Abstract
Background
Lung cancer is one of the most preventable causes of death globally both in developed and developing countries. Although it is well established that smokers develop lung cancer, there are some smokers who are free from the disease risk. The predisposition to lung cancer is attributed to genetic polymorphisms in xenobiotic metabolizing genes. Reports on assessment of xenobiotic metabolizing genes like Cytochrome P 450 1A1 (CYP1A1), Glutathione -S -transferase M1 (GSTM1) and T1 (GSTT1) polymorphisms from India are meagre, and reports from Andhra Pradesh are lacking.
Methods and results
Assessment of polymorphisms in CYP1A1, GSTM1 and GSTT1 in NSCLC patients and healthy individuals specific to population of Andhra Pradesh, a South Indian state was attempted by multiplex PCR and RFLP, and this is the first study which tried to correlate oxidative stress with the polymorphisms in xenobiotic metabolizing genes. Results showed that CYP1A1 m1 ‘CC’ genotype was significantly associated with lung cancer susceptibility with a 2.3-fold risk, CYP1A1 m2 ‘AG’ gene polymorphisms with 8.8-fold risk and GSTT1 (−/−) genotype demonstrated a twofold risk of disease susceptibility.
Conclusions
A combined role of genetic polymorphisms and smoking status can be attributed for the cause of lung cancer. Further, the association between oxidative stress and genetic polymorphisms showed a correlation between GSTT1 and super oxide dismutase activity; CYP1A1 m1, m2 and GSTT1 with glutathione peroxidase activity; CYP1A1 m1 and GSTM1 with melondialdehyde levels; and CYP1A1 m1 and GSTT1 with 8-oxo-7,8-dihydro-2′-deoxyguanosine. A higher risk of lung cancer seems to be associated with combined gene polymorphisms of phase I and phase II enzymes than that ascribed to single gene polymorphism.
Similar content being viewed by others
Background
Xenobiotic metabolism is the process of detoxification of endogenous or exogenous carcinogens/poisons and occurs in two phases. In Phase I, cytochrome P450 oxidases amend the xenobiotics by introducing a polar or reactive group. In Phase II, the modified xenobiotics are conjugated to polar compounds facilitated by enzymes such as glutathione S-transferases [1]. Among the phase I enzymes, cytochrome P450 1A1 (CYP1A1) plays a vital role in the activation of polycyclic aromatic hydrocarbons (PAHs) to convert them to carcinogens [2]. The phase II enzymes involve glutathione-S-transferases (GSTs) which are divided into five classes (alpha, mu, pi, theta and zeta), and catalyse the conjugation of highly reactive PAHs to soluble glutathiones [3]. Among the GSTs, GSTM1 preferentially detoxifies carcinogens (epoxides and hydroxylated derivatives) derived from tobacco, whereas GSTT1 causes the biotransformation of many toxins such as butadiene and ethylene oxides (ingredients of tobacco smoke) [4]. The balance between the phase I and phase II enzymes is crucial to determine the amount of reactive intermediates that are formed in the cell. Any aberrations due to genetic polymorphisms affect the activities of these enzymes; thereby, increasing the risk of cancer in an individual and gene–gene interactions of phase I and phase II enzymes together with life style habits can be synergistic risk factors.
Among all the cancers, carcinoma of the lung is responsible for the high death rate throughout the world [5]. Although tobacco consumption is considered to be the significant aetiological factor for lung cancer [6], not all smokers develop lung cancer. Risk is dependent on the extent of smoking, environmental factors (carcinogen exposure) and most prominently genetic factors. Genetic polymorphisms in the enzymes involved in metabolic activation and detoxification were found to immensely contribute to the risk of developing lung cancer [7]. These polymorphisms cause inter-individual differences in the bio-activation and detoxification of pro-carcinogens, which are in turn responsible for the varied susceptibilities to lung cancer [8, 9]. Among the xenobiotic metabolizing enzymes, CYP1A1, GSTM1 and GSTT1 have been projected as the potential modulators of cancer susceptibility [10]. Although these enzymes play a crucial role in bio-activation and detoxification of chemical carcinogens present in tobacco smoke, the role of Glutathione–S transferase genes in modulating the risk of cancer has been debated owing to inter-individual, geographical, ethnic and demographical differences throughout the world. The association between CYP1A1 and GSTM1 polymorphisms in lung cancer was reported [11, 12]. However, GSTT1 deficiency was demonstrated (GSTT1 null) not to increase the risk of lung cancer [13, 14]. The frequencies of CYP1A1 and GSTM1 gene polymorphisms were found to vary among different ethnic populations [15, 16]. Among Asians, CYP1A1 2A and CYP1A1 2C genetic polymorphisms are common, whereas in Caucasians, the variation in CYP1A1 2C is rare [16, 17]. Similarly, GSTM1 null type is more common in Asians than in Caucasians [18]. Null genotype represents the homozygous deletion of the gene. The inter-relation between CYP1A1 polymorphism, tobacco smoking and lung cancer was found to be high in Japanese and Chinese populations, whereas the same was not observed in Caucasians [17, 19–22]. The risk association between GSTM1 null genotype with squamous cell and small cell carcinomas in Asians was found to be significant [23, 24]. Further, a combination of GSTM1 null genotype with CYP1A1 polymorphisms augmented lung cancer risk [25, 26].
In the Indian context, studies on association of lung cancer and genetic polymorphisms are limited. In a North Indian cohort, the risk of CYP1A1 gene polymorphism in 100 patients with lung cancer was assessed, and a 2.68-fold risk was observed for CYP1A1 2C allele and in the presence of a single copy of the variant CYP1A1 (CYP1A1 * 1/2A) and for null GSTT1 genes, a threefold increased risk of lung cancer was demonstrated [27]. Another group from North India demonstrated that the risk of lung cancer is associated with CYP1B1 and GSTM1 polymorphisms in the population [28]. CYP2E1 polymorphisms in six ethnic groups of South Indian population were demonstrated [29]. A study from Kerala in 146 lung cancer patients indicated that CYP1A1 MspI homozygous variant allele and GSTT1 null deletion frequency were significantly higher in smoking-induced lung cancer patients compared with other populations [30]. The Southern part of India is largely composed of five states, namely, Andhra Pradesh (Telangana + Andhra Pradesh), Tamilnadu, Kerala, Karnataka and Maharashtra, where the environmental conditions, economy, food habits and life style vary a lot. The limitations of systematic studies that correlate the association between CYP450 and GST gene polymorphisms and the risk of lung cancer include (1) limited number of subjects from different areas which are not representative of the entire population; (2) subjects exposed to different environmental conditions; and (3) different gene polymorphisms being evaluated. Besides ethnic background, life style and dietary habits also contribute to the increased risk of lung cancer. The dietary habits, environmental factors and tobacco consumption vary between the Northern and Southern regions of India. Tobacco consumption is rampant in both North and South Indian populations [31]. Although studies reported the association of lung cancer and gene polymorphisms in South Indian population, the samples were drawn from a tertiary hospital located in the capital city of a particular state. Hence, the entire South Indian population was not represented. In view of the above, it becomes imperative to determine the association between the gene polymorphisms of enzymes that are associated with detoxification of tobacco-related carcinogens and the risk of lung cancer in the state of Andhra Pradesh to generate more data to arrive at a plausible conclusion. Further, the samples were collected from a single hospital that received patients from the entire state of Andhra Pradesh, and this ensured homogeneity of the samples obtained.
