Abstract
Background
Orthotopic heart transplantation (HTX) is the gold standard to treat end-stage heart failure. Numerous risk stratification tools have been developed in the past years. However, their clinical utility is limited by their poor discriminative ability. High sensitivity troponin T (hsTnT) is the most specific biomarker to detect myocardial cell injury. However, its prognostic relevance after HTX is not fully elucidated. Thus, this study evaluated the predictive value of postoperative hsTnT for 1-year survival and days alive and out of hospital (DAOH) after HTX.
Methods
This retrospective cohort study included patients who underwent HTX at the University Hospital Duesseldorf, Germany between 2011 and 2021. The main exposure was hsTnT concentration at 48 h after HTX. The primary endpoints were mortality and DAOH within 1 year after surgery. Receiver operating characteristic (ROC) curve analysis, logistic regression model and linear regression with adjustment for risk index for mortality prediction after cardiac transplantation (IMPACT) were performed.
Results
Out of 231 patients screened, 212 were included into analysis (mean age 55 ± 11 years, 73% male). One-year mortality was 19.7% (40 patients) and median DAOH was 298 days (229–322). ROC analysis revealed strongest discrimination for mortality by hsTnT at 48 h after HTX [AUC = 0.79 95% CI 0.71–0.87]. According to Youden Index, the cutoff for hsTnT at 48 h and mortality was 1640 ng/l. After adjustment for IMPACT score multivariate logistic and linear regression showed independent associations between hsTnT and mortality/DAOH with odds ratio of 8.10 [95%CI 2.99–21.89] and unstandardized regression coefficient of −1.54 [95%CI −2.02 to −1.06], respectively.
Conclusion
Postoperative hsTnT might be suitable as an early prognostic marker after HTX and is independently associated with 1-year mortality and poor DAOH.
Similar content being viewed by others
Introduction
Orthotopic heart transplantation (HTX) is still the gold standard therapy for end-stage heart failure. Unfortunately, there is an ongoing donor shortage which limits the number of HTX and leads to an increasing number of patients on HTX waiting lists [1]. In addition, the treatment of end-stage heart failure is continuously improving so that the patients undergoing HTX are getting older and have more comorbidities with an increased risk for postoperative complications [2, 3]. To optimize perioperative risk stratification and early re-estimation of risk, the development of risk prediction models and the identification of perioperative prognostic factors became more and more important [4, 5, 6]. Numerous risk stratification tools have been developed in the past years, but their clinical use is often limited by insufficient predictive values [5]. In this context, the Index for Mortality Prediction After Cardiac Transplantation (IMPACT) score was introduced as validated tool for prediction of 1-year mortality after HTX [7, 8]. However, some studies report poor-to-moderate discrimination for mortality in their cohorts [9,10,11].
Previously biomarkers were established as another possibility to support perioperative stratification and early re-estimation of risk. A sensitive biomarker for myocardial cell injury is high-sensitivity troponin T (hsTnT) [12]. Postoperative troponin release has been investigated extensively in cardiac and non-cardiac surgery and is associated with adverse events [13, 14, 15]. Recently, Devereaux et al. investigated the prognostic value of high-sensitivity troponin I (hsTnI) in patients undergoing cardiac surgery and showed that levels of hsTnI were independently associated with mortality [16]. The role of hsTnT as a prognostic factor after HTX, however, is not clear and recent literature is ambiguous [17]. Therefore, we conducted this analysis to evaluate whether hsTnT is a suitable marker for risk stratification and prognosis after HTX.
Methods
This retrospective single-center cohort study was approved by the University of Duesseldorf’s ethics committee (reference number: 4567) and complies with the International Society for Heart and Lung Transplantation (ISHLT) ethics statement. Data were extracted from the local prospective HTX database. All patients had given written informed consent to be registered in this database. Reporting of this work corresponds to the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines [18].
Patient population
All patients aged ≥ 18 years who underwent HTX at the University Hospital Duesseldorf, Germany, in a time period from September 2010 to August 2021 were considered for inclusion. Patients with missing data regarding survival, DAOH and hsTnT measurements, as well as patients without completed 1-year follow-up were excluded from analysis. In all patients HTX was conducted using bicaval technique and traditional cold storage was used for donor organ preservation.
