Abstract
Acinetobacter baumannii is without a doubt one of the most problematic bacteria causing hospital-acquired nosocomial infections in today's healthcare system. To solve the high prevalence of multi-drug resistant (MDR) in A. baumannii, we investigated one of the medicinal plants traditionally used as antibacterial agent; namely Murraya koenigii (L.) Sprengel. The total methanolic extracts of seeds and pericarps were prepared and their anti-bacterial activity was assessed using the agar diffusion method and minimum inhibitory concentration (MIC) was then calculated as compared to tigecycline. Then, an in-vivo murine model was established which confirmed the promising activity of M. koenigii seeds in demonstrating anti-bacterial and anti-inflammatory actions. The histopathological study of lungs, scoring of pulmonary lesions, counting of bacterial loads after infection by multi-drug resistant A. baumannii all provided evidence to support these findings. LC–MS/MS profiling coupled to molecular networking and chemometrics detected the presence of carbazole alkaloids, and coumarins as dominate metabolites of the active seed extracts. Positively correlated metabolites to antibacterial potential were 6-(2ʹ,3ʹ-dihydroxy-3-methylbutyl)-8-prenylumbelliferone, scopoline, and 5-methoxymurrayatin. An in-silico study was also performed on the crystal structure of MurF from A. baumannii (PDB ID: 4QF5), the studied structures of the mentioned extracts revealed good docking interaction at the active site suggestive of competition with the ATP ligand. These collective findings suggest that extracts of Murraya koenigii (L.) Sprengel seed is a novel prospective for the discovery of drug candidates against infections caused by MDR A. baumannii.
Similar content being viewed by others
Introduction
Acinetobacter baumannii is a prominent and increasingly prevalent bacterium that causes severe illness and mortality [1]. It is one of the most common nosocomial diseases due to its capacity to develop mechanisms of resistance to many last-line antimicrobial treatments containing carbapenems [2,3,4]. In 2017, the World Health Organization (WHO) designated this bacteria as a priority-1 pathogen [5]. The bacteria lead to a wide range of infections, including ventilator-associated pneumonia, soft tissue, skin, wound, and urinary tract infections [6].
Most A. baumannii infections occur in seriously ill patients in the intensive care unit setting accounting for up to 20% of infections in ICUs worldwide [7]. Furthermore, the prevalence of A. baumannii infections in the community has been steadily growing. The multi-drug-resistant A. baumannii (MDRAB) phenotype may invade both biotic and abiotic surfaces and form as biofilm. Numerous anti-bacterial medications, such as tigecycline, carbapenem, polymyxin, and non-antibiotic therapy, are in demand due to A. baumannii's pathogenicity [8]. The detection of lactamase, low permeability of the outer membrane (OM), and effective pump systems are the primary causes of MDRAB resistance to traditional antibacterial drugs [8].
Colistin and tigecycline are antibiotics of last resort used to treat a variety of multidrug-resistant bacteria, although there have been reports of antibiotic resistance against these drugs worldwide [9]. In perspectives of therapeutic strategies, herbal medicines are one of the probable approaches, which is an efficient alternative to develop several bioactive derivatives.
Curry (Murraya koenigii (L.) Sprengel) is a small aromatic shrub in the Rutaceae family [10]. Phytochemical study of its pericarps and seeds extracts revealed the existence of alkaloids, flavonoids, and phenolic contents, all of which have enormous potential to improve consumer health and reduce illness risks [11]. As such Murraya species can be considered as rich source of antibacterial compounds including carbazole alkaloids, and phenolics [12,13,14].
It is known that MurF is essential during peptidoglycan biosynthesis. It is an appealing target for multiple resistant bacterial treatment [15]. Discovery of novel therapeutic compounds is urgently needed to overcome bacterial resistance. Therefore, an in-silico approach using the C-Docker protocol in Discovery Studio 4.0 Software can be performed on the metabolites that are positively correlated with the antibacterial activity.
In continuation of our teams attempts to explore novel alternatives to last-resort therapies to avoid treatment failure against MDR infection [16,17,18,19,20,21], here we investigated Murraya koenigii (L.) Sprengel seeds and pericarps as anti-A. baumannii, where their chemical profiles were studied using LC/MS/MS. In silico studies and correlation analysis revealed that 6-(2ʹ,3ʹ-dihydroxy-3-methylbutyl)-8-prenylumbelliferone, scopoline, and 5-methoxymurrayatin as the most promising bioactive antibacterial metabolites.
