Sci. 2015;60(7):1948–57.
Forte D, Ciciarello M, Valerii MC, De Fazio L, Cavazza E, Giordano R, Parazzi V, et al. Human cord blood-derived platelet lysate enhances the therapeutic activity of adipose-derived mesenchymal stromal cells isolated from Crohn’s disease patients in a mouse model of colitis. Stem Cell Res Ther. 2015;6(1):1–16.
Yousefi-Ahmadipour A, Rashidian A, Mirzaei MR, Farsinejad A, PourMohammadi-Nejad F, Ghazi-Khansari M, Ai J, et al. Combination therapy of mesenchymal stromal cells and sulfasalazine attenuates trinitrobenzene sulfonic acid induced colitis in the rat: The S1P pathway. J Cell Physiol. 2019;234(7):11078–91.
Wu Q, Xie S, Zhu Y, Chen J, Tian J, Xiong S, Wu C, et al. Wogonin strengthens the therapeutic effects of mesenchymal stem cells in DSS-induced colitis via promoting IL-10 production. Oxid Med Cell Longev. 2021;2021:14.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–26.
Schäfer R, Spohn G, Baer PC. Mesenchymal stem/stromal cells in regenerative medicine: can preconditioning strategies improve therapeutic efficacy. Transfus Med Hemother. 2016;43(4):256–67.
Lin T, Pajarinen J, Nabeshima A, Lu L, Nathan K, Jämsen E, Yao Z, et al. Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis. Stem Cell Res Ther. 2017;8(1):1–9.
Sun X, Fang B, Zhao X, Zhang G, Ma H. Preconditioning of mesenchymal stem cells by sevoflurane to improve their therapeutic potential. PLoS ONE. 2014;9(3): e90667.
Li S, Deng Y, Feng J, Ye W. Oxidative preconditioning promotes bone marrow mesenchymal stem cells migration and prevents apoptosis. Cell Biol Int. 2009;33(3):411–8.
Ying J, You Q, Wang Z, Hu Z. Hypoxic preconditioning promotes the immunosuppressive effects of mesenchymal stem cells in mice with colitis. Res Vet Sci. 2022;144:157–63.
Qian W, Huang L, Xu Y, Lu W, Wen W, Guo Z, Zhu W, et al. Hypoxic ASCs-derived exosomes attenuate colitis by regulating macrophage polarization via miR-216a-5p/HMGB1 axis. Inflamm Bowel Dis. 2022. https://doi.org/10.1093/ibd/izac225.
Ye Y, Zhang X, Su D, Ren Y, Cheng F, Yao Y, Shi G, et al. Therapeutic efficacy of human adipose mesenchymal stem cells in Crohn’s colon fibrosis is improved by IFN-γ and kynurenic acid priming through indoleamine 2,3-dioxygenase-1 signaling. Stem Cell Res Ther. 2022;13(1):465.
Chinnadurai R, Rajan D, Qayed M, Arafat D, Garcia M, Liu Y, Kugathasan S, et al. Potency analysis of mesenchymal stromal cells using a combinatorial assay matrix approach. Cell Rep. 2018;22(9):2504–17.
Bertolo A, Pavlicek D, Gemperli A, Baur M, Pötzel T, Stoyanov J. Increased motility of mesenchymal stem cells is correlated with inhibition of stimulated peripheral blood mononuclear cells in vitro. J Stem Cells Regen Med. 2017;13(2):62–74.
Shin TH, Ahn JS, Oh SJ, Shin YY, Yang JW, Kang MJ, Kim JM, et al. TNF-α priming elicits robust immunomodulatory potential of human tonsil-derived mesenchymal stem cells to alleviate murine colitis. Biomedicines. 2020;8(12):561.
Li Y, Soendergaard C, Bergenheim FH, Aronoff DM, Milne G, Riis LB, Seidelin JB, et al. COX-2-PGE(2) signaling impairs intestinal epithelial regeneration and associates with TNF inhibitor responsiveness in ulcerative colitis. EBioMedicine. 2018;36:497–507.
Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128(5):1445–61.