Besides genetic factors, biochemical markers such as oxidative stress and antioxidant responses were also implicated in the development of lung cancer which showed changes in the oxidant and antioxidant statuses in the peripheral lymphocytes of non-small cell lung cancer (NSCLC) patients [32]. However, till date, studies that demonstrate the association between genetic and biochemical interactions and the risk of NSCLC were not reported. Hence, for the first time, in this study, we analysed such associations which might serve as predictive markers, contributing to differential susceptibility toward PAH and tobacco-induced cancers.
Methods
Study population
The subjects of the present study included 246 newly diagnosed and previously untreated NSCLC patients referred to Indo-American cancer Hospital from various regions of Andhra Pradesh, India during the period June 2008–2012. 98.2 % of the patients and all the control subjects included in our study were natives of Andhra Pradesh. All the patients were rated as positive for NSCLC by histological analyses and were classified using revised lung cancer staging system [33]. The co-morbid conditions in NSCLC patients included 24 (9.75 %) of them being diabetic, 30 (12.19 %) hypertensive and 2 (0.81 %) patients having hypothyroidism. Age- and sex-matched healthy controls (n = 250) were enroled from the general population of the same geographical region. Routine medical check-up was conducted, and history of illness was recorded by a health practitioner. Those who appeared apparently healthy without any history of cancer or other chronic diseases were considered as normal. The co-morbid conditions among controls included 8 (3.2 %) of them being diabetic, 11 (4.4 %) being hypertensive, and none having hypothyroidism. Study subjects who were used to smoking at the time of diagnosis were considered as smokers and those who had smoked at least 100 cigarettes in their life time were considered as ex-smokers. Among the NSCLC smokers, 51 (48.11 %) and 47 (44.34 %) consumed cigarettes and bidis, respectively, and 8 (7.55 %) consumed both. In the case of NSCLC patients who are ex-smokers, the cigarette and bidi consumers were 26 (61.9 %) and 15 (35.72 %), and 1 (2.38 %) consumed both bidi as well as cigarette. Pack years were computed as the number of cigarettes smoked per day multiplied by the duration of smoking in years, and the average tobacco consumption was expressed in pack years. Among the control smokers, 48 (76.19 %) consumed cigarettes, while 15 (23.81 %) were bidi smokers. In case of ex-smokers, 8 (66.66 %) were cigarette smokers, while 4 (33.34 %) were bidi smokers.
Ethics statement
The study was carried out with the approval of Institutional Ethics Committees of Indo-American Cancer Hospital and Institute of Genetics and Hospital for Genetic Diseases. Educated and informed consent was obtained from all the subjects of the study. A standard questionnaire was used to document the socio-demographical characteristics such as age, sex, lifestyle (alcohol, diet, etc.), occupational exposure (working hours/day, years of exposure, use of protective measures, etc.), history of smoking, number of cigarettes per day and duration of smoking.
Molecular analysis of CYP1A1 m1, m2, GSTM1 and GSTT1 gene polymorphisms
Blood collection and DNA isolation
2 ml of whole blood was collected in vacutainers (BD Biosciences) containing ethylenediamine tetra acetic acid (EDTA) for DNA isolation, and 3 ml was collected in heparinized vacutainers for the assessment of oxidative stress markers from healthy controls and NSCLC patients. Genomic DNA was isolated (Flexi gene extraction kit, QIAGEN) from 300 μl of whole blood and was stored in −80 °C until further use.
CYP1A1 m1 and m2 genotyping
Genotyping for CYP1A1 m1 and m2 genes (rs4646903 and rs1048943) was carried out as described earlier [34]. The primers’ sequences used for m1 site were M1F (5′-CAG TGA AGA GGT GTA GCC GCT-3′) and M1R (5′-TAG GAG TCT TGT CTC ATG CCT-3′) and for m2 site were M2F (5′- TTC CAC CCG TTG CAG GAT AGC C-3′) and M2R (5′-CTG TCT CCC TCT GGT TAC AGG AAG-3′). The PCR amplification was carried out in 25-µl reaction mixture consisting of 100 ng template of DNA, 10 µM of each primer, 0.2 mM each dNTP, 2.4 mM MgCl2, 1 U Taq DNA polymerase with 1× reaction buffer (Bangalore Genei). The PCR cycle consisted of 1 min at 94 °C, 1 min at 61 °C (for CYP1A1 m1)/63 °C (for CYP1A1 m2) and 1 min at 72 °C with initial denaturation of 5 min at 94 °C and final extension of 10 min at 72 °C. The PCR amplicons generated for m1 (340 bp) and m2 (204 bp) were subjected to restriction digestion. Msp1 and BsrD1 restriction enzymes were used to detect polymorphisms in the CYP1A1 m1 and m2, respectively. The reaction mixtures were incubated at 37 °C for 12 h, electrophoresed on 3.0 % agarose gel and stained with ethidium bromide (Sigma Aldrich, USA) for visualization. All the sampling experiments were done in duplicates. Restriction digestion was repeated in cases which were unclear. Positive samples were included in each run of PCR as well as restriction digestion to ensure that the samples were properly digested.