High-sensitivity troponin T measurements
Main exposure was postoperative hsTnT measured in ng/L after HTX at different time points. At our institution hsTnT is routinely measured preoperatively, within the first 12 h, day 1, day 2 and day 3 after HTX. Measurements were performed in the central laboratory of the University hospital Duesseldorf.
IMPACT score calculation
The risk index for mortality prediction after cardiac transplantation (IMPACT) is a score validated to predict 1-year mortality after HTX from preoperative recipient risk factors. This score assigns varying points for 10 variables: age, serum bilirubin, creatinine clearance, dialysis, sex, heart failure etiology, preoperative infection, race, circulatory support and type of ventricular assist device. The score was calculated for each HTX patient as described before with a maximum of 50 points [7, 8].
Outcomes
The primary outcome of this study was mortality during the first year after HTX. Days alive and out of hospital (DAOH) at 1 year after HTX was the secondary endpoint of this study. Calculation of DAOH was conducted by summing up all days of hospitalization in the first year after HTX and subtracting them from 365 days, as described before [19,20,21]. In case of mortality, the number of days the patient did not survive and of days spent in hospital were subtracted from 365 days.
Statistical analysis
Statistical analysis was performed using IBM SPSS© software version 25.0 (Armonk, NY, USA), GraphPad Prism© version 8.02 (La Jolla, California, USA), MedCalc® Statistical Software version 20.114 (MedCalc Software Ltd, Ostend, Belgium) and R Statistical Software (v4.1.2; R Core Team 2021). Patients characteristics with continuous variables were presented as mean ± standard deviation (SD) or as median and interquartile ranges (IQR, 25–75%), as appropriate. Categorical variables were presented as numbers (n) with corresponding percentages (%) in brackets. Fisher’s exact test or unpaired t-tests were used to test for differences between dichotomous or continuous variables between groups defined by survival status. For analysis of the primary endpoint, receiver operating characteristic (ROC) analyses were performed for hsTnT levels within 12 h, 24 h, 48 h and 72 h after HTX. Cutoff values for troponin levels were determined by Youden index. The cutoff of the hsTnT time point with the strongest discrimination for 1-year mortality in ROC analysis was added to a logistic regression model, with adjustment using the continuous IMPACT score. The net reclassification improvement (NRI) and the net absolute reclassification improvement (NARI) of the mortality prediction model by adding the postoperative troponin cutoff were assessed. Discrimination (ROC-AUC) of the models with and without postoperative hsTnT was quantified and compared using Delong test. To compare net benefit of using these models to detect patients’ risk for 1-year mortality, a decision curve analysis was performed for both models.
For analysis of DAOH patients were classified by hsTnT cutoff. DAOH was compared using non-parametric Mann–Whitney U test. Association of continuous hsTnT elevation per 100 ng/L with DAOH was adjusted by the continuous IMPACT score using multivariable linear regression. For all statistical tests, a p < 0.05 was considered significant.
Results
Study cohort and characteristics
In total 231 patients underwent HTX at the University Hospital Duesseldorf during the time period of September 2010 and December 2021. Thereof, 19 (8%) patients had to be excluded according to the exclusion criteria. Among the 212 included patients, mean age was 55 ± 11 years and 155 (73%) were male. During first year after HTX, 40 patients (19%) died. Causes of death were: graft dysfunction (7 patients), septic shock (9 patients), major bleeding complications (6 patients with intracranial hemorrhage, 1 patient with gastrointestinal bleeding, 1 patient with ECMO cannulation site bleeding), bowel ischemia (3 patients), cerebral hypoxia (3 patients) and unknown causes (10 patients who died out of hospital). Overall median DAOH was 298 days (229–322). Detailed patient characteristics for survivors and non-survivors are presented in Table 1 (Additional file 1: Figure S1, Table 1).
HsTnT levels of survivors and non-survivors after HTX
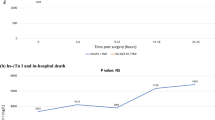
Baseline levels of hsTnT did not differ significantly between survivors and non-survivors. In contrast, postoperative hsTnT levels were significantly higher in patients who died as compared to survivors at 12 h, 24 h, 48 h and 72 h after HTX. Detailed results are presented in Fig. 1.