Material and methods
Plant material
Seeds and pericarps of Murraya koenigii (L.) Spreng. (Family Rutaceae) were collected in September 2022 from Orman botanic garden (Giza, Egypt), authenticated by Mrs. Therese Labib, Botanical Specialist and Consultant at Orman and Qubba Botanical Gardens, Egypt. The voucher specimen was deposited in the herbarium of Cairo University’s Pharmacognosy Department, Faculty of Pharmacy (#5.5.2022I).
Preparation of the extracts
Fresh pericarps (350 g) and 150 g of fresh seeds were air-dried and extracted by methanol (3 × 5 L) by maceration for 3 days. The collected extracts were filtered and evaporated under reduced pressure to give 28, and 13 g of total methanolic extract of M. koenigii pericarps and seeds, respectively.
Chemical profiling, molecular networking and metabolites annotation
Aliquots of the samples (2 mg) were re-suspended in 1000 μL methanol: water (UPLC-grade, 1:1, v/v) and moved to the autosampler, 2 μL was injected and separated on RP High Strength Silica (HSS)T3 C18 column (100 mm × 2.1 mm having 1.7 μm diameter particles, Waters), using a Waters Acquity UPLC system. The mass spectra were obtained by full scan MS in positive ionization mode on an exact high resolution Orbitrap-type MS (Thermo-Fisher, Bremen, Germany) [22]. Metabolites were identified using their mass spectra, and by comparison with our in-house database and the references literature.
To obtain the online workflow, the mzXML files (Additional file 1: Table S1) were uploaded to the GNPS online platform [23, 24]. The constructed MN and its settings are available at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=0e03844516104989b9d6a810ce3d96bc. For further processing and visualisation, network files were loaded into the open-source software platform Cytoscape 3.9.1 (https://cytoscape.org/download.html) [25].
Microorganisms
Extensively drug-resistant A. baumannii (XDRAB) strains clinical isolate (Additional file 1: Fig. S1) was collected from a tertiary care center in Cairo from a post covid secondary bacterial infected patient and characterized in our previous work [26].
In vitro antibacterial assay
Preliminary screening of antibacterial activity was first screened for inhibitory zone by the agar disc-diffusion method according to CLSI guidelines as described before [27, 28]. Furthermore, the microbroth dilution method was implemented to determine MIC (the minimum bacteria growth inhibitory drug concentration) [29].
In vivo antibacterial assay
Male Balb C mice (22 to 25 g, 8 weeks) were obtained from the Egyptian Drug Authority, and kept in a standard controlled condition. The protocol was approved by the Animal Experiment Ethics Committee of the National Hepatology & Tropical Medicine Research Institute (NHTMRI) for Research Ethics Committee (REC) (#NHTMRI REC A5-2023). All C57BL/6 mice were anesthetized by inhaling isoflurane [30]. Then the mice were in the position of head up and upright, and A. baumnii suspension was dripped into the nasal cavity for 1 × 109 cfu in 50 μL of phosphate-buffered saline (PBS). The mice in Sham group were dripped with the same volume of normal saline by the same method. After inoculation, the mice kept their heads upright for 20 s to ensure that bacterial suspension or normal saline could enter both lungs evenly due to gravity. When the mice woke up, they were placed in the cage to eat freely. After an incubation period of 4 h, all the infected mice were randomly divided into five groups (n = 10) as follows:
(1) The Sham control group (without treatment).
(2) Untreated group.
(3 and 4) The plant groups (oral administration at 200 mg/kg every 24 h).
(5) The Tigecyclin treatment group (subcutaneous administration of Tigecyclin at 75,000 U/kg every 12 h).
The Sham and model group were administrated by oral gavage with the same volume of normal saline. After 72 h of drug administration, the animals were sacrificed by cervical dislocation, lungs were collected for biochemical and histopathological examinations [31].
Biochemical examination Interferon-gamma (IFN-γ) (CEK1476), tumor necrosis factor alpha (TNF-α) (CSB-E04741m), IL-6 (CSB-E04639m), IL10 (CSB-E04594m), IL12 (MBS2568055), and Myeloperoxidase (MPO) (MBS700747) were measured following the manufacturer's guides.