GSTM1 and GSTT1 genotyping
The GSTM1 and GSTT1 gene deletions (rs4025935 and rs71748309) were analysed simultaneously by multiplex PCR [35]. To detect the GSTM1 deletion, the primers used were GSTM1 F (5′-GAACTCCCTGAAAAGCTAAAGC-3′) and GSTM1 R (5′-GTTGGGCTCAAATATACGGTGG-3′). For GSTT1, the primers used were GSTT1 F (5′-TTCCTTACTGGTCCTCACATCTC-3′) and GSTT1R (5′-TCACCGGATCATGGCCAGCA-3′). The PCR amplicons were electrophoresed on a 4 % agarose gel, stained with ethidium bromide, and the results were documented using a gel documentation system (Bio-Rad). The presence of GSTM1 and that of GSTT1 genes were indicated by the resulting 215- and 480-bp PCR amplicons, respectively. A DNA sample with GSTM1 and GSTT1 alleles present was run as a positive control in each run. As an internal control, human albumin gene (HAB) was amplified (350 bp) using the primers HAB F (5′-CAACTTCATCCACGTTCACC-3′) and HAB R (5′-GAAGAGCCAAGGACAG GTAC-3′) for the authentication of multiplex PCR.
Estimation of 8-oxodG, lipid peroxidation and antioxidant enzymes
8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) levels in the urine samples of healthy controls and NSCLC patients were measured in 125 patients and 100 controls using commercially available kits (Japan Institute for the Control of Aging, Shizuoka, Japan). Lipid peroxidation products were measured in the plasma of 246 patients and 250 controls as described earlier [32]. Red cell superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities were estimated in 238 NSCLC patients and 250 controls using SOD-525 and GPx-340 spectrophotometric assay kits according to the manufacturer’s instructions (Bioxytech; OXIS International, Portland, USA). Haemoglobin (Hb) concentrations were measured by a commercially available kit (Sigma, St. Louis, MO, USA).
Statistical analyses
The data were analysed using the SPSS 15.0 program (SPSS, Chicago, IL). The significance of the differences between controls and patients end point means were analysed using Student’s t test. ANOVA (analysis of variance) was used for comparisons among the three or more groups. Multiple regression analysis was done to investigate the associations of the independent variables. Pearson correlation analysis was used for testing relationships between genotypes in patients and controls. The results were considered to be significant at p values of less than 0.05 (indicated by *). The differences in the distribution of genotype frequencies were calculated using the χ2 test. Genotype frequencies were checked for deviation from Hardy–Weinberg equilibrium and were not significantly different from those predicted. Odds ratios and 95 % confidence interval (95 % CI) were calculated to assess the relationship between CYP1A1 and GST gene polymorphisms.
Results
General characteristic features of the study group
The general characteristic features of NSCLC patients (n = 246) who had no previous history of diagnosis and healthy controls (n = 250) included in this study are given in Table 1. NSCLC was predominant in males, affecting older men in the age group of 60–70 years. Age of onset of the disease was lower in women compared to males (55 vs. 58.91), although it was not statistically significant. The risk estimation for patients without co-morbid conditions compared to controls without co-morbid conditions was 3.58-fold (OR 3.58; 95 % CI 2.058, 6.24; p ≤ 0.001).
Molecular analysis of CYP1A1 m1, m2, GSTM1 and GSTT1 genes
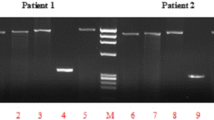
CYP1A1m1 and m2 polymorphisms were detected by RFLP. PCR amplification for CYP1A1 m1 produces 340-bp amplicons. A gain of Msp1 restriction site in the polymorphic allele resulted in 340-bp products for homozygous major type (TT), 200 and 140 bp for homozygous minor (CC), respectively (Fig. 1). BsrD1 restriction enzyme-based digestion was used to detect the CYP1A1m2 polymorphisms. In the case of ‘GG’ (homozygous minor), due to loss of the restriction sites, a single amplicon of 204 bp was obtained, whereas in the ‘AA’ (homozygous major) allele will generate two amplicons of sizes, 149 and 55 bp (Fig. 2). Multiplex PCR-based approach was employed to determine the genetic polymorphisms of GSTM1 and GSTT1 genes. Amplicons of 215 bp and 480 bp indicated the presence of GSTM1 and GSTT1 genes (Fig. 3).
Amplifications of CYP1A1 m1 and the RFLP products of the polymorphic forms: a PCR for CYP1A1 m1 (340 bp) in multiple samples. b CYP1A1 m1 polymorphisms were detected by RFLP. The 340-bp PCR product was digested with Msp1 enzyme. Lanes 4 and 9 represent homozygous major type (TT; 340 bp); Lanes 3 and 12 represent homozygous minor (CC; 200 and 140 bp); Lanes 1, 2, 5, 6, 7, 8, 10, 11, 13, 14 and 15 represent heterozygous type (TC; 340 bp, 200 and 140 bp)
Amplifications of CYP1A1 m2 and the RFLP products of the polymorphic forms: a PCR amplification for CYP1A1 m2 (204 bp). b CYP1A1 m2 polymorphism detected by RFLP. The 204-bp PCR product was digested with BsrDI enzyme. The cleavage site is lost in case of variants to give a single amplicon, whereas the wild-type allele generates 149- and 55-bp fragments. Lane 5 represents homozygous minor (GG); Lane 7 represents the heterozygote (AG); Lanes 2–4, 6 and 8–13 represent homozygous major (AA)
GSTM1 and GSTT1 polymorphisms: The GSTM1 and GSTT1 gene deletions were analysed simultaneously by multiplex PCR. Amplicons of 215 bp and 480 bp indicate, respectively, GSTM1 and GSTT1. Lane 1, 100-bp DNA ladder; Lanes 2 and 14, GSTT1 Null type (TN); Lanes 3–13 and 15–18: GSTT1 Wild type (TW); Lanes 2–9 and 13–18: GSTM1 wild type (MW); Lanes 10–12: GSTM1 Null type (MN). Albumin (350 bp) was used as an internal control
Genotyping distribution of CYP1A1 m1 (T3801C 3′ noncoding region)
The homozygous major (TT), heterozygous (TC) and homozygous minor (CC) genotype frequencies of CYP1A1 m1 gene in healthy controls were 57.2, 37.2 and 5.6 %, respectively, whereas the same in NSCLC patients were 49.59, 38.62 and 11.78 %, respectively (Table 2). The ‘CC’ genotype was significantly higher in the NSCLC patients compared to healthy controls (p = 0.007, χ2 = 5.98, OR 2.25, 95 % CI 1.16–4.37) with 2.25-fold risk of disease susceptibility.