Postoperative levels of hsTnT of survivors and non-survivors. The graphs depict high-sensitivity troponin (hs-TnT) concentrations for survivors (triangles) and non-survivors (dots) across different time points with corresponding standard errors. Preoperative hs-TnT values did not differ significantly between groups [baseline hsTnT—survivors 165 ± 687 ng/L vs. non-survivors 228 ± 906 ng/L, p = 0.638]. Postoperative values at timepoints 12 h, 24 h, 48 h and 72 h after surgery were significantly higher in non-survivors as compared to survivors [hsTnT 12 h—survivors 5089 ± 5305 ng/L vs. non-survivors 7972 ± 7419 ng/L, p = 0.005; hsTnT 24 h—survivors 3309 ± 2934 ng/L vs. non-survivors 7266 ± 7942 ng/L, p ≤ 0.0001; hsTnT 48 h—survivors 1911 ± 1598 ng/L vs. non-survivors 5115 ± 5196 ng/L, p ≤ 0.0001; hsTnT 72 h—survivors 1363 ± 1565 ng/L vs. non-survivors 3651 ± 4126 ng/L, p ≤ 0.0001]
ROC analysis for postoperative hsTnT and 1-year mortality
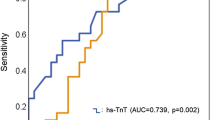
We performed ROC analysis for hsTnT levels and 1-year mortality at sampling time points 12 h, 24 h, 48 h and 72 h after HTX, respectively. All postoperative hsTnT showed a significant discrimination for 1-year mortality [hsTnT12h—AUC = 0.66, 95% CI 0.56–0.75; hsTnT24h—AUC = 0.74, 95% CI 0.66–0.82; hsTnT48h—AUC = 0.79, 95% CI 0.71–0.87; hsTnT72h—AUC = 0.77, 95% CI 0.68–0.86]. The hsTnT levels at 48 h after HTX showed numerically strongest discrimination for 1-year mortality regarding the AUC. Youden Index determined a cutoff of 1640 ng/L for hsTnT at 48 h after HTX (Fig. 2).
Receiver operating characteristic curves of postoperative hsTnT and 1-year mortality. The figure shows the receiver operating characteristics (ROC) curves for association of different hs-TnT sampling time points with 1-year mortality after heart transplantation. The areas under the curves (AUC) are as follows: hsTnT 12 h AUC = 0.66 (CI 0.56–0.75); hsTnT 24 h AUC = 0.74 (CI 0.66–0.82); hsTnT 48 h AUC = 0.79 (CI 0.71–0.87); hsTnT 72 h AUC = 0.77 (CI 0.68–0.86). The numerically strongest discrimination ability is given for hsTnT values at 48 h after heart transplantation
Binary logistic regression model for hsTnT and 1-year mortality
Binary logistic regression model was performed with hsTnT cutoff at 48 h after HTX as independent variable and 1-year mortality as dependent variable. In univariate analysis hsTnT levels at 48 h after HTX showed significant association with 1-year mortality [OR 8.84 95% CI 3.31–23.66, p ≤ 0.0001]. After adjustment for continuous IMPACT score, association of hsTnT with mortality remained significant [hsTnT48h—OR 8.10 95% CI 2.99–21.89, p ≤ 0.0001; IMPACT score—OR 1.09 95% CI 1.01–1.18, p = 0.025]. We analyzed in how far risk prediction for 1-year mortality by IMPACT score was improved by the addition of hsTnT levels at 48 h after HTX are added to the logistic regression model. The NRI for the model including hsTnT was 7.6% (95% CI 4.1–12.6) for non-events and 27.5% (95% CI 14.6–43.9) for events. Regarding NARI, the model was able to identify 114/1000 patients more at risk for 1-year mortality. Corresponding reclassification tables were added to the supplements. In ROC analysis the AUC for the model including troponin was significantly higher as compared to the model only including IMPACT score [IMPACT score—AUC = 0.65 95% CI 0.56–0.74; IMPACT score with hsTnT48h—AUC = 0.77 95% CI 0.70–0.84; difference between areas: 0.12, 95% CI 0.04–0.19, p = 0.0016]. The net benefit curve suggested highest net benefit for the combined use of IMPACT and hsTnT (Figs. 3, 4, Additional file 1: Table S1).