Histopathology and lesion score Hematoxylin and eosin (H&E) staining was performed [32], and examined by Leica DM4 B light microscope (Leica, Germany). Images were captured by Leica DMC 4500 digital camera (Leica, Germany). The severity of the detected lesions was evaluated as follow (−) = absent, (+) = mild, (++) = moderate and (+++) = severe (Qualitative scoring system).
Pulmonary bacterial loads Lungs were removed aspetically and homogenized with a tissue homogenizer in 5 ml of sterila saline. Homogenized lung samples are then serially diluted in sterile saline and plated on Lauria bertani agar plates (Additional file 1: Fig. S2) [33].
In silico study The crystal structure of MurF from Acinetobacter baumannii complexed with ATP was successfully downloaded from Protein Data Bank (PDB ID: 4QF5) [15]. The protein was cleaned. Also, hydrogen atoms was added to complete any missing residues in amino acids. It’s worth noting that water molecules was removed and all unneeded molecules. Force Field CHARMm and MMFF94 partial charge was successfully applied. Protein was prepared and minimized; the active site was well defined as ATP is the main ligand. The ligand ATP was removed before docking of the tested compounds.
Statistical analysis Analysis of Variance (ANOVA) was used to establish statistical significance. The aligned peak list obtained by MZmine software and was exported as a CSV file, with information about retention time, the feature ID number, peak intensity, and mass-to-charge ratio (m/z). All variables were log10-transformed scaled to Pareto variance before PCA and OPLS-DA. The web-based platform Metaboanalyst 5.0 (https://www.metaboanalyst.ca/) was utilized for Multivariate data analysis [34].
Results
Chemical profiling
The methanolic extracts of the M. koenigii seeds (MKS) and pericarps (MKF) were analyzed using UPLC-MS/MS (Table 1), where the mass spectra in positive modes resulted in the identification of 102 compounds viz. 40 alkaloids, 35 phenolics (11 flavonoids, five phenolic acids, and 19 coumarins), five organic acids, five phospholipids, three fatty acids, ten amino acids, one quinoline derivative, one sugar, one vitamin, and one fatty amide. The mass fragmentation of those compounds was compared to reference papers as cited in Table 1.
The most prominent compounds belong to the carbazole alkaloids class; mukoline, koenimbine, murrayanine, and murrastanine A (peaks # 72, # 76, # 101, and # 102) in seeds where, koenimbine, isomahanine, 9-formyl-3-methyl carbazole, and koenine (peaks # 65, 76, # 73, # 85, and # 54) with Mexoticin (# 94) as coumarin were the prominent in pericarps. In the supplemental materials shown in Additional file 1: Figs. S3–S6, the representative MS/MS spectra of selected compounds among the major classes are shown.
The molecular networking approach was applied, and this enabled the direct visual examination of MS/MS data, as well as the observation of metabolite distribution among the various extracts (Fig. 1). It categorized molecules into families or clusters based on the similarity of their MS/MS fragmentation patterns.
A Molecular networking established using LC–MS/MS data in the positive ESI mode. B Selected nodes and clusters have been zoomed in. Single nodes are used to represent molecules that do not form groups. Nodes were also colored by organ type (seeds and pericarps) and labelled with their precursor m/z values. The nodes were also represented as a pie chart to show the semi-relative abundance of the identified molecule ions, with the borders indicating the mass differences between the connected nodes
In vitro anti-A. baumannii study
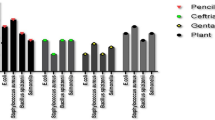
Upon performing antibacterial susceptibility by Kirby’s Bauer disc diffusion, M. koenigii seed extracts showed high antibacterial activity (21 mm) and could diminish bacterial growth with A. baumannii being sensitive towards the sample as well as tigecycline (20 mm). By contrast, M. koenigii fruit extracts were ineffective with inhibition zones of 10 mm (Table 2 and Additional file 1: Fig. S7). Furthermore, MICs for M. koenigii seeds and fruit extracts were 32, and 125 ug/ml, respectively (Table 2).
In vivo anti-A. baumannii study
Hematological biomarkers
M. koenigii seed extracts and tigecycline envoked few changes in regard to these parameters. By contrast, the fruit extract showed higher levels of hematological biomarkers revealing higher inflammation and lower antibacterial activity (Fig. 2).