Genotyping distribution of CYP1A1 m2 (Exon 7 Ile462Val)
The frequencies of CYP1A1 m2 homozygous major (AA), heterozygous (AG) and homozygous minor (GG) genotypes in healthy controls were 78.4, 13.6 and 8 %, whereas the same were 29.67, 58.14 and 12.19 % in NSCLC patients, respectively (Table 3). Interestingly, the heterozygous ‘AG’ genotype was significantly higher in NSCLC group compared to healthy controls (p < 0.001, χ2 = 106.9, OR 8.82, 95 % CI 5.67–13.72) with an estimated 8.8-fold risk of developing lung cancer in individuals with this genotype.
Risk associated with additive effect of CYP1A1 m1 and CYP1A1 m2 polymorphisms within the same gene
Healthy controls displayed higher percentage of homozygous major (TT/AA) genotype combination (44.8 %) followed by the combination of homo/hetero (TT/AG; 38 %), and hetero/homo (TC/AA; 29.2 %), among all SNP combinations (Additional file 1: Table S1). Interestingly, in NSCLC patients, the frequency of homo/hetero genotypes and hetero/homo (TT/AG; 29.26 % and TC/AA; 14.63 %) was more common, followed by homozygous major (TT/AA; 13.41 %) genotypes. The frequency of homozygous minor genotype ‘CC/GG’ (p = 0.02, χ2 = 4.79, OR 12.48, CI 0.69–224.5) in patients demonstrated a 12-fold risk of developing lung cancer compared to the controls. The combination of ‘CC/AG’ (p = 0.004, χ2 = 6.89, OR 6.89, CI 2.01–23.6) showed a 6.9-fold risk of susceptibility to lung cancer, while heterozygous m1/m2 ‘TC/AG’ (p = 0.001, χ2 = 28.33, OR 5.18, CI 2.69–10.00) presented with a five fold risk.
Genotypic distributions of GSTM1 and GSTT1 genes
GSTM1 gene was found to be present in 76 % of the healthy controls and 73.98 % of NSCLC patients (Table 4). GSTT1 gene was present in 89.6 % of healthy controls and 81.3 % of NSCLC patients and individuals lacking this gene were at a twofold risk of developing lung cancer (p = 0.008, χ2 = 6.86, OR 1.98, CI 1.18–3.32) (Table 5).
Risk associated with combination of the two glutathione-S-transferase gene polymorphisms
Combined frequencies of GSTM1 and GSTT1 polymorphisms Wild/Wild, Wild/Null, Null/Wild and Null/Null in healthy control were 66.80, 10.40, 23.60 and 1.6 %, respectively, whereas in NSCLC patients the frequencies were 59.34, 14.63, 21.95 and 4.06 %, respectively. It was clear that the GSTM1 Wild/GSTT1 Wild genotype followed by GSTM1 Null/GSTT1 Wild combinations were more predominant in both healthy controls and NSCLC patients (Additional file 1: Table S2). The disease association was found between GSTM1 Wild/GSTT1 Null genotype (p = 0.01, OR 1.97) and GSTM1 Null/GSTT1 Null (p = 0.04, OR 2.6) combinations, indicating a 1.97- and 2.6-fold risk of disease susceptibility, respectively (Additional file 1: Table S2).
Risk of NSCLC associated with CYP1A1, GSTM1 and GSTT1 genotypes stratified by smoking exposure
Patients who were non-smokers and having a CYP1A1 m1 (T/C) (OR 1.82, 95 % CI 1.08, 3.07) and CYP1A1 m2 (A/G) (OR 12.39 95 % CI 6.53, 23.51) genotypes had an increased lung cancer. Lung cancer patients who smoked and having CYP1A1 m1 T/C, C/C and CYP1A1 m2 A/G, G/G and GSTT1 null (−/−) genotypes were at higher risk compared to the controls (Table 6).
Association of CYP1A1, GSTM1 and GSTT1 genotypes stratified by histology
In all the three pathological subtypes, CYP1A1m2 A/G, GSTM1 (+/+) wild and GSTT1 (+/+) wild were the predominant genotypes (Table 7).
Risk of NSCLC associations with combination of CYP1A1 and GST genes
The combinations of genotypes having a profound effect were CYP1A1 m2 A/G + GSTM1 wild (+/+); CYP1A1 m2 A/G + GSTM1 null (−/−); and CYP1A1 m2 G/G + GSTT1 wild (+/+) with estimated risks of sixfold, sixfold and 10.5-fold, respectively (Additional file 1: Table S3).
In the case of three genotype combinations, CYP1A1 m1 T/T + CYP1A1 m2 G/G + GSTM1 null (−/−) showed a 19-fold risk; CYP1A1 m1 T/C + CYP1A1 m2 G/G + GSTM1 wild (+/+) showed 11.6-fold risk and CYP1A1 m1 G/G + CYP1A1 m2 A/G + GSTM1 wild (+/+) showed a 10.5-fold risk of disease susceptibility (Additional file 1: Table S4).
The overall risk of NSCLC associated with three genotype combinations of CYP1A1 m1, m2 and/or GSTT1 genes ranged from 3.48 to 10.55 (Additional file 1: Tables S5, S6). When analysed for the overall risk with four genotype combinations, it ranged from 5.22 to 13.89 (Additional file 1: Tables S7–S9). Spearman coefficient correlation indicated CYP1A1 m2 gene significantly correlated with GSTM1 and GSTT1 genes (Table 8).
Impact of gene polymorphisms on oxidative stress markers
The impact of CYP1A1 m1, CYP1A1m2, GSTM1 and GSTT1 gene polymorphisms on superoxide dismutase activity (Table 9), Glutathione peroxidase activity (Table 10), MDA (Table 11) and 8-OHdG levels (Table 12) were assessed between controls and lung cancer patients. In NSCLC patients, there was a significant difference between the SOD levels of GSTT1 wild (+/+) vs null (−/−); GPx activities between CYP1A1 m1 T/T vs T/C and T/T vs C/C; CYP1A1 m2 A/A vs A/G and A/A vs G/G genotypes and GSTT1 wild vs null genotypes. Mean MDA levels were significantly different with respect to CYP1A1 m1 and GSTM1 genotypes. The difference in 8-OHdG levels between the genotypes was significant only for CYP1A1 m2 gene and GSTT1 gene polymorphisms. The difference between the genotypes of different genes for SOD, GPx, MDA and 8-OHdG levels were not significant in the control group.