Receiver operating characteristic curves of two prediction models for 1-year mortality. The figure shows the receiver operating characteristics (ROC) curves of risk prediction models for 1-year mortality. While on its own the IMPACT score has a ROC- a moderate discrimination ability (AUC = 0.65 95% CI 0.56–0.74), adding hsTnT levels at 48 h after heart transplantation to the model improves its performance (AUC = 0.79; CI 0.71–0.87)
Decision curves for different models of risk prediction after HTX. The figure shows a decision curve analysis for mortality prediction model with IMPACT score (turquoise) and IMPACT score with hsTnT (purple). The x-axis shows the threshold probability for 1-year mortality while the y-axis shows the net benefit of the models. Beyond a threshold of 5% the combined model of IMPACT and hsTnT shows the greatest net benefit. The red line depicts a model in which all patients would be treated and the green line represents a model in which none patient will be treated
Association of postoperative hsTnT and DAOH
In univariate analysis hsTnT levels higher than predefined cutoff by Youden Index were associated with lower DAOH at 48 h after HTX [hsTnT48h—below cutoff 317 (283–328) days vs. above cutoff 278 (14–308) days, p ≤ 0.0001]. Results for other hsTnT sampling timepoints are presented in the supplements. In a multivariable linear regression model, association of continuous hsTnT elevation (per 100 ng/L) and DAOH remained significant when adjusted for points on the IMPACT score [per 100n g/L hsTnT elevation—regression coefficient: -1.54, 95% CI −2.02 to −1.06, p ≤ 0.0001; IMPACT score—regression coefficient: −4.79, 95% CI −7.83 to −1.76, p = 0.002] (Fig. 5, Additional file 1: Figure S2, Table 2).
Association of hsTnT levels above cutoff and days alive and out of hospital. The box-plot shows significantly fewer DAOH for patients above the determined cutoff of hs-TnT at 48 h after heart transplantation [hsTnT 48 h—below cutoff 317 (283–328) days vs. above cutoff 278 (14–308) days, p ≤ 0.0001]. Black dots represent individual DAOH values of patients, the upper end of the boxes shows the median while error bars depict interquartile ranges. The hsTnT cutoff was determined by Youden index
Discussion
The present study suggested that early hsTnT levels at 48 h after HTX are independently associated with mortality and DAOH after HTX. Moreover, this study showed that the addition of hsTnT improved risk prediction for mortality and DAOH over IMPACT score.
Referring to the current literature, the prognostic value of troponin after HTX is underexplored. A recent systematic review by Liu and colleagues identified only three studies including a total of 372 patients that investigated the association between elevated troponin levels and mortality. As these studies revealed significant heterogeneities, the authors decided not to perform a meta-analysis [17]. Labarrere et al. investigated the value of persistent troponin I (TnI) levels in 110 HTX patients during first year after HTX. They found that persistent TnI levels greater than 0.5 ng/ml were associated with development of coronary artery disease and graft failure. However, as patients were only included if they had survived the first year after HTX, association of postoperatively elevated TnI and early mortality was not investigated [22]. Another study investigated the prognostic value of postoperative hsTnT for 1-year mortality in 141 HTX patients. They identified that elevated hsTnT levels at 6 weeks after HTX were highly associated with 1-year mortality. Again, association of early postoperative levels of hsTnT was not investigated by the authors [23]. The last study by Franeková et al. demonstrated an association of hsTnT levels at 10 days after HTX and 1-year mortality [24]. However, an earlier postoperative assessment of risk might be favorable as it might change clinical practice for patients at risk.