Hematological biomarkers of seeds and pericarps of M. koenigii as compared to Tigecycline in Acinetobacter baumannii murine animal model. Data are expressed as mean ± SD. Statistical analysis was carried out by one-way ANOVA followed by Tukey's multiple comparison test. aSignificant difference from normal group at p < 0.05. bSignificant difference from infected group at p < 0.05. abSignificant difference from normal group and infected group at p < 0.05
Histopathology
As illustrated in Fig. 3 and Table 3, no histopathological changes were observed in lung sections from normal group as normal bronchioles and alveoli were observed. By contrast, the untreated group exhibited severe diffuse pneumonia manifested by heavy inflammatory cells infiltrations that masked lung tissue with discreet area of pulmonary necrosis. The alveolar lumens were heavily infiltrated by inflammatory cells with presence of bacterial colonies. Blood vessels with severely congested. M. koenigii seeds extract treated mice showed marked improvement as most of the studied sections demonstrated mild interstitial mononuclear inflammatory cells infiltrations with clear alveolar lumen and mild vascular congestion. The M. koenigii pericarps extract treated group exhibited mild improvement as the examined tissue samples showed moderate inflammatory cells infiltrations in the interstitial tissue as well as multifocal areas of pneumonia. Tigecycline S/C group showed marked improvement as most of the examined sections were apparently normal, only a few sections exhibited mild interstitial infiltration with mononuclear inflammatory cells.
Photomicrographs of lung by hematoxylin and eosin stain (H&E) showing normal structure of lung in normal group (a), intense inflammatory cells infiltration (black arrow) and severe congestion (red arrow) in untreated group (b), mild interstitial pneumonia in M. koenigii seeds treated group (c), moderate thickening of interalveolar wall with mononuclear inflammatory cells (green arrow) in M. koenigii pericarps treated group (d), mild interstitial pneumonia in Tigecycline S/C group (e)
Pulmonary bacterial loads
M. koenigii seeds showed better anti A. baumannii than pericarps which had a moderate activity as revealed from bacterial loads results (Table 4).
Multivariate data analysis
Untargeted metabolomic analyses usually generate a complex dataset in terms of features and their corresponding intensities, thus, various dimensionality reduction methods including principal component analysis (PCA) and hierarchical cluster analysis (HCA) are performed to ease the process of visualizing the data. PCA results are shown in Fig. 4A. As can be shown, PC1 and PC2 (87.1% and 10.1% of the total variance, respectively) well explained the variation of the samples (n = 3) as analyzed by LC/MS analysis. HCA are presented in Fig. 4B. These separated the samples into two clusters, revealing different chromatographic patterns of both organs.
The results of unsupervised analysis was further verified using the supervised orthogonal partial least squares discriminant analysis (OPLS-DA), Offering valuable insights into the distinguishing metabolites between the tested samples. The OPLS-DA score plot (Fig. 5A) explained 65.9% of the total variance and 15.3 of the orthogonal total variances, where seeds and pericarps segregated into two different nonoverlapping clusters. The loadings S-plot (Fig. 5B) was used to compare variable magnitude vs reliability, with axes displayed from the predictive component being the covariance P [1] versus the correlation P(cor) [1]. Isogirinimbine, scopoline, sinapine, isomahanine, mukoline, Murrastinine B, 8, 8″-bis koenigine, N-Methylaniline, sinensetin, and 5-Methoxymurrayatin were the discriminating metabolites of seeds. Furthermore, quercetin, isoquercitrin, toddalenone, bismurrayafoline A, norrangiformic acid, meranzin hydrate, 8,8'-Bismurrayamine A, dimethyl ether, Murrastinine C/ murrayacine, koenigine, and mexoticin were the discriminating metabolites in pericarps.
Pearson's correlation coefficients (r) were next used to determine the correlation between the abundance of the annotated metabolites in M. koenigii seed and fruit extracts and antibacterial activity, with Pearson's correlation coefficient (r) was ≥ 0.9 at p < 0.05. The compounds that discriminated fruit extract were 6-(2′,3′-dihydroxy-3-methylbutyl)-8-prenylumbelliferone, scopoline, 5-demethylnobiletin, quercetin-O-pentoside, vanillin, mexoticin, koenigine, murrastinine C/ murrayacine, 8,8'-Bismurrayamine A, dimethyl ether, meranzin hydrate, norrangiformic acid, bismurrayafoline A, mukoeic acid, koenidine/ koenigicine, toddalenone, quercetin, quercetin-3-O-arabinoglucoside, and paniculatin. While the compounds discriminating theseed extract were tyrosine, isogirinimbine, isoquercetrin, 9-hydroxy-10,12-octadecadienoic acid, sucrose, koenimbine, and sinapine (Fig. 5C).