Multiple regression analysis of different variants in lung cancer patients
Multiple regression analysis was performed by taking age, gender, smoking status, alcohol consumption, dietary habits, occupation, family history, stage of the disease and histology (Table 13). We observed that smoking, histology, stage of the disease, MDA levels, GPx activities and polymorphisms in CYP1A1 m1 and GSTT1 genes were the strongest predicting factors for increased free radical generation and imbalances in antioxidant defence causing oxidative stress and leading to disease susceptibility in lung cancer patients. Other variables did not have any impact as reflected by lack of significance.
Discussion
Xenobiotic metabolising enzymes expedites purging of a variety of toxic substances, thereby gaining prominence in the pathophysiology of cancer. Hence, gene polymorphisms in the enzymes that are intricate in the metabolism of carcinogens may regulate an individual’s predisposition to cancer including lung cancer [36]. Besides this, environmental and life style insults also contribute to the predisposition of lung cancer. Cigarette smoke contain PAHs which can be metabolically activated to highly reactive compounds capable of binding to DNA and initiating the carcinogenic process [37, 38]. Among the variety of xenobiotic metabolising enzymes, CYP1A1, GSTM1 and GSTT1 have been implicated to modulate the risk of lung cancer because of their potential involvement in carcinogenesis metabolism. Globally many studies reported on the association among gene interactions and lung cancer in different populations, but the conclusions were conflicting [18]. In the Indian context, risk assessment between gene polymorphisms and lung cancer was investigated in Northern and Southern Indian populations. CYP1A1, GSTM1 and GSTT1 polymorphisms and the association with lung cancer in the South Indian population (patients reporting to a specific hospital in Thiruvananthapuram, the capital city of Kerala state) was reported [30], suggesting the risk in the specific population of that state. However, there are genotypic, life style and environmental differences in the populations of the five states (Andhra Pradesh, Tamilnadu, Kerala, Karnataka and Maharashtra) of South India. Hence, we conducted systematic analyses on the associations of CYP1A1, GSTM1 and GSTT1 polymorphisms with the risk of NSCLC in the population of Andhra Pradesh.
In the present study, a high frequency of CYP1A1 m1 homozygous minor genotype (C/C) was recorded among NSCLC patients. Association of lung cancer risk with homozygosity of CYP1A1 variant alleles was reported in Chilean and Caucasian populations [39–41]. Likewise, in the North and South Indian populations, the association of CYP1A1 polymorphism with lung cancer risk was reported [30, 42, 43]. Further, in the current study, evaluation of the genotypic frequencies in lung cancer patients from Andhra Pradesh have shown a higher frequency and a significant association of CYP1A1 m2 heterozygous ‘AG’ genotype. Similar observations (higher frequency of CYP1A1 m2 (A/G) allele) were reported in lung cancer patients from Korea [19]. On the same lines, CYP1A1 m2 (G/G) allele frequency was demonstrated to be lower in Caucasians than Japanese [44]. Heterozygous and homozygous minor CYP1A1 m2 genotypes were on the higher side in Chilean lung cancer patients [25]. The higher frequency of this gene was also reported in the Southern and Northern Indian lung cancer patients [42, 45]. Results of our study are in parallel with the observations made in different populations worldwide, and it is possible that the mutated genotype of CYP1A1 plays an important role in the aetiology of lung cancer in the population of Andhra Pradesh state.
In the current study, GSTM1 wild and null genotypes were detected, respectively, in 73.98 and 26.1 % of lung cancer patients. A similar trend was observed in the healthy controls (76 % wild type and 24 % null type, respectively). Similarly, GSTM1 null genotype was not associated with the increased risk of lung cancer, and the proportions of the NSCLC patients and healthy controls exhibiting GSTM1 null genotype were apparently equal. Similar trends were observed in the South and North Indian population cohorts [30, 46]. On the contrary, GSTM1 null or deletion genotype was reported to be prevalent in about 50 % of Caucasians, 33 % of African Americans and 45 % of Japanese [47] lung cancer patients. We found that GSTT1 null genotype was high in lung cancer patients compared to the controls, which is consistent [30, 41, 48] and in conflict [42, 49] with previous reports. GSTT1 deletion polymorphisms was reported in 13–28 % of Caucasians [18]. Similarly, the frequencies of homozygous deletions (null genotype) for GSTM1 and GSTT1 were found to be 22.4 % and 17.6 %, respectively, in the South Indian population; 54 % and 13 %, respectively, in the East Indian population [50, 51]; 41 % and 21.5 %, respectively, in the North Indian population [46]. Results of our study and others indicate that in the Indian context, the risk of lung cancer is more associated with GSTT1 polymorphism rather than GSTM1 genotype.
A majority of the patients included in our study were bidi smokers (made of crude particles of dried tobacco leaves wrapped in a tendu or temburni leaf and rich in tar and nicotines); and bidi smoking is known to generate stronger carcinogen load than cigarette [52]. Our data clearly indicate that individuals who were smokers and had CYP1A1m1 T/C, C/C or CYP1A1m2 A/G, G/G genotypes and GSTT1 null genes were at higher risk of disease susceptibility to lung cancer. A threefold risk of lung cancer associated with CYP1A1 m1 genotype was reported [46]. Further, increased risk of lung cancer in heavy smokers [34, 42] and light smokers [20, 53] with CYP1A1 m1 allele was demonstrated. In the present study, no risk of lung cancer was associated with GSTM1 null genotype in smokers and non-smokers. The association between GSTM1 genotype and cumulative smoking is controversial [47]. Stronger associations were reported in casual smokers [42] and low smoking exposed individuals [53], whereas such an association was not evident in other reports [54]. Results of our study indicate that CYP1A1 polymorphisms rather than GSTM1 polymorphisms and smoking contribute to the higher risk of lung cancer. Our results are in accordance with another study from North India where the relative risk for the carriers of variant CYP1A1 genotypes was high [55]. The disease association among combination of GST genes in the lung cancer patients was found between the Wild/Null and Null/Null types. The increased risk due to deletions of GST may result in less detoxification of xenobiotics, thereby making the individual more susceptible to toxic substances present in the environment.
