Abstract
Objectives
There are limited data about nosocomial coinfections of COVID-19 cases monitored in the intensive care unit. This study aims to investigate coinfections in COVID-19 patients followed in an intensive care unit of a university hospital.
Methods
This study analyzed retrospectively the data of coinfections of 351 COVID-19 patients in the period 28.02.2020–15.01.2021 in a tertiary care intensive care unit in a university hospital.
Results
Bacterial coinfections were present in 216 of the 351 cases. One hundred and thirty of these cases were evaluated as nosocomial infections. On the third day the Sequential Organ Failure Assessment Score, usage of invasive mechanical ventilation and presence of septic shock were significantly higher in the coinfected group. The neutrophil/lymphocyte ratio, polymorphonuclear leukocyte count, procalcitonin, ferritin, and blood urea nitrogen values were significantly higher in the coinfection group. White blood cells (WBC) (OR: 1.075, 95% CI 1.032–1.121, p = 0.001) and ICU hospitalization day (OR: 1.114, 95% CI 1.063–1.167, p < 0.001) were found to be independent risk factors for coinfection in the multivariate logistic regression analysis. The rates of hospitalization day on the day of arrival, the 21st day, as well as total mortality (p = 0.004), were significantly higher in the coinfected group.
Conclusion
Bacterial coinfections of COVID-19 patients in the intensive care unit remain a problem. Identifying the infectious agent, classifying colonizations and infections, and using the proper treatment of antibiotics are of great importance in the case management of COVID-19 patients in the intensive care unit.
Similar content being viewed by others
Introduction
The ongoing COVID-19 pandemic caused by the SARS-CoV-2 virus is causing significant morbidity and mortality worldwide [1]. Intensive care monitoring is crucial because of severe pneumonia, acute respiratory distress syndrome (ARDS), and cytokine storms seen in the clinical course of the disease. In critical patients, bacterial and fungal coinfections can add to the clinical picture because of mechanical ventilation, the immune condition in the disease, and the predisposition caused by possible steroid use [2]. The SARS-CoV-2 infection causes damage primarily to B cells, T cells, and NK cells, causing a deterioration in the host’s immune system. Decreased lymphocyte count and impaired host immune response may cause COVID-19 coinfections [3]. Mortality related to high coinfection rates is higher in severe cases compared to moderate cases [4, 5]. In these severe cases, we could see secondary infections because of the use of invasive catheters and multidrug-resistant strains, such as Acinetobacter baumannii, Escherichia coli, Pseudomonas aeruginosa, and Enterococcus spp. Few studies have identified bacterial coinfections observed in COVID-19 cases monitored in intensive care due to extremely limited data. The incidence of nosocomial infections in COVID-19 intensive care units varies between %14 and 54 in different studies [2, 4, 5]. This study was planned to investigate bacterial coinfections in critical COVID-19 cases monitored in the intensive care unit.
Methods
This study collected data from COVID-19 observed in the period 28.02.2020–15.01.2021 in a tertiary care intensive care unit (ICU) of a university hospital. There were 351 cases with COVID-19 lung involvement in the chest computed tomography (CT) scan and/or COVID-19-positive polymerase chain reaction (PCR) test. The data were retrospectively analyzed. The cases were monitored by anesthesiologists and infectious disease specialists for infection development. The specification and the classification of infections into community-acquired or nosocomial origins were made according to CDC and European Intensive Care Hospital Associated Surveillance Protocols [6,7,8]. The presence of sepsis was determined according to the definitions of Sepsis-3 [9]. Tracheal aspirate, phlegm, urine, wound site sample, and blood and catheter tip cultures were taken to determine the focus of the infection. A subgroup analysis was performed between the group of patients diagnosed with COVID-19 upon arrival or who were considered cases of coinfection by positive results after 48 h in the culture from clinical samples and the group that developed bacterial coinfection 48 h after hospitalization.