In addition, the metabolites that distinguished between active and inactive extracts were further validated by computing the VIP scores obtained from the OPLS-DA modelling of the active seed extract against the inactive fruit extract. As can be concluded, the discriminating metabolites were relevant to explain the variance when also having VIP scores > 1at p < 0.05 (Fig. 5C). The metabolites positively correlated to the antibacterial activity are shown in Table 5.
In silico study
The tested ligands were prepared, then docked into the binding site of MurF (A. baumannii). The binding mode of the tested compounds was analyzed and compared to that of the ligand to investigate the antibacterial activity. Ten compounds showed best results regarding the binding mode to the key amino acids in the active site, compared to the ATP ligand. The C-Docker results of the ATP ligand (E = − 142.63 kcal/mol) was used to evaluate the docking interaction of the test compounds as shown in Fig. 6. Where the main reported binding interactions including Hydrogen Bond Acceptor interaction (HBA) with Ser123, Hydrogen Bond Donor (HBD) interaction with His292, and HBA interaction with Asn296. In addition to HBD with Asp341 and HBA with Lys448 in the central domain was accomplished. While in the C-terminal domain, pi donor hydrogen bond interaction with Ser349 and hydrophobic interaction with Ala352 was noticed. Moreover, charge interactions with Lys125, and Arg327 in the central domain was confirmed [15]. In addition to, 3HBA with Asn344, 1 HBA withThr126, Thr127, Asn150 via the phosphate groups of ATP. And 1 HBD with Gln295 and pi donor hydrogen bond with Thr348.
The docked tested compounds revealed the essential interactions compared to ATP showing comparable competitive behavior with promising predicted antibacterial activity (Fig. 7a, b). Where, 6-(2',3'-Dihydroxy-3-methylbutyl)-8-prenylumbelliferone, scopoline, and 5-methoxymurrayatin were the key discriminators.
a The 3D binding interactions on MurF of Acinetobacter baumannii (PDB ID: 4QF5) of A Sinapine (E = -47.80 kcal/mol), B Sinestin (E = − 50.65 kcal/mol), C Iso-Koenigine (E = − 41.70 kcal/mol), D Isogirinimbine (E = − 31.65 kcal/mol), E Mahanimbidine (E = − 43.82 kcal/mol) and F Scopolin (E = − 56.92 kcal/mol). b The 3D binding interactions on MurF of Acinetobacter baumannii (PDB ID: 4QF5) of G Ferulic acid (E = − 59.15 kcal/mol), H Mukonine (E = − 38.45 kcal/mol), I 5-Methoxymurrayatin (E = − 55.74 kcal/mol), J Quercitrin (E = − 36.41 kcal/mol), K Murrastinine B (E = − 39.39 kcal/mol) and L 6-(2',3'-dihydroxy-3-methylbutyl)-8-prenylumbelliferone (E = − 55.44 kcal/mol)
While the two bulky compounds named: 8,8ʺ-biskoenigine and murrafoline A, failed to interact within the active site and showed no docking results. Ferulic acid showed the best binding interaction energy (E = − 59.15 kcal/mol), revealing the most stability at the binding pocket. Also, scopolin (E = − 56.92 kcal/mol), 5-methoxymurrayatin (E = − 55.74 kcal/mol), 6-(2',3'-dihydroxy-3-methylbutyl)-8-prenylumbelliferone (E = − 55.44 kcal/mol), sinestin (E = − 50.65 kcal/mol) and sinapine (E = − 47.80 kcal/mol) showed good binding interaction energy (Fig. 8a). The visual inspection of the binding mode of the tested compounds confirmed the essential binding interactions with the reported amino acid residues at the active site compared to ATP ligand (Fig. 8b).