Postoperative troponin release has been extensively studied in non-cardiac surgery before [25, 26]. In these studies, early postoperative troponin elevation above the upper limit of normal was associated with major adverse events like mortality. In cardiac surgery however, this upper limit of normal troponin concentration is frequently exceeded by myocardial trauma due to surgery, with not necessarily higher risk for mortality [13]. Therefore, Devereaux et al. defined new cutoffs for association of troponin and 30-day mortality after cardiac surgery corresponding with an hsTnI level 218 times the upper reference limit [16]. Our recent study now adds data for association of early hsTnT with 1-year mortality after HTX. Patients who died within the first year after HTX had significantly higher troponin values at each timepoint of measurement. These findings were independently associated when adjusted for the IMPACT score. IMPACT score showed weak-to-moderate association with 1-year mortality in our cohort. This goes in line with previous reports, describing similar AUC in ROC analysis [9, 11]. Addition of hsTnT level at 48 h improved the discrimination ability of IMPACT score. The net benefit using the combined model was also higher to identify patients at risk for 1-year mortality. Recently, similar risk prediction models and decision curve analyses for mortality were presented as effective to guide palliative care consultation [27, 28]. Additionally, hsTnT levels were independently associated with low DAOH. This is an important finding, as this complements the current knowledge on more patient-centered outcomes in the field of end-stage heart failure and HTX surgery [21, 29, 30].
In the Eurotransplant area, the responsible parties discuss the implementation of a cardiac allocation score (CAS) which is supposed to optimize the allocation of the limited donor organs. In the lung transplantation setting, a similar score already exists (Lung allocation score (LAS)) which is also used to prioritize waiting list candidates 12 years and older based on a combination of waitlist urgency and post-transplant survival. Based on the data of this study, postoperative hsTnT may also be included into such a score for early re-estimation of postoperative risk and prognosis. Further studies should focus on potential interventions depending on hsTnT values that might be able to prevent complications and finally to reduce mortality after HTX. These may include intensified monitoring or standardized protocols for strict follow-up of these patients.
Strengths and limitations
Strengths of the presented data include first, standardized troponin measurement at multiple time points with high data completeness; second, complete 12-month follow-up. Further, we did not only address mortality but also DOAH, a more patient-centered endpoint to quantify life impact [19]. We are aware of the following limitations. First, as a single-center study, sample size and number of events was limited. However, only 2 variables (troponin and IMPACT) were included into the logistic model that can therefore be considered robust. Second, we cannot exclude that any external hospitalization took place. However, HTX patients are very closely connected to our center so that we consider the risk of misclassification bias was very limited. Further, although DAOH is a measure of life impact, we did not collect data on quality of life, another relevant patient-centered outcome. Finally, we chose hsTnT at 48 h after HTX as primary biomarker for our analysis as it showed the numerically strongest discrimination for 1-year mortality. However, ROC-AUC did not significantly differ from values at 24 h and 72 h. In this context an earlier timepoint like 24 h after HTX might be favorable for early re-estimation of postoperative risk in the clinical setting. Therefore, optimal cutoff and sampling time point should be investigated in a larger cohort.
The generalizability of these findings may be hampered by the single-center design. However, characteristics such as 1-year mortality were in line with the current literature.
Conclusion
Early hsTnT levels after HTX surgery are independently associated with poor 1-year survival and reduced DAOH. Therefore, early hsTnT concentrations might be useful for early risk reassessment to tailor postoperative therapy or decision-making in the intensive care unit.
Availability of data and materials
All relevant data are included in the present manuscript or in the supplements. Raw data are available upon reasonable request by the first author R.M.
References
Bakhtiyar SS, Godfrey EL, Ahmed S, Lamba H, Morgan J, Loor G, Civitello A, Cheema FH, Etheridge WB, Goss J, et al. Survival on the heart transplant waiting list. JAMA Cardiol. 2020;5(11):1227–35.
Alvarez P, Kitai T, Okamoto T, Niikawa H, McCurry KR, Papamichail A, Doulamis I, Briasoulis A. Trends, risk factors, and outcomes of post-operative stroke after heart transplantation: an analysis of the UNOS database. ESC Heart Fail. 2021;8(5):4211–7.
Khush KK, Hsich E, Potena L, Cherikh WS, Chambers DC, Harhay MO, Hayes D Jr, Perch M, Sadavarte A, Toll A, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-eighth adult heart transplantation report—2021; focus on recipient characteristics. J Heart Lung Transplant. 2021;40(10):1035–49.