Dai L, King DW, Perera DS, Lubowski DZ, Burcher E, Liu L. Inverse expression of prostaglandin E2-related enzymes highlights differences between diverticulitis and inflammatory bowel disease. Dig Dis Sci. 2015;60(5):1236–46.
Fuenzalida P, Kurte M, Fernández-O’ryan C, Ibañez C, Gauthier-Abeliuk M, Vega-Letter AM, Gonzalez P, et al. Toll-like receptor 3 pre-conditioning increases the therapeutic efficacy of umbilical cord mesenchymal stromal cells in a dextran sulfate sodium-induced colitis model. Cytotherapy. 2016;18(5):630–41.
Qiu Y, Guo J, Mao R, Chao K, Chen BL, He Y, Zeng ZR, et al. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS-induced colitis via the TLR3-Jagged-1-Notch-1 pathway. Mucosal Immunol. 2017;10(3):727–42.
Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, et al. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9(6):473–81.
Nie H, An F, Mei J, Yang C, Zhan Q, Zhang Q. IL-1β pretreatment improves the efficacy of mesenchymal stem cells on acute liver failure by enhancing CXCR4 expression. Stem Cells Int. 2020;2020:11.
Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, et al. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 2017;8(1):79.
Cheng W, Su J, Hu Y, Huang Q, Shi H, Wang L, Ren J. Interleukin-25 primed mesenchymal stem cells achieve better therapeutic effects on dextran sulfate sodium-induced colitis via inhibiting Th17 immune response and inducing T regulatory cell phenotype. Am J Transl Res. 2017;9(9):4149.
Kaiwen W, Zhaoliang S, Yinxia Z, Siamak SS, Zhijun J, Yuan X, Heng Y, et al. Changes and significance of IL-25 in chicken collagen II-induced experimental arthritis (CIA). Rheumatol Int. 2012;32(8):2331–8.
Deng C, Peng N, Tang Y, Yu N, Wang C, Cai X, Zhang L, et al. Roles of IL-25 in type 2 inflammation and autoimmune pathogenesis. Front Immunol. 2021;12: 691559.
Su J, Xie C, Fan Y, Cheng W, Hu Y, Huang Q, Shi H, et al. Interleukin-25 enhances the capacity of mesenchymal stem cells to induce intestinal epithelial cell regeneration. Am J Transl Res. 2017;9(12):5320–31.
Lim J-Y, Kim B-S, Ryu D-B, Kim TW, Park G, Min C-K. The therapeutic efficacy of mesenchymal stromal cells on experimental colitis was improved by the IFN-γ and poly (I: C) priming through promoting the expression of indoleamine 2, 3-dioxygenase. Stem Cell Res Ther. 2021;12(1):1–13.
Xu C, Feng C, Huang P, Li Y, Liu R, Liu C, Han Y, et al. TNFα and IFNγ rapidly activate PI3K-AKT signaling to drive glycolysis that confers mesenchymal stem cells enhanced anti-inflammatory property. Stem Cell Res Ther. 2022;13(1):491.
Cross RK, Wilson KT. Nitric oxide in inflammatory bowel disease. Inflamm Bowel Dis. 2003;9(3):179–89.
Kim YJ, Kim EH, Hahm KB. Oxidative stress in inflammation-based gastrointestinal tract diseases: challenges and opportunities. J Gastroenterol Hepatol. 2012;27(6):1004–10.
Kolios G, Valatas V, Ward SG. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology. 2004;113(4):427–37.
Middleton SJ, Shorthouse M, Hunter JO. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993;341(8843):465–6.
Boughton-Smith N, Evans S, Whittle B, Moncada S, Hawkey C, Cole A, Balsitis M. Nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Lancet. 1993;342(8867):338-e2.
Nowakowski A, Walczak P, Janowski M, Lukomska B. Genetic engineering of mesenchymal stem cells for regenerative medicine. Stem Cells Dev. 2015;24(19):2219–42.
Hassanzadeh A, Shamlou S, Yousefi N, Nikoo M, Verdi J. Genetically-modified stem cell in regenerative medicine and cancer therapy; a new era. Cure Gene Ther. 2022. https://doi.org/10.2174/1566523221666210707125342.