a The 2D binding mode of A Sinapine (E = − 47.80 kcal/mol), B Sinestin (E = − 50.65 kcal/mol), C Iso-Koenigine (E = − 41.70 kcal/mol), D Isogirinimbine (E = − 31.65 kcal/mol), E Mahanimbidine (E = − 43.82 kcal/mol) and F Scopolin (E = − 56.92 kcal/mol) on with the essential amino acids in the active site of MurF of Acinetobacter baumannii (PDB ID: 4QF5). b. The 2D binding mode of G ferulic acid (E = − 59.15 kcal/mol), H mukonine (E = − 38.45 kcal/mol), I 5-methoxymurrayatin (E = − 55.74 kcal/mol), J quercitrin (E = − 36.41 kcal/mol), K murrastinine B (E = − 39.39 kcal/mol) and L 6-(2',3'-dihydroxy-3-methylbutyl)-8-prenylumbelliferone (E = − 55.44 kcal/mol) on MurF of Acinetobacter baumannii (PDB ID: 4QF5)
Discussion
Murraya koenigii (L.) Sprengel or the Curry plant is a potential medicinal plant, which belongs to the family Rutaceae, highly known for its characteristic aroma and bioactive compounds. Phytochemical analysis of the plant has revealed the presence of proteins, carbohydrates, vitamins, alkaloids, phenolics, and flavonoids providing enormous possibilities to improve consumer health and reduce illness risks [11]. Carbazole alkaloids such as koenine, mukoeic acid, mahanimbine, koenimbine, murrayazolidine, murrayazoline, murrayacine and girinimbine have been identified as biologically active compounds with antioxidant, antimicrobial, anti-inflammatory, anthelmintic, antidiarrheal, hepatoprotective, analgesic, and cytotoxic properties [12]. Carbazoles such as mahanimbine, girinimbine, koenimbine, isomahanine and mahanine were isolated from the pulp of pericarps and seeds of M. koenigii. Coumarins were isolated and characterized from the seeds [12, 35, 36], as were a wide range of phospholipids and fatty acids [37]. In our investigation of extracts from the seeds and pericarps of M. koenigii, LC/MS/MS analysis allowed the identification and quantification of 40 carbazole alkaloids, 19 coumarins, 11 flavonoids, and five phenolic acids.
Murraya koenigii carbazole alkaloids and coumarins have been demonstrated to be antibacterial, with even greater efficacy than the antibiotics Amikacin and Gentamicin against Staphylococcus, Streptococcus, Escherichia coli, Pseudomonas, Klebsiella, and Proteus sp. [38]. So, the presence of some coumarins such as murrayanone, and scopoline and carbazole alkaloids as mukonine, and iso-koenigine demonstrates the potential of seeds than pericarps as anti A. baumannii in our study. Plants of the family Rutaceae are widely used in various parts against Gram-positive and Gram-negative harmful bacterial strains as extracts from this family are dominated by the two important bioactive classes of compounds namely the coumarins and carbazole alkaloids [39, 40]. In-vitro and in-vivo studies revealed promising activity of the seed extracts which were almost as active as the standard Tigecycline. Multidrug-resistant A. baumannii is a pathogen that causes severe infections in critically ill people and is infamous for propagating epidemically [41]. By producing pro-inflammatory cytokines, the host activates the innate and adaptive immune systems. However, because over-activation of pro-inflammatory cytokines leads to multi-organ failure, macrophages' early anti-inflammatory action is crucial in fighting infections [42]. Cytokines are key mediators in infections, divided into proinflammatory and anti-inflammatory mediators. We have chosen four proinflammatory mediators, as tumor necrosis factor (TNF)-α, myeloperoxidase (MPO), interleukin (IL)-6, and interferon (IFN)-γ and two anti-inflammatory mediators, including IL-10 and IL-12, to assess the cytokine profile of A. baumannii-infected mice and the protective roles of our treatments [41]. Pro-inflammatory cytokines such as IFN γ, MPO, IL-6, IL-1β, and TNF-α are elevated in A. baumannii-infected mice which contributed to apoptosis in the lung tissues [43,44,45]. In contrast, the anti-inflammatory mediators (IL-10) were decreased in A. baumannii-infected mice. Low levels of IL-10, and IL-12 at the first day of A. baumannii infection have been attributed to the morbidity and mortality in mice infected with A. baumannii strains. IL-10 has also been implicated in vaccine-induced protection against A. baumannii challenge, where pro-inflammatory cytokines (TNF- and IL-6) levels were significantly reduced and anti-inflammatory cytokine IL-10 levels were significantly increased in lungs and serum, resulting in decreased severity and slow progression of disease [46]. These protective effects were reflected in the hematological parameters measured, and from the histopathological study also where the treatments alleviated the inflammatory cells infiltration, pulmonary necrosis, and vascular congestion. In addition to the reduction of pulmonary bacterial loads and decreasing the high mortality rates after infection with highly resistant A. baumannii strain. The majority of the positively linked biomarker metabolites have been shown to have antibacterial