Analysing multiple gene interactions provide better understanding to assess the risks associated with lung cancer risk. In our study, the combinations of two (CYP1A1 m2 and GSTM1) or three (CYP1A1 m1, CYP1A1 m2 and GSTM1/GSTT1) genotypes had a profound effect on susceptibility to lung cancer up to 14-fold depending on the genotypic interaction. Correlations between lung cancer risk and combinations of CYP1A1, GSTM1 and GSTT1 is of particular interest since these genotypes suggest that alterations in the action of phases I and II enzymes lead to defective metabolism of xenobiotic compounds, thereby potentiating the cancer risk. It was suggested that individuals having polymorphisms in more than one of these genes are at higher risk than having for only one gene [56]. Polymorphisms of MspI and exon7-Val of CYP1A1 and GSTM1 null genotypes and increased lung cancer risk was evidenced in summarized data of 46 studies of Chinese populations [56]. In an Indian population study, a twofold risk of lung cancer was found in individuals displaying variations in the CYP1A1 and GSTM1 genes [42]. CYP1A1, GSTM1, GSTP1 and GSTT1 polymorphisms and their association to lung cancer in a cohort of North Indian population was reported [46]. Similarly, in a study involving South Indian population, a 4.4-fold increased risk of the GSTM1 null, GSTT1 null, CYP1A1 homozygous major genotype combination and a 3.5-fold increased risk, although not statistically significant, in individuals possessing the GSTM1 null, GSTT1 null, CYP1A1 homozygous minor genotype combination [30, 57] were reported. Results of our study are in agreement with the reported data and clearly indicate strong associations of CYP1A1, GSTM1 and GSTT1 genetic polymorphism with NSCLC.
The role of oxidative stress in the pathophysiology of a variety of cancers including lung cancer was well documented [32]. Genetic polymorphisms of metabolic enzymes and oxidative stress markers in occupational exposure were reported [58]. However, the association between oxidative stress and genetic polymorphisms with respect to lung cancer was not documented. We previously demonstrated that 8-oxo-dG and malondialdehyde levels were increased and red cell superoxide dismutase and glutathione peroxidase activities were significantly decreased in lung cancer patients [32]. Hence, in this study, the genotypes of polymorphic markers were stratified with respect to oxidative markers to evaluate whether the inter-individual variation of oxidants and antioxidants could lead to disease susceptibility. To the best of our knowledge, this is the first study to assess the association of polymorphism of CYP1A1 and GST genes with respect to SOD, GPx, MDA and 8-oxo-dG levels in lung cancer patients from India. We found an association between GSTT1 null genotype and SOD activity, CYP1A1 m1, m2 and GSTT1 and GPx activity, MDA levels and CYP1A1 m1 & GSTM1, 8-oxo-dG and CYP1A1 m1 and GSTT1 gene polymorphisms. Although no information is available on the association of oxidants, antioxidants and gene polymorphisms, some information is available on the association of gene polymorphisms and urinay 8-oxo-dG levels. While some studies [59–65] demonstrated the influence of gene polymorphisms on urinary 8-oxo-dG levels, some other studies did not show such an association [66–68]. It is possible that deletion polymorphisms of GSTM1 and GSTT1 (null genotype) results in no functional enzymatic activity, thereby failing to detoxify several xenobiotics including tobacco smoke constituents and finally leading to increased generation of ROS and lowered GPx activity and \({\text{O}}_{2}^{ - }\) scavenging activity of SOD. Results of our study provide strong association between gene polymorphisms, oxidant and antioxidant status and the risk of developing NSCLC, which hitherto was not reported.
The limitations of our study are that the healthy controls and NSCLC patients were in the ratio of 1:1, and some of the NSCLC patients had co-morbid conditions. Further studies with large sample size can provide concrete data on the combined effect of genetic polymorphisms and NSCLC. The effect of co-morbid conditions to the contribution of gene polymorphisms observed cannot be ruled out.
In conclusion, we report that in the population of Andhra Pradesh, the South Indian state, a higher risk of lung cancer was associated with combined gene polymorphisms of phase I and phase II enzymes, than with a single susceptible gene. Risk assessment of NSCLC can be related to gene polymorphisms and oxidant status. This finding may have an important implication for the prevention of smoking and occupational exposures in susceptible individuals.
References
Mannervik B, Danielson UH. Glutathione transferases–structure and catalytic activity. CRC Crit Rev Biochem. 1988;23:283–337.
Gonzalez FJ. Molecular genetics of the P-450 superfamily. Pharmacol Ther. 1990;45:1–38.
Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600.
Guengerich FP, Thier R, Persmark M, Taylor JB, Pemble SE, et al. Conjugation of carcinogens by theta class glutathione s-transferases: mechanisms and relevance to variations in human risk. Pharmacogenetics. 1995;5:S103–7.
Bethesda M. SEER Cancer Statistics Review. National Institutes of Health. 1973–1998.
Gupta D, Boffetta P, Gaborieau V, Jindal SK. Risk factors of lung cancer in Chandigarh, India. Indian J Med Res. 2001;113:142–50.
Raunio H, Husgafvel-Pursiainen K, Anttila S, Hietanen E, Hirvonen A, et al. Diagnosis of polymorphisms in carcinogen-activating and inactivating enzymes and cancer susceptibility–a review. Gene. 1995;159:113–21.
Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, et al. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–84.
Barouki R, Morel Y. Repression of cytochrome P450 1A1 gene expression by oxidative stress: mechanisms and biological implications. Biochem Pharmacol. 2001;61:511–6.
Taningher M, Malacarne D, Izzotti A, Ugolini D, Parodi S. Drug metabolism polymorphisms as modulators of cancer susceptibility. Mutat Res. 1999;436:227–61.
Seidegard J, Pero RW, Markowitz MM, Roush G, Miller DG, et al. Isoenzyme(s) of glutathione transferase (class Mu) as a marker for the susceptibility to lung cancer: a follow up study. Carcinogenesis. 1990;11:33–6.
Bell DA, Thompson CL, Taylor J, Miller CR, Perera F, et al. Genetic monitoring of human polymorphic cancer susceptibility genes by polymerase chain reaction: application to glutathione transferase mu. Environ Health Perspect. 1992;98:113–7.
Vineis P, d’Errico A, Malats N, Boffetta P. Overall evaluation and research perspectives. IARC Sci Publ. 1999;148:403–8.
Habdous M, Siest G, Herbeth B, Vincent-Viry M, Visvikis S. Glutathione S-transferases genetic polymorphisms and human diseases: overview of epidemiological studies. Ann Biol Clin (Paris). 2004;62:15–24.
Inoue K, Asao T, Shimada T. Ethnic-related differences in the frequency distribution of genetic polymorphisms in the CYP1A1 and CYP1B1 genes in Japanese and Caucasian populations. Xenobiotica. 2000;30:285–95.