Chen Z, Chen Q, Du H, Xu L, Wan J. Mesenchymal stem cells and CXC chemokine receptor 4 overexpression improved the therapeutic effect on colitis via mucosa repair. Exp Ther Med. 2018;16(2):821–9.
Li Q, Lian Y, Deng Y, Chen J, Wu T, Lai X, Zheng B, et al. mRNA-engineered mesenchymal stromal cells expressing CXCR2 enhances cell migration and improves recovery in IBD. Mol Ther-Nucleic Acids. 2021;26:222–36.
Poritz LS, Harris LR, Kelly AA, Koltun WA. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig Dis Sci. 2011;56(10):2802.
Zhao Ml, Chen T, Zhang TH, Tian F, Wan X. H19 overexpression improved efficacy of mesenchymal stem cells in ulcerative colitis by modulating the miR-141/ICAM-1 and miR-139/CXCR4 axes. Dis Markers. 2021;2021:7107705.
Li X, Wang H, Zhang Y, Zhang J, Qi S, Zhang Y, Gao M-Q. Overexpression of lncRNA H19 changes basic characteristics and affects immune response of bovine mammary epithelial cells. PeerJ. 2019;7: e6715.
Zhou L, Yan F, Jiang R, Liu J, Cai L, Wang Y. Administration of Nrf-2-modified hair-follicle MSCs ameliorates DSS-induced ulcerative colitis in rats. Oxid Med Cell Longev. 2021;2021:9930187.
Wang H, Sun R-T, Li Y, Yang Y-F, Xiao F-J, Zhang Y-K, Wang S-X, et al. HGF gene modification in mesenchymal stem cells reduces radiation-induced intestinal injury by modulating immunity. PLoS ONE. 2015;10(5): e0124420.
Gómez-Ferrer M, Amaro-Prellezo E, Dorronsoro A, Sánchez-Sánchez R, Vicente Á, Cosín-Roger J, Barrachina MD, et al. HIF-overexpression and pro-inflammatory priming in human mesenchymal stromal cells improves the healing properties of extracellular vesicles in experimental Crohn’s disease. Int J Mol Sci. 2021;22(20):11269.
Chen Y, Song Y, Miao H, Xu Y, Lv M, Wang T, Hou Y. Gene delivery with IFN-γ-expression plasmids enhances the therapeutic effects of MSCs on DSS-induced mouse colitis. Inflamm Res. 2015;64(9):671–81.
Yan Y, Zhao N, He X, Guo H, Zhang Z, Liu T. Mesenchymal stem cell expression of interleukin-35 protects against ulcerative colitis by suppressing mucosal immune responses. Cytotherapy. 2018;20(7):911–8.
Li Y, Wang Y, Liu Y, Wang Y, Zuo X, Li Y, Lu X. The possible role of the novel cytokines il-35 and il-37 in inflammatory bowel disease. Mediators Inflamm. 2014;2014:10.
Wirtz S, Billmeier U, Mchedlidze T, Blumberg RS, Neurath MF. Interleukin-35 mediates mucosal immune responses that protect against T-cell–dependent colitis. Gastroenterology. 2011;141(5):1875–86.
Wang Y, Mao Y, Zhang J, Shi G, Cheng L, Lin Y, Li Y, et al. IL-35 recombinant protein reverses inflammatory bowel disease and psoriasis through regulation of inflammatory cytokines and immune cells. J Cell Mol Med. 2018;22(2):1014–25.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. 2020;9(3):660.
Hassanzadeh A, Rahman HS, Markov A, Endjun JJ, Zekiy AO, Chartrand MS, Beheshtkhoo N, et al. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res Ther. 2021;12(1):1–22.
Moghadasi S, Elveny M, Rahman HS, Suksatan W, Jalil AT, Abdelbasset WK, Yumashev AV, et al. A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. J Transl Med. 2021;19(1):302.
Ma ZJ, Wang YH, Li ZG, Wang Y, Li BY, Kang HY, Wu XY. Immunosuppressive effect of exosomes from mesenchymal stromal cells in defined medium on experimental colitis. Int J Stem Cells. 2019;12(3):440.