Patients were divided into the coinfection group (n = 216) and the non-coinfection group (n = 135). In addition to demographic data, comorbid diseases, SOFA (Sequential Organ Failure Assessment Score) scores on days 0 and 3, and mechanical ventilation usage were investigated. The correlation between the coinfection group and the non-coinfection group was examined in terms of infection sites, active microorganisms, antibiotic treatments, chest CT scans, laboratory findings, intensive care unit hospitalization times, septic shock development status, and mortality rates.
Statistical analysis
Statistical tests were performed using SPSS version 19 (SPSS Inc., Chicago, IL, USA). Continuous variables are shown as mean value ± SD and categorical variables as the number of cases and percentage of the total number of patients. A student t-test or Mann–Whitney U test was used to compare parametric values between the two groups as appropriate. A Chi-square test was applied to compare categorical variables. Logistic regression analysis was used to identify independent predictors for coinfection. The factors entered into the multivariate model included those with p-values < 0.1 from the univariate analysis and variables with known predictive value. Also, spearman correlation analysis was performed to determine correlations among continuous variables and identify potential confounding factors. A two-sided p < 0.05 were considered statistically significant.
Power analysis
The study needed to recruit 71 participants for each group to have 80.3% power with a 5% type 1 error level when assuming a coinfection rate of 45% in the ICU. The power of the study increased to 97.4% with the selection of 216 patients in the coinfection (+) group and 135 patients in the coinfection (−) group with a 5% type 1 error level.
Results
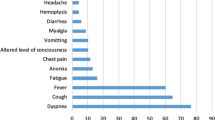
Of the 351 patients, the mean age of the patients who developed coinfection was 66.0 ± 14.6 years, and the patients without coinfection were 63.6 ± 14.4 years. There were 84 (39%) female patients in the coinfection group and 50 (37%) in the non-coinfection group. There was no significant relationship in terms of the development of coinfection according to age and gender characteristics (p = 0.148, and p = 0.728). The most common comorbid diseases in the coinfection group were diabetes mellitus (DM) with 94 (61%) patients, hypertension (HT) with 118 (55%) patients, and coronary heart disease (CDH) with 57 (26%) patients. There was no statistically significant correlation between the coinfection group and the non-coinfection group regarding comorbid diseases. There was no statistical increase in coinfection in the postoperative cases monitored in the COVID-19 intensive care unit. The Sequential Organ Failure Assessment (SOFA) score, evaluated as a disease severity criterion, showed that, while there was no significant correlation between arrival SOFA scores (SOFA 0) and coinfection development, on day 3, SOFA scores were significantly higher in the coinfection group (p = 0.001). While the coinfection rate was not significant in the group using a high-flow nasal cannula (HFNC), a continuous positive airway pressure (CPAP) machine, or reservoir masks, it was significantly higher in cases with invasive mechanical ventilation usage (p < 0.005). Patients were monitored after 48 h of hospitalization in the ICU. Bloodstream infections and surgical site infections (SSI) were also monitored after 48 h of hospitalization, and infections that developed within 30 days after surgery were considered SSIs. In the coinfection group, the rate of community-acquired infections detected during hospitalization was 40%, while the nosocomial infection rate was 60% (Table 1). The most common causative microorganisms of community-acquired infections were Staphylococcus aureus and Streptococcus pneumonia. The subgroup analysis of the nosocomial coinfections indicated pulmonary infection in 85 (24%) cases, bloodstream infection in 48 (14%) cases, urinary tract infection (UTI) in 33 (9%) cases, and catheter-associated urinary tract infection (CAUTI) in 6 cases. The distribution of infectious agents was as follows: Acinetobacter spp. in 63cases, Enterococcus spp. in 24 cases, Klebsiella pneumonia in 16 cases, Methicillin-resistant Staphylococcus aureus (MRSA) in 9 cases, Methicillin-resistant Staphylococcus epidermidis (MRSA) in 13 cases, E. coli in 11cases, and Pseudomonas aeruginosa in 10 cases. The distribution of microorganisms according to the areas of infection is given in Table 2. The most common antibiotics used in nosocomial coinfected cases were piperacillin–tazobactam in 37 cases, meropenem in 78 cases, teicoplanin in 52 cases, colistin in 41 cases, and fosfomycin in 8 cases (Table 3). The mean duration of treatment was 11.4 ± 6.8 days in the coinfected group. The resistance patterns of the isolated microorganisms were as follows: carbapenem, cephalosporin resistance of gram-negative microorganisms, and methicillin resistance of staphylococci were 13.8%, 6.9%, and 17.1%, respectively. No significant correlation was found between the group with and without coinfection in terms of steroid usage, dose, and treatment duration.