Hung RJ, Boffetta P, Brockmoller J, Butkiewicz D, Cascorbi I, et al. CYP1A1 and GSTM1 genetic polymorphisms and lung cancer risk in Caucasian non-smokers: a pooled analysis. Carcinogenesis. 2003;24:875–82.
Song N, Tan W, Xing D, Lin D. CYP1A1 polymorphism and risk of lung cancer in relation to tobacco smoking: a case–control study in China. Carcinogenesis. 2002;22:11–6.
Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10:1239–48.
Hong YS, Chang JH, Kwon OJ, Ham YA, Choi JH. Polymorphism of the CYP1A1 and glutathione-S-transferase gene in Korean lung cancer patients. Exp Mol Med. 1998;30:192–8.
Sugimura H, Wakai K, Genka K, Nagura K, Igarashi H, et al. Association of Ile462Val (Exon 7) polymorphism of cytochrome P450 IA1 with lung cancer in the Asian population: further evidence from a case-control study in Okinawa. Cancer Epidemiol Biomarkers Prev. 1998;7:413–7.
Shields PG, Caporaso NE, Falk RT, Sugimura H, Trivers GE, et al. Lung cancer, race, and a CYP1A1 genetic polymorphism. Cancer Epidemiol Biomarkers Prev. 1993;2:481–5.
Bouchardy C, Wikman H, Benhamou S, Hirvonen A, Dayer P, et al. CYP1A1 genetic polymorphisms, tobacco smoking and lung cancer risk in a French Caucasian population. Biomarkers. 1997;2:131–4.
Benhamou S, Lee WJ, Alexandrie AK, Boffetta P, Bouchardy C, et al. Meta- and pooled analyses of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancer risk. Carcinogenesis. 2002;23:1343–50.
Vineis P, Malats M, Lang M, d’Errico A, Caporaso N, et al. Metabolic polymorphisms and susceptibility to cancer. IARC Sci Publ. 1999;148:1–150.
Quinones L, Lucas D, Godoy J, Caceres D, Berthou F, et al. CYP1A1, CYP2E1 and GSTM1 genetic polymorphisms. The effect of single and combined genotypes on lung cancer susceptibility in Chilean people. Cancer Lett. 2001;174:35–44.
Yang XR, Wacholder S, Xu Z, Dean M, Clark V, et al. CYP1A1 and GSTM1 polymorphisms in relation to lung cancer risk in Chinese women. Cancer Lett. 2004;214:197–204.
Sobti RC, Onsory K, Al-Badran AI, Kaur P, Watanabe M, et al. CYP17, SRD5A2, CYP1B1, and CYP2D6 gene polymorphisms with prostate cancer risk in North Indian population. DNA Cell Biol. 2006;25:287–94.
Shah PP, Singh AP, Singh M, Mathur N, Mishra BN, et al. Association of functionally important polymorphisms in cytochrome P4501B1 with lung cancer. Mutat Res. 2008;643:4–10.
Lakkakula S, Maram R, Munirajan AK, Pathapati RM, Visveswara SB, et al. Functional PstI/RsaI polymorphisms in the CYP2E1 gene among south Indian populations. Asian Pac J Cancer Prev. 2013;14:179–82.
Sreeja L, Syamala V, Hariharan S, Madhavan J, Devan SC, et al. Possible risk modification by CYP1A1, GSTM1 and GSTT1 gene polymorphisms in lung cancer susceptibility in a South Indian population. J Hum Genet. 2005;50:618–27.
Kyaing NN, Islam MA, Sinha DN, Rinchen S. Social, economic and legal dimensions of tobacco and its control in South-East Asia region. Indian J Public Health. 2011;55:161–8.
Peddireddy V, Siva Prasad B, Gundimeda SD, Penagaluru PR, Mundluru HP. Assessment of 8-oxo-7, 8-dihydro-2′-deoxyguanosine and malondialdehyde levels as oxidative stress markers and antioxidant status in non-small cell lung cancer. Biomarkers. 2012;17:261–8.
Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13–5.
Song N, Tan W, Xing D, Lin D. CYP 1A1 polymorphism and risk of lung cancer in relation to tobacco smoking: a case-control study in China. Carcinogenesis. 2001;22:11–6.
Teixeira JP, Gaspar J, Martinho G, Silva S, Rodrigues S, et al. Aromatic DNA adduct levels in coke oven workers: correlation with polymorphisms in genes GSTP1, GSTM1, GSTT1 and CYP1A1. Mutat Res. 2002;517:147–55.
Schneider J, Bernges U, Philipp M, Woitowitz HJ. GSTM1, GSTT1, and GSTP1 polymorphism and lung cancer risk in relation to tobacco smoking. Cancer Lett. 2004;208:65–74.
Alexandrie AK, Nyberg F, Warholm M, Rannug A. Influence of CYP1A1, GSTM1, GSTT1, and NQO1 genotypes and cumulative smoking dose on lung cancer risk in a Swedish population. Cancer Epidemiol Biomarkers Prev. 2004;13:908–14.
Alexandrie AK, Rannug A, Juronen E, Tasa G, Warholm M. Detection and characterization of a novel functional polymorphism in the GSTT1 gene. Pharmacogenetics. 2002;12:613–9.
Xu X, Kelsey KT, Wiencke JK, Wain JC, Christiani DC. Cytochrome P450 CYP1A1 MspI polymorphism and lung cancer susceptibility. Cancer Epidemiol Biomarkers Prev. 1996;5:687–92.
Quinones L, Berthou F, Varela N, Simon B, Gil L, et al. Ethnic susceptibility to lung cancer: differences in CYP2E1, CYP1A1 and GSTM1 genetic polymorphisms between French Caucasian and Chilean populations. Cancer Lett. 1999;141:167–71.
Taioli E, Gaspari L, Benhamou S, Boffetta P, Brockmoller J, et al. Polymorphisms in CYP1A1, GSTM1, GSTT1 and lung cancer below the age of 45 years. Int J Epidemiol. 2003;32:60–3.
Sobti RC, Sharma S, Joshi A, Jindal SK, Janmeja A. Genetic polymorphism of the CYP1A1, CYP2E1, GSTM1 and GSTT1 genes and lung cancer susceptibility in a north indian population. Mol Cell Biochem. 2004;266:1–9.
Sobti RC, Kaur P, Kaur S, Janmeja AK, Jindal SK, et al. Combined effect of GSTM1, GSTT1 and GSTP1 polymorphisms on histological subtypes of lung cancer. Biomarkers. 2008;13:282–95.