Lund LH. Optimizing outcomes after heart transplantation. Eur J Heart Fail. 2018;20(2):395–7.
Aleksova N, Alba AC, Molinero VM, Connolly K, Orchanian-Cheff A, Badiwala M, Ross HJ, Duero Posada JG. Risk prediction models for survival after heart transplantation: a systematic review. Am J Transplant. 2020;20(4):1137–51.
Ayers B, Sandholm T, Gosev I, Prasad S, Kilic A. Using machine learning to improve survival prediction after heart transplantation. J Card Surg. 2021;36(11):4113–20.
Kilic A, Allen JG, Weiss ES. Validation of the United States-derived index for mortality prediction after cardiac transplantation (IMPACT) using international registry data. J Heart Lung Transplant. 2013;32(5):492–8.
Weiss ES, Allen JG, Arnaoutakis GJ, George TJ, Russell SD, Shah AS, Conte JV. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT). Ann Thorac Surg. 2011;92(3):914–21.
Nguyen LS, Coutance G, Ouldamar S, Zahr N, Brechot N, Galeone A, Bougle A, Lebreton G, Leprince P, Varnous S. Performance of existing risk scores around heart transplantation: validation study in a 4-year cohort. Transpl Int. 2018;31(5):520–30.
Zheng S, Tang H, Zheng Z, Song Y, Huang J, Liao Z, Liu S. Validation of existing risk scores for mortality prediction after a heart transplant in a Chinese population. Interact Cardiovasc Thorac Surg. 2022;34(5):909–18.
Ortiz-Bautista C, Muniz J, Almenar-Bonet L, Crespo-Leiro MG, Sobrino-Marquez JM, Farrero-Torres M, Garcia-Cosio MD, Diaz-Molina B, Zegri-Reiriz I, Gonzalez-Vilchez F, et al. Utility of the IMPACT score for predicting heart transplant mortality. Analysis on a contemporary cohort of the Spanish heart transplant registry. Clin Transplant. 2022;36:e14774.
Michailovich CA. Diagnostic role and methods of detection of cardiac troponins: an opinion from historical and current points of view. Curr Cardiol Rev. 2022. https://doi.org/10.2174/1573403X18666220610164946.
Lurati Buse GA, Koller MT, Grapow M, Bolliger D, Seeberger M, Filipovic M. The prognostic value of troponin release after adult cardiac surgery—a meta-analysis. Eur J Cardiothorac Surg. 2010;37(2):399–406.
Borg Caruana C, Jackson SM, Ngyuen Khuong J, Campbell R, Liu Z, Ramson DM, Douglas N, Kok J, Perry LA, Penny-Dimri JC. Systematic review and meta-analysis of postoperative troponin as a predictor of mortality and major adverse cardiac events after vascular surgery. J Vasc Surg. 2020;72(3):1132-1143.e1131.
Ekeloef S, Alamili M, Devereaux PJ, Gogenur I. Troponin elevations after non-cardiac, non-vascular surgery are predictive of major adverse cardiac events and mortality: a systematic review and meta-analysis. Br J Anaesth. 2016;117(5):559–68.
Devereaux PJ, Lamy A, Chan MTV, Allard RV, Lomivorotov VV, Landoni G, Zheng H, Paparella D, McGillion MH, Belley-Cote EP, et al. High-sensitivity troponin I after cardiac surgery and 30-day mortality. N Engl J Med. 2022;386(9):827–36.
Liu Z, Perry LA, Penny-Dimri JC, Handscombe M, Overmars I, Plummer M, Segal R, Smith JA. Prognostic significance of elevated troponin in adult heart transplant recipients: a systematic review and meta-analysis. Exp Clin Transplant. 2022;20(7):633–41.
Ghaferi AA, Schwartz TA, Pawlik TM. STROBE reporting guidelines for observational studies. JAMA Surg. 2021;156(6):577–8.
Ariti CA, Cleland JG, Pocock SJ, Pfeffer MA, Swedberg K, Granger CB, McMurray JJ, Michelson EL, Ostergren J, Yusuf S. Days alive and out of hospital and the patient journey in patients with heart failure: Insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Am Heart J. 2011;162(5):900–6.