action against distinct strains of bacteria including sinapine [47], sinensetin [48], quercitrin [49], ferulic acid inhibits tetK and MsrA efflux pumps of multidrug resistance strains [50] in addition to scopolin which is a coumarin derivative that could inhibit the activity of p-glycoprotein (p-gp) and other multidrug resistance proteins [51]. Validation was confirmed by re-docking of the ligand in the target active site showing RMSD value = 0.5A° as good validation result. It was reported from the previous literature [52] that the binding mode of ATP at the active site is mainly concerned with the interface between the Central and the C-terminal domains of MurF, showing several interactions with amino acid residues. Where MurF structure is mainly composed of three domains named: the N-terminal, the Central, and the C-terminal domains, respectively [15]. It is known that MurF is essential during the last step in the biosynthesis of monomeric precursor of peptidoglycan within peptidoglycan biosynthesis since it adds (in an ATP-dependent manner) D-Ala-D-Ala dipeptide to UDP-N-acetylmuramyl-L-Ala-γ-D-Glu-m-DAP. What makes MurF became an attractive target to develop novel antibiotics. Here, we used computer added drug design (CADD) applying molecular simulation via working on the crystal structure of the Acinetobacter baumannii MurF (AbMurF)-ATP complex [15]. The molecular docking study of the tested compounds revealed twelve promising compounds with comparable and competitive binding mode to ATP as ligand in the binding site. Furthermore, the potential of cabazole alkaloids as antibacterial against multidrug resistant strains was reported several potential actions helping to solve the MDR problems worldwide [19]. Therefore, in order to gain insight to the potential of positively correlated carbazoles including isogirinimbine, 8, 8''- bis koenigine, mukonine, murrastinine B, iso-Koenigine, mahanimbidine, and murrafoline, a further detailed studies of these compounds are required. It is worth highlighting the prophylactic efficacy against A. baumannii infection in a murine model of Murraya koenigii (L.) Sprengel seeds and pericarps is reported here for the first time.
Trying to deal with the unmanageable multidrug resistance towards the existing antibacterial agents, novel approaches towards the discovery of new targets are required in order to prevent and treat A. baumannii infections. While clinical monitoring, developed antibiotics have been successfully targeting enzymes involved in the synthesis of peptidoglycan.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Vázquez-López R, Solano-Gálvez SG, Juárez Vignon-Whaley JJ, Abello Vaamonde JA, Padró Alonzo LA, Rivera Reséndiz A et al (2020) Acinetobacter baumannii resistance: a real challenge for clinicians. Antibiotics 9:205
Ayoub Moubareck C, Hammoudi HD (2020) Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics 9:119
Ibrahim S, Al-Saryi N, Al-Kadmy IM, Aziz SN (2021) Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol Biol Rep 48:6987–6998
Morris FC, Dexter C, Kostoulias X, Uddin MI, Peleg AY (2019) The mechanisms of disease caused by Acinetobacter baumannii. Front Microbiol 10:1601
Ibrahim ME (2019) Prevalence of Acinetobacter baumannii in Saudi Arabia: risk factors, antimicrobial resistance patterns and mechanisms of carbapenem resistance. Ann Clin Microbiol Antimicrob 18:1
Qi L, Li H, Zhang C, Liang B, Li J, Wang L et al (2016) Relationship between antibiotic resistance, biofilm formation, and biofilm-specific resistance in Acinetobacter baumannii. Front Microbiol 7:483
Garnacho-Montero J, Timsit J-F (2019) Managing Acinetobacter baumannii infections. Curr Opin Infect Dis 32:69–76
Lee C-R, Lee JH, Park M, Park KS, Bae IK, Kim YB et al (2017) Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front Cell Infect Microbiol 7:55
Sato Y, Ubagai T, Tansho-Nagakawa S, Yoshino Y, Ono Y (2021) Effects of colistin and tigecycline on multidrug-resistant Acinetobacter baumannii biofilms: advantages and disadvantages of their combination. Sci Rep 11:11700
Arulmoorthy K, Raja K, Sundareswaran S (2019) Physiological and biochemical changes in desiccation sensitive curry leaf (Murraya koenigii (L.) Sprengel) seeds. J Phytol 11:38–41
Datta HS, Bora D, Purkayastha MD, Choudhury M, Neog M (2023) Murraya koenigii (L.) Spreng. Himalayan fruits and berries. Elsevier, Amsterdam, pp 271–287
Gahlawat DK, Jakhar S, Dahiya P (2014) Murraya koenigii (L.) Spreng: an ethnobotanical, phytochemical and pharmacological review. J Pharmacogn Phytochem 3:109–119
Abeysinghe D, Kumara K, Kaushalya K, Chandrika U, Alwis D (2021) Phytochemical screening, total polyphenol, flavonoid content, in vitro antioxidant and antibacterial activities of Sri Lankan varieties of Murraya koenigii and Micromelum minutum leaves. Heliyon. https://doi.org/10.1016/j.heliyon.2021.e07449
Ilangovan SS, Krishna P, Koushika Das SS. A review on anti-microbial properties of Murraya Koenigii. Am J Pharm Res 2016;6.