Barnhoorn M, Plug L, Muller-de Jonge E, Bos E, van der Meulen-de Jong A, Verspaget H, Hawinkels L. OP22 Mesenchymal stromal cell-derived exosomes stimulate epithelial regeneration in vitro and reduce experimental colitis. J Crohns Colitis. 2019;13(1):S015.
Mao F, Wu Y, Tang X, Kang J, Zhang B, Yan Y, Qian H, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int. 2017;2017:5356760.
Ikarashi S, Tsuchiya A, Kawata Y, Kojima Y, Watanabe T, Takeuchi S, Igarashi K, et al. Effects of human adipose tissue-derived and umbilical cord tissue-derived mesenchymal stem cells in a dextran sulfate sodium-induced mouse model. Biores Open Access. 2019;8(1):185–99.
Sendon-Lago J, Rio LG, Eiro N, Diaz-Rodriguez P, Avila L, Gonzalez LO, Vizoso FJ, et al. Tailored hydrogels as delivery platforms for conditioned medium from mesenchymal stem cells in a model of acute colitis in mice. Pharmaceutics. 2021;13(8):1127.
Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Ghaffari Khaligh S, Hashemi SM. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236(8):5906–20.
Hsueh PT, Lin HH, Wang HH, Liu CL, Ni WF, Liu JK, Chang HH, et al. Immune imbalance of global gene expression, and cytokine, chemokine and selectin levels in the brains of offspring with social deficits via maternal immune activation. Genes Brain Behav. 2018;17(7): e12479.
Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162(2):597–608.
Vallance BA, Gunawan MI, Hewlett B, Bercik P, Van Kampen C, Galeazzi F, Sime PJ, et al. TGF-beta1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G116-28.
Yang R, Huang H, Cui S, Zhou Y, Zhang T, Zhou Y. IFN-γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death Dis. 2020;11(7):1–12.
Sun C-M, Wu J, Zhang H, Shi G, Chen Z-T. Circulating miR-125a but not miR-125b is decreased in active disease status and negatively correlates with disease severity as well as inflammatory cytokines in patients with Crohn’s disease. World J Gastroenterol. 2017;23(44):7888.
Han J, Theiss AL. Stat3: friend or foe in colitis and colitis-associated cancer? Inflamm Bowel Dis. 2014;20(12):2405–11.
Cai X, Zhang ZY, Yuan JT, Ocansey DK, Tu Q, Zhang X, Qian H, et al. hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis. Stem Cell Res Ther. 2021;12(1):1–16.
Wang D, Xue H, Tan J, Liu P, Qiao C, Pang C, Zhang L. Bone marrow mesenchymal stem cells-derived exosomes containing miR-539-5p inhibit pyroptosis through NLRP3/caspase-1 signalling to alleviate inflammatory bowel disease. Inflamm Res. 2022;71(7–8):833–46.
Xu Y, Tang X, Fang A, Yan J, Kofi Wiredu Ocansey D, Zhang X, Mao F. HucMSC-Ex carrying miR-203a-3p.2 ameliorates colitis through the suppression of caspase11/4-induced macrophage pyroptosis. Int Immunopharmacol. 2022;110:108925.
Chen Y, Qin X, An Q, Yi J, Feng F, Yin D, An N, et al. Mesenchymal stromal cells directly promote inflammation by canonical NLRP3 and non-canonical caspase-11 inflammasomes. EBioMedicine. 2018;32:31–42.
Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, Robertson AA, et al. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8(1):1–15.
Wu D, Wu K, Zhu Q, Xiao W, Shan Q, Yan Z, Wu J, et al. Formononetin administration ameliorates dextran sulfate sodium-induced acute colitis by inhibiting NLRP3 inflammasome signaling pathway. Mediators Inflamm. 2018;2018:3048532.
Lim CX, Lee B, Geiger O, Passegger C, Beitzinger M, Romberger J, Stracke A, et al. miR-181a modulation of ERK-MAPK signaling sustains DC-SIGN expression and limits activation of monocyte-derived dendritic cells. Cell Rep. 2020;30(11):3793-805.e5.
Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50(8):1–9.
Liu H, Liang Z, Wang F, Zhou C, Zheng X, Hu T, He X, et al. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 2019;4(24): e131273.