Okada T, Kawashima K, Fukushi S, Minakuchi T, Nishimura S. Association between a cytochrome P450 CYPIA1 genotype and incidence of lung cancer. Pharmacogenetics. 1994;4:333–40.
Sreelekha TT, Ramadas K, Pandey M, Thomas G, Nalinakumari KR, et al. Genetic polymorphism of CYP1A1, GSTM1 and GSTT1 genes in Indian oral cancer. Oral Oncol. 2001;37:593–8.
Kumar M, Agarwal SK, Goel SK. Lung cancer risk in north Indian population: role of genetic polymorphisms and smoking. Mol Cell Biochem. 2009;322:73–9.
Nelson HH, Wiencke JK, Christiani DC, Cheng TJ, Zuo ZF, et al. Ethnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase theta. Carcinogenesis. 1995;16:1243–5.
Sorensen M, Autrup H, Tjonneland A, Overvad K, Raaschou-Nielsen O. Glutathione S-transferase T1 null-genotype is associated with an increased risk of lung cancer. Int J Cancer. 2004;110:219–24.
Lan Q, He X, Costa DJ, Tian L, Rothman N, et al. Indoor coal combustion emissions, GSTM1 and GSTT1 genotypes, and lung cancer risk: a case-control study in Xuan Wei, China. Cancer Epidemiol Biomarkers Prev. 2000;9:605–8.
Ghosh P, Basu A, Mahata J, Basu S, Sengupta M, et al. Cytogenetic damage and genetic variants in the individuals susceptible to arsenic-induced cancer through drinking water. Int J Cancer. 2006;118:2470–8.
Vettriselvi V, Vijayalakshmi K, Paul SF. Genetic variation of GSTM1, GSTT1 and GSTP1 genes in a South Indian population. Asian Pac J Cancer Prev. 2006;7:325–8.
Peddireddy V, Badabagni SP, Gundimeda SD, Sultana S, Kadali K, et al. Genetic instability in peripheral lymphocytes as biological marker for non-small cell lung cancer patients in the South Indian state of Andhra Pradesh. Int J Biol Markers. 2014;29:e345–53.
Nakachi K, Imai K, Hayashi S, Kawajiri K. Polymorphisms of the CYP1A1 and glutathione S-transferase genes associated with susceptibility to lung cancer in relation to cigarette dose in a Japanese population. Cancer Res. 1993;53:2994–9.
Brockmoller J, Kerb R, Drakoulis N, Nitz M, Roots I. Genotype and phenotype of glutathione S-transferase class mu isoenzymes mu and psi in lung cancer patients and controls. Cancer Res. 1993;53:1004–11.
Sobti RC, Sharma S, Joshi A, Jindal SK, Janmeja A. CYP1A1 and CYP2D6 polymorphism and risk of lung cancer in a North Indian population. Biomarkers. 2003;8:415–28.
Shi X, Zhou S, Wang Z, Zhou Z, Wang Z. CYP1A1 and GSTM1 polymorphisms and lung cancer risk in Chinese populations: a meta-analysis. Lung Cancer. 2008;59:155–63.
Dialyna IA, Miyakis S, Georgatou N, Spandidos DA. Genetic polymorphisms of CYP1A1, GSTM1 and GSTT1 genes and lung cancer risk. Oncol Rep. 2003;10:1829–35.
Prasad SB, Vidyullatha P, Vani GT, Devi RP, Rani UP, et al. Association of gene polymorphism in detoxification enzymes and urinary 8-OHdG levels in traffic policemen exposed to vehicular exhaust. Inhal Toxicol. 2013;25:1–8.
Chiou HY, Hsueh YM, Hsieh LL, Hsu LI, Hsu YH, et al. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat Res. 1997;386:197–207.
Matsui A, Ikeda T, Enomoto K, Hosoda K, Nakashima H, et al. Increased formation of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, in human breast cancer tissue and its relationship to GSTP1 and COMT genotypes. Cancer Lett. 2000;151:87–95.
Bergamaschi E, De Palma G, Mozzoni P, Vanni S, Vettori MV, et al. Polymorphism of quinone-metabolizing enzymes and susceptibility to ozone-induced acute effects. Am J Respir Crit Care Med. 2001;163:1426–31.
Kim YD, Lee CH, Nan HM, Kang JW, Kim H. Effects of genetic polymorphisms in metabolic enzymes on the relationships between 8-hydroxydeoxyguanosine levels in human leukocytes and urinary 1-hydroxypyrene and 2-naphthol concentrations. J Occup Health. 2003;45:160–7.
Breton CV, Kile ML, Catalano PJ, Hoffman E, Quamruzzaman Q, et al. GSTM1 and APE1 genotypes affect arsenic-induced oxidative stress: a repeated measures study. Environ Health. 2007;6:39.
Buthbumrung N, Mahidol C, Navasumrit P, Promvijit J, Hunsonti P, et al. Oxidative DNA damage and influence of genetic polymorphisms among urban and rural schoolchildren exposed to benzene. Chem Biol Interact. 2008;172:185–94.
Park EY, Hong YC, Lee KH, Im MW, Ha E, et al. Maternal exposure to environmental tobacco smoke, GSTM1/T1 polymorphisms and oxidative stress. Reprod Toxicol. 2008;26:197–202.
Loft S, Moller P. Oxidative DNA damage and human cancer: need for cohort studies. Antioxid Redox Signal. 2006;8:1021–31.
Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9.
Kuo HW, Chou SY, Hu TW, Wu FY, Chen DJ. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) and genetic polymorphisms in breast cancer patients. Mutat Res. 2007;631:62–8.
Authors’ contributions
VP collected blood samples, designed and conducted experiments, surveyed literature and developed text of the manuscript. BSP helped for statistical analysis. SDG, VM, PPR and HPM refined the write up. All authors read and approved the final manuscript.
Acknowledgements
We thank all the study subjects who voluntarily participated in the study. We also thank the Director, the Institute of Genetics for providing necessary infrastructure to carry out the study. Special thanks are offered also to Dr. Venkata Karunakar Kolla for his statistical assistance.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Peddireddy, V., Badabagni, S.P., Gundimeda, S.D. et al. Association of CYP1A1, GSTM1 and GSTT1 gene polymorphisms with risk of non-small cell lung cancer in Andhra Pradesh region of South India. Eur J Med Res 21, 17 (2016). https://doi.org/10.1186/s40001-016-0209-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-016-0209-x