M’Pembele R, Roth S, Stroda A, Buse GL, Sixt SU, Westenfeld R, Polzin A, Rellecke P, Tudorache I, Hollmann MW, et al. Life impact of VA-ECMO due to primary graft dysfunction in patients after orthotopic heart transplantation. ESC Heart Fail. 2022;9(1):695–703.
Roth S, M’Pembele R, Nucaro A, Stroda A, Tenge T, Lurati Buse G, Sixt SU, Westenfeld R, Rellecke P, Tudorache I, et al. Impact of cardiopulmonary resuscitation of donors on days alive and out of hospital after orthotopic heart transplantation. J Clin Med. 2022;11(13):3853.
Labarrere CA, Nelson DR, Cox CJ, Pitts D, Kirlin P, Halbrook H. Cardiac-specific troponin I levels and risk of coronary artery disease and graft failure following heart transplantation. JAMA. 2000;284(4):457–64.
Erbel C, Taskin R, Doesch A, Dengler TJ, Wangler S, Akhavanpoor M, Ruhparwar A, Giannitsis E, Katus HA, Gleissner CA. High-sensitive troponin T measurements early after heart transplantation predict short- and long-term survival. Transpl Int. 2013;26(3):267–72.
Franekova J, Hoskova L, Secnik P Jr, Pazdernik M, Kotrbata M, Kubicek Z, Jabor A. The role of timely measurement of galectin-3, NT-proBNP, cystatin C and hsTnT in predicting prognosis and heart function after heart transplantation. Clin Chem Lab Med. 2016;54(2):339–44.
Puelacher C, Lurati Buse G, Seeberger D, Sazgary L, Marbot S, Lampart A, Espinola J, Kindler C, Hammerer A, Seeberger E, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221–32.
Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295–304.
Allyn J, Ferdynus C, Bohrer M, Dalban C, Valance D, Allou N. Simplified acute physiology score II as predictor of mortality in intensive care units: a decision curve analysis. PLoS ONE. 2016;11(10):e0164828.
Carolinas Trauma Network Research G, Konda SR, Seymour R, Manoli A, Gales J, Karunakar MA. Development of a middle-age and geriatric trauma mortality risk score a tool to guide palliative care consultations. Bull Hosp Jt Dis (2013). 2016;74(4):298–305.
Roth S, M’Pembele R, Stroda A, Voit J, Lurati Buse G, Sixt SU, Westenfeld R, Polzin A, Rellecke P, Tudorache I, et al. Days alive and out of hospital after left ventricular assist device implantation. ESC Heart Fail. 2022;9(4):2455–63.
Mahmoudi R, Moitie T, Dorent R, Guillemin F, Couchoud C. Implementation of patient-reported outcome measures in a heart transplant recipient registry: first step toward a patient-centered approach. Clin Transplant. 2022;36(8):e14708.
Acknowledgements
None.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research received no external funding.
Author information
Authors and Affiliations
Contributions
RM: concept/design, data collection, data analysis/interpretation, statistics, writing of article. SR: concept/design, data collection, data analysis/interpretation, critical revision of article. AN: data collection, data analysis, writing and critical revision of article. AS: data collection, data analysis, critical revision of article. TT: data collection, data analysis, critical revision of article. GLB: statistics and methodology, critical revision of article. FB, DS, CB, IT, HA: data collection, critical revision of article. AL: drafting article, data collection, critical revision of article. RH and UB: concept/design, data interpretation, critical revision of article. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the University Hospital Duesseldorf (Chair: Professor Thomas Hohlfeld; reference number: 4567; date of approval: 25.01.2021).
Consent to participate
Not applicable.
Competing interests
The authors declare no competing or financial competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Study Flowchart. Figure S2. Association of hsTnT levels at different timepoints above cutoff and days alive and out of hospital. Table S1. Reclassification tables of a 1-year mortality prediction model using IMPACT compared to a model using IMPACT and hsTnT.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
M’Pembele, R., Roth, S., Nucaro, A. et al. Postoperative high-sensitivity troponin T predicts 1-year mortality and days alive and out of hospital after orthotopic heart transplantation. Eur J Med Res 28, 16 (2023). https://doi.org/10.1186/s40001-022-00978-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00978-4