Cha S-S, An YJ, Jeong C-S, Yu JH, Chung KM (2014) ATP-binding mode including a carbamoylated lysine and two Mg2+ ions, and substrate-binding mode in Acinetobacter baumannii MurF. Biochem Biophys Res Commun 450:1045–1050
El-Shiekh RA, Hassan M, Hashem RA, Abdel-Sattar E (2021) Bioguided isolation of antibiofilm and antibacterial pregnane glycosides from Caralluma quadrangula: disarming multidrug-resistant pathogens. Antibiotics 10:811
Salem MA, El-Shiekh RA, Hashem RA, Hassan M (2021) In vivo antibacterial activity of star anise (Illicium verum Hook.) Extract Using Murine MRSA skin infection model in relation to its metabolite profile. Infect Drug Resist. https://doi.org/10.2147/IDR.S285940
El-Shiekh RA, Elhemely MA, Naguib IA, Bukhari SI, Elshimy R (2023) Luteolin 4′-neohesperidoside inhibits clinically isolated resistant bacteria in vitro and in vivo. Molecules 28:2609
Elshimy R, Khawagi WY, Naguib IA, Bukhari SI, El-Shiekh RA (2023) 9-Methoxyellipticine: antibacterial bioactive compound isolated from Ochrosia elliptica Labill. Roots. Metabolites 13:643
Ali NB, El-Shiekh RA, Ashour RM, El-Gayed SH, Abdel-Sattar E, Hassan M (2023) In vitro and in vivo antibiofilm activity of red onion scales: an agro-food waste. Molecules 28:355
El-Shiekh RA, Elshimy R (2023) Therapeutic effects of Stemmoside C against Salmonella enterica serotype Typhimurium infected BALB/c mice. Steroids 199:109296
Salem MA, Jüppner J, Bajdzienko K, Giavalisco P (2016) Protocol: a fast, comprehensive and reproducible one-step extraction method for the rapid preparation of polar and semi-polar metabolites, lipids, proteins, starch and cell wall polymers from a single sample. Plant Methods 12:45
Pluskal T, Castillo S, Villar-Briones A, Orešič M (2010) MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform 11:1–11
Nothias L-F, Petras D, Schmid R, Dührkop K, Rainer J, Sarvepalli A et al (2020) Feature-based molecular networking in the GNPS analysis environment. Nat Methods 17:905–908
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Farouk F, El Shimy R, Abdel-Motaleb A, Essam S, Azzazy HM (2020) Detection of Acinetobacter baumannii in fresh produce using modified magnetic nanoparticles and PCR. Anal Biochem 609:113890
Bauer A (1966) Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 45:149–158
Rana E, Rania AK, Hamdallah Z, Alaa E-DSH, Tarek HE (2018) Study on prevalence and genetic discrimination of methicillin-resistant Staphylococcus aureus (MRSA) in Egyptian hospitals. Afr J Microbiol Res 12:629–646
Wiegand I, Hilpert K, Hancock RE (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175
Knapp S, de Vos AF, van der Windt GJ, Florquin S, Randle J, van der Poll T (2006) Caspase-1 is an important mediator of lung inflammation during Acinetobacter baumannii pneumonia. Inflammatory response to severe bacterial infections 81
Harris G, KuoLee R, Xu HH (2017) Chen W (2017) Mouse models of Acinetobacter baumannii infection. Curr Protoc Microbiol 46:6G.3.1-6G.3.23
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier health sciences
Kang M-J, Jang A-R, Park J-Y, Ahn J-H, Lee T-S, Kim D-Y