Coyle P, Philcox J, Carey L, Rofe A. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59(4):627–47.
Inoue KI, Takano H, Shimada A, Wada E, Yanagisawa R, Sakurai M, Satoh M, et al. Role of metallothionein in coagulatory disturbance and systemic inflammation induced by lipopolysaccharide in mice. FASEB J. 2006;20(3):533–5.
Cockram PE, Kist M, Prakash S, Chen S-H, Wertz IE, Vucic D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28(2):591–605.
Xiao Y, Huang Q, Wu Z, Chen W. Roles of protein ubiquitination in inflammatory bowel disease. Immunobiology. 2020;225:152026.
Fu Z, Liao W, Ma H, Wang Z, Jiang M, Feng X, Zhang W. Inhibition of neddylation plays protective role in lipopolysaccharide-induced kidney damage through CRL-mediated NF-κB pathways. Am J Transl Res. 2019;11(5):2830.
Wan P, Zhu X-D, Liu X-P, Yu T, Yan R-W, Guo Y, Bai A-P. Inhibition of neddylation ameliorates DSS-induced colitis. Cell Mol Immunol. 2018;15(6):649–50.
Wang G, Yuan J, Cai X, Xu Z, Wang J, Ocansey DK, Yan Y, et al. HucMSC-exosomes carrying miR-326 inhibit neddylation to relieve inflammatory bowel disease in mice. Clin Transl Med. 2020;10(2): e113.
Ocansey DKW, Zhang Z, Xu X, Liu L, Amoah S, Chen X, Wang B, et al. Mesenchymal stem cell-derived exosome mitigates colitis via the modulation of the gut metagenomics-metabolomics-farnesoid X receptor axis. Biomater Sci. 2022;10(17):4822–36.
Li P, Zhang HY, Gao JZ, Du WQ, Tang D, Wang W, Wang LH. Mesenchymal stem cells-derived extracellular vesicles containing miR-378a-3p inhibit the occurrence of inflammatory bowel disease by targeting GATA2. J Cell Mol Med. 2022;26(11):3133–46.
Zhang L, Yuan J, Kofi Wiredu Ocansey D, Lu B, Wan A, Chen X, Zhang X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells regulate lymphangiogenesis via the miR-302d-3p/VEGFR3/AKT axis to ameliorate inflammatory bowel disease. Int Immunopharmacol. 2022;110:109066.
Zhu F, Wei C, Wu H, Shuai B, Yu T, Gao F, Yuan Y, et al. Hypoxic mesenchymal stem cell-derived exosomes alleviate ulcerative colitis injury by limiting intestinal epithelial cells reactive oxygen species accumulation and DNA damage through HIF-1α. Int Immunopharmacol. 2022;113(Pt A): 109426.
Vieujean S, Loly J-P, Boutaffala L, Pariente B, Reenaers C, Briquet A, Lechanteur C, et al. P293 local mesenchymal stem cells injection in Crohn’s disease strictures: a phase I-II, open-label clinical study. J Crohns Colitis. 2021;15(Supplement_1):S323–4.
Lightner AL, Dozois EJ, Dietz AB, Fletcher JG, Friton J, Butler G, Faubion WA. Matrix-delivered autologous mesenchymal stem cell therapy for refractory rectovaginal Crohn’s fistulas. Inflamm Bowel Dis. 2020;26(5):670–7.
Ko JZ-H, Johnson S, Dave M. Efficacy and safety of mesenchymal stem/stromal cell therapy for inflammatory bowel diseases: an up-to-date systematic review. Biomolecules. 2021;11(1):82.
Farge D, Loisel S, Resche-Rigon M, Lansiaux P, Colmegna I, Langlais D, Charles C, et al. Safety and preliminary efficacy of allogeneic bone marrow-derived multipotent mesenchymal stromal cells for systemic sclerosis: a single-centre, open-label, dose-escalation, proof-of-concept, phase 1/2 study. Lancet Rheumatol. 2022. https://doi.org/10.1016/S2665-9913(21)00326-X.