The examination of chest computed tomography (CT) findings showed that 21 (10%) of coinfected cases had less than 50% lung involvement, 108 cases had 50% or more involvement, and 82 cases had ARDS findings. Statistically, there was no significant difference between the coinfected and non-coinfected groups according to their radiological findings (p = 0.153). Patients diagnosed with septic shock were significantly higher in the coinfected group (Table 1, p < 0.001).
According to laboratory data, neutrophil/lymphocyte ratio (p < 0.001), polymorphonuclear leukocyte (PNL) level (p < 0.001), procalcitonin positivity (p = 0.013), high ferritin levels (p = 0.002), and high BUN levels (blood urea nitrogen) (p < 0.001) were significantly correlated in the coinfected group (Table 4). White blood cells (WBC) (OR: 1.075, 95% CI 1.032–1.121, p = 0.001) and ICU hospitalization day (OR: 1.114, 95% CI 1.063–1.167, p < 0.001) were identified as independent risk factors for coinfection in the logistic regression analysis (Table 5 and Fig. 1). While no difference was detected between both groups when observing the mortality components on the 7th day, mortality on the 21st day was significantly higher in the coinfected group (p = 0.005). Total mortality was significantly higher in the coinfected group than in the non-coinfected group (Table 1, p = 0.004). The distribution of the mortality causes in the coinfected group was 40 cases from respiratory failure, 41 cases from septic shock, 50 cases from multiorgan failure, and 5 cases from sudden cardiac death (p = 0.003).
Discussion
The presented study showed that bacterial coinfections developed frequently, especially in severe cases requiring intensive care monitoring for COVID-19.
Bacterial coinfections in viral pneumonia are especially common in patients in ICUs [10]. Primary infection or secondary bacterial pneumonia rates are 11–35% in patients infected with respiratory viruses [11]. This also applies to SARS-CoV-2 infection. Zhang and his colleagues reported a higher rate of bacterial coinfection (25%) in severe cases than in mild and moderate cases (0.8%) [12].
In this retrospective study, we screened COVID-19 cases with bacterial infections that developed within 48 h after attendance in a university hospital ICU. Infection was detected in 216 out of 351 cases, of which 130 (37%) were identified as nosocomial. This ratio is aligned with the literature [13]. Bardi et al. revealed that of the 140 critically ill patients with COVID-19 41% had bacterial or fungal infections on the 9th day of the intensive care unit. Also in a meta-analysis, including 30 studies and 3834 patients, 7% of hospitalized COVID-19 patients had a bacterial coinfection and this rate was higher in the COVID-19 intensive care unit [2]. In the analysis carried out in this group, the SOFA score was higher in the coinfected group on day 3. The SOFA score is a scoring system applied in intensive care patients with organ failure, and it has been reported in the literature that the disease is more serious in cases with a high SOFA score [14]. Bacterial coinfections increase the severity of the disease and speed up the progression to organ failure. Using HFNC, CPAP, and reservoir masks did not increase the development of coinfection. In addition, the coinfection rate was significantly higher in patients undergoing invasive mechanical ventilation. The increase in duration in the ICU increases and invasive treatments, such as mechanical ventilation, are applied, causing a risk for ventilator-related pneumonia [15]. These data are quite similar to the results of our study.
We found a statistically significant higher septic shock rate in the group with coinfection. It is a fact that bacterial factors have an important role in sepsis and septic shock, in this study, the incidence of septic shock diagnosis was found to be higher, in cases with a proven bacterial infection as expected.
In an evaluation of the infection sites, pulmonary infections were the most prevalent. This is compatible with the rate reported in the literature, which is in the range of 0–50% [16, 17]. However, one of the inclusion criteria in the study was a “positive CT scan.” These criteria increase the rate of pulmonary COVID-19 involvement and subsequently, possible coinfections. Therefore, the rate of pulmonary coinfection might be overestimated in the presented study. Blood, urine, and catheter culture positivity are the next most common site of infection in our study. The blood culture positivity rate is 3.8–33.5% in the literature [17]. Our blood culture positivity rate was similar to the literature. Some of these positivities suggest a positive result due to being contaminated with the skin flora and being taken under favorable conditions. Bardi et al. claimed that in the COVID-19 intensive care unit the most common infections were bloodstream infections (25%), pulmonary infections (23%), and urinary tract infections (8%) [13]. In our study, they reported a septic shock rate of 60% in the bacterial coinfected group and stated that bacterial coinfections were associated with a high SOFA score [13]. Congruent to our study, Humieres et al. and Baccolini et al. reported that pneumonia and subsequent bloodstream infection were the most common nosocomial coinfections [17, 18]. The distribution of infectious agents in nosocomial coinfections was as follows: Acinetobacter spp. in 63 (48%) cases, Enterococcus spp. in 24 (18%) cases, Klebsiella pneumonia in 16 (12%) cases, Methicillin-resistant Staphylococcus aureus (MRSA) in 9 (7%) cases, Methicillin-resistant Staphylococcus epidermidis (MRSA) in 13 (10%) cases, E. coli in 11 (8%) cases, and Pseudomonas aeruginosa in 10 (8%) cases.
In their 254-case series, which is compatible with our study, Baskaran et al. isolated 139 microorganisms from 83 patients, the most common of which were nosocomial pathogens, such as Klebsiella pneumonia and Escherichia coli [19]. In another study, Pseudomonas aeruginosa was the most commonly identified as a factor of nosocomial pneumonia [20]. As in our study, other studies have reported Acinetobacter baumannii as the most common nosocomial pathogen [18, 21]. Chen et al. reported that Acinetobacter baumannii and Klebsiella pneumonia were the most common bacteria that caused coinfection in 99 cases [22].
The duration of hospitalization in the intensive care unit and on the 21st day, as well as total mortality, were significantly higher in the coinfected group. The group with bacterial coinfection had a higher SOFA score on day 3, higher usage of invasive mechanical ventilation, prolonged hospitalization in the intensive care unit, higher incidence of septic shock, and a higher mortality rate on the 21st day. Similarly, mechanical ventilation and prolonged hospitalization duration in the intensive care unit were independent risk factors for coinfection [23]. The total mortality rate reported in the study by Bardi et al. in coinfected patients was similar to our study [13].
In severe COVID-19 cases, leukocyte count, neutrophil/lymphocyte ratio, procalcitonin, CRP, and ferritin elevation are reported in the literature [24, 25]. In our study using laboratory markers, the parameters that were detected as significantly higher in coinfected patients compared to the non-coinfected group were neutrophil/lymphocyte ratio, PNL, procalcitonin, BUN, and ferritin. When the literature was examined, Elabbadi et al. stated that they did not find any significant difference between the group with and without lymphopenia in terms of laboratory parameters in the COVID-19 cases followed up in the ICU [20].
In the COVID-19 pandemic, bacterial coinfections develop frequently, especially in severe cases requiring intensive care monitoring, increasing mortality drastically. There are insufficient data in the literature on these coinfections. Identifying the infectious agent, classifying colonizations and infections, and using the proper treatment of antibiotics are of great importance in case management. In addition, laboratory markers that may indicate infection should be considered in follow-up studies. Unnecessary antibiotic use should be avoided considering comorbid diseases, accompanying ARDS, and the multiorgan deficiencies of these cases. It is possible to reduce COVID-19-related mortality with appropriate and timely diagnosis and treatment.