Dige A, Hougaard HT, Agnholt J, Pedersen BG, Tencerova M, Kassem M, Krogh K, et al. Efficacy of injection of freshly collected autologous adipose tissue into perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2019;156(8):2208-16.e1.
Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant. 2013;22(2):279–85.
Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59(12):1662–9.
Dhere T, Copland I, Garcia M, Chiang KY, Chinnadurai R, Prasad M, Galipeau J, et al. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn’s disease—a phase 1 trial with three doses. Aliment Pharmacol Ther. 2016;44(5):471–81.
Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical cord mesenchymal stem cell treatment for Crohn’s disease: a randomized controlled clinical trial. Gut Liver. 2018;12(1):73–8.
Molendijk I, Bonsing BA, Roelofs H, Peeters KC, Wasser MN, Dijkstra G, van der Woude CJ, et al. Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2015;149(4):918-27.e6.
Barnhoorn MC, Wasser M, Roelofs H, Maljaars PWJ, Molendijk I, Bonsing BA, Oosten LEM, et al. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J Crohns Colitis. 2020;14(1):64–70.
de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28(3):313–23.
Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12(1):64–71.
Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388(10051):1281–90.
Garcia-Olmo D, Gilaberte I, Binek M. Follow-up study to evaluate the long-term safety and efficacy of Darvadstrocel (mesenchymal stem cell treatment) in patients with perianal fistulizing Crohn’s disease: aDMIRE-CD phase 3 randomized controlled trial. Dis Colon Rectum. 2022;65(5):713–20.
Kim M, Yun HW, Park DY, Choi BH, Min BH. Three-dimensional spheroid culture increases exosome secretion from mesenchymal stem cells. Tissue Eng Regen Med. 2018;15(4):427–36.
Cao J, Wang B, Tang T, Lv L, Ding Z, Li Z, Hu R, et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 2020;11(1):206.
Liang L, Dong C, Chen X, Fang Z, Xu J, Liu M, Zhang X, et al. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transplant. 2011;20(9):1395–408.
Cao X, Duan L, Hou H, Liu Y, Chen S, Zhang S, Liu Y, et al. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE2-mediated M2 macrophage polarization. Theranostics. 2020;10(17):7697.
Castelo-Branco MT, Soares ID, Lopes DV, Buongusto F, Martinusso CA, de Rosario A, Souza SA, et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS ONE. 2012;7(3): e33360.
Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, et al. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326(2):523–31.
Liu W, Zhang S, Gu S, Sang L, Dai C. Mesenchymal stem cells recruit macrophages to alleviate experimental colitis through TGFβ1. Cell Physiol Biochem. 2015;35(3):858–65.
Tang RJ, Shen SN, Zhao XY, Nie YZ, Xu YJ, Ren J, Lv MM, et al. Mesenchymal stem cells-regulated Treg cells suppress colitis-associated colorectal cancer. Stem Cell Res Ther. 2015;6(1):1–11.
Song W-J, Li Q, Ryu M-O, Ahn J-O, Bhang DH, Jung YC, Youn H-Y. TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates DSS-induced colitis by inducing M2 macrophage polarization in mice. Sci Rep. 2017;7(1):1–14.
Chen Z, He X, He X, Chen X, Lin X, Zou Y, Wu X, et al. Bone marrow mesenchymal stem cells ameliorate colitis-associated tumorigenesis in mice. Biochem Biophys Res Commun. 2014;450(4):1402–8.
Li L, Liu S, Xu Y, Zhang A, Jiang J, Tan W, Xing J, et al. Human umbilical cord-derived mesenchymal stem cells downregulate inflammatory responses by shifting the Treg/Th17 profile in experimental colitis. Pharmacology. 2013;92(5–6):257–64.
Barnhoorn M, de Jonge-Muller E, Molendijk I, van Gulijk M, Lebbink O, Janson S, Schoonderwoerd M, et al. Endoscopic administration of mesenchymal stromal cells reduces inflammation in experimental colitis. Inflamm Bowel Dis. 2018;24(8):1755–67.
Lee HJ, Oh SH, Jang HW, Kwon J-H, Lee KJ, Kim CH, Park SJ, et al. Long-term effects of bone marrow-derived mesenchymal stem cells in dextran sulfate sodium-induced murine chronic colitis. Gut Liver. 2016;10(3):412.
Robinson AM, Rahman AA, Miller S, Stavely R, Sakkal S, Nurgali K. The neuroprotective effects of human bone marrow mesenchymal stem cells are dose-dependent in TNBS colitis. Stem Cell Res Ther. 2017;8(1):1–18.
Stavely R, Robinson AM, Miller S, Boyd R, Sakkal S, Nurgali K. Human adult stem cells derived from adipose tissue and bone marrow attenuate enteric neuropathy in the guinea-pig model of acute colitis. Stem Cell Res Ther. 2015;6(1):1–21.
Liu L, Zheng L, Zhang H, Shih DQ, Zhang X. Mesenchymal stem cell transplantation improves chronic colitis-associated complications through inhibiting the activity of toll-like receptor-4 in mice. BMC Gastroenterol. 2018;18(1):1–4.
González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136(3):978–89.
Onishi R, Ohnishi S, Higashi R, Watari M, Yamahara K, Okubo N, Nakagawa K, et al. Human amnion-derived mesenchymal stem cell transplantation ameliorates dextran sulfate sodium-induced severe colitis in rats. Cell Transplant. 2015;24(12):2601–14.
Stavely R, Robinson AM, Miller S, Boyd R, Sakkal S, Nurgali K. Allogeneic guinea pig mesenchymal stem cells ameliorate neurological changes in experimental colitis. Stem Cell Res Ther. 2015;6(1):1–21.
Liao Y, Lei J, Liu M, Lin W, Hong D, Tuo Y, Jiang MH, et al. Mesenchymal stromal cells mitigate experimental colitis via insulin-like growth factor binding protein 7-mediated immunosuppression. Mol Ther. 2016;24(10):1860–72.
Kang J, Zhang Z, Wang J, Wang G, Yan Y, Qian H, Zhang X, et al. hucMSCs attenuate IBD through releasing miR148b-5p to inhibit the expression of 15-lox-1 in macrophages. Mediators Inflamm. 2019;2019:6953963.
Jia K, Wang Y, Tong X, Wang R. KGF is delivered to inflammatory and induces the epithelial hyperplasia in trinitrobenzene sulfonic acid-induced ulcerative colitis rats. Drug Des Dev Ther. 2020;14:217.
Zhang Y, Chen J, Fu H, Kuang S, He F, Zhang M, Shen Z, et al. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Oral Sci. 2021;13(1):1–15.
Barnhoorn M, Plug L, Muller-de Jonge E, Molenkamp D, Bos E, Schoonderwoerd M, Corver W, et al. Mesenchymal stromal cell-derived exosomes contribute to epithelial regeneration in experimental inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2020;9(4):715-7. e8.
Yu H, Yang X, Xiao X, Xu M, Yang Y, Xue C, Li X, et al. Human adipose mesenchymal stem cell-derived exosomes protect mice from DSS-Induced inflammatory bowel disease by promoting intestinal-stem-cell and epithelial regeneration. Aging Dis. 2021;12(6):1423.
Cao L, Xu H, Wang G, Liu M, Tian D, Yuan Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int Immunopharmacol. 2019;72:264–74.
Chang C-L, Chen C-H, Chiang JY, Sun C-K, Chen Y-L, Chen K-H, Sung P-H, et al. Synergistic effect of combined melatonin and adipose-derived mesenchymal stem cell (ADMSC)-derived exosomes on amelioration of dextran sulfate sodium (DSS)-induced acute colitis. Am J Transl Res. 2019;11(5):2706.
Acknowledgements
Not applicable.
Funding
No funders.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and the main idea of the work. MVK, SR, and MF, drafted the main text, figures, and tables. AR supervised the work and provided comments and additional scientific information. MVK and AR also reviewed and revised the text. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Saadh, M.J., Mikhailova, M.V., Rasoolzadegan, S. et al. Therapeutic potential of mesenchymal stem/stromal cells (MSCs)-based cell therapy for inflammatory bowel diseases (IBD) therapy. Eur J Med Res 28, 47 (2023). https://doi.org/10.1186/s40001-023-01008-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01008-7