Availability of data and materials
The data sets used during the current study are available from the corresponding author upon reasonable request.
References
Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med. 2020;382:1708–20.
Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–9.
He Z, Zhao C, Dong Q, Zhuang H, Song S, Peng G, Dwyer DE. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9:323–30.
Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;1(180):934–43.
De Santis V, Corona A, Vitale D, et al. Bacterial infections in critically ill patients with SARS-2-COVID-19 infection: results of a prospective observational multicenter study. Infection. 2022;50:139–48.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32.
Center for Disease and Prevention Control. National Healthcare Safety Network (NHSN) Overview. Patient Safety Component Manual. 2016.
Europian Centre for Disease Prevention and Control. European Surveillance of Healthcare-Associated Infections in Intensive Care Units—HAI-Net ICU protocol, version 1.02. Stockholm. ECDC. 2015.
Singer M, Deutschman CS, Seymour CW, et al. The Third International consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–10.
Chen X, Liao B, Cheng L, et al. The microbial coinfection in COVID-19. Appl Microbiol Biot. 2020;104:7777–85.
Klein EY, Monteforte B, Gupta A, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10:394–403.
Zhang G, Hu C, Luo L, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127: 104364.
Bardi T, Pintado V, Gomez-Rojo M, et al. Nosocomial infections associated with COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis. 2021;40:495–502.
Yang Z, Hu Q, Huang F, Xiong S, Sun Y. The prognostic value of the SOFA score in patients with COVID-19: a retrospective, observational study. Medicine. 2021;100: e26900.
Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46:888–906.
Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–22.
D’Humières C, Patrier J, Lortat-Jacob B, et al. Two original observations concerning bacterial infections in COVID-19 patients hospitalized in intensive care units during the first wave of the epidemic in France. PLoS ONE. 2021;16: e0250728.
Baccolini V, Migliara G, Isonne C, et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10:87.
Baskaran V, Lawrence H, Lansbury LE, et al. Co-infection in critically ill patients with COVID-19: an observational cohort study from England. J Med Microbiol. 2021;70: 001350.
Elabbadi A, Turpin M, Gerotziafas GT, Teulier M, Voiriot G, Fartoukh M. Bacterial coinfection in critically ill COVID-19 patients with severe pneumonia. Infection. 2021;49:559–62.
Yang S, Hua M, Liu X, Du C, Pu L, Xiang P, Wang L, Liu J. Bacterial and fungal co-infections among COVID-19 patients in the intensive care unit. Microbes Infect. 2021;23: 104806.
Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;393:507–13.
Saade A, Moratelli G, Dumas G, Mabrouki A, Tudesq JJ, Zafrani L, Azoulay E, Darmon M. Infectious events in patients with severe COVID-19: results of a cohort of patients with a high prevalence of underlying immune defect. Ann Intensive Care. 2021;1:83.
Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389–99.
Tjendra Y, Al Mana AF, Espejo AP, Akgun Y, Millan NC, Gomez-Fernandez C, Cray C. Predicting disease severity and outcome in COVID-19 patients: a review of multiple biomarkers. Arch Pathol Lab Med. 2020;144:1465–74.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
BK, BO, and SG made substantial contributions to the conception and design of the work and acquisition and analysis of data; AS interpretation of data; and AST the creation of new software used in the work; MA, NAK, and TD revised it. All the authors approved the submitted version (and any substantially modified version that involves the author’s contribution to the study) and agreed both to be personally accountable for the author’s contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical approval was taken from Katip Celebi University Hospital Ethics Committee and the Ethical Approval Number is: 0131, date: 04.03.2021.
Consent for publication
Our study is retrospective, and we collected data from patient records.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Karaca, B., Aksun, M., Karahan, N.A. et al. Are bacterial coinfections really rare in COVID-19 intensive care units?. Eur J Med Res 28, 43 (2023). https://doi.org/10.1186/s40001-023-01004-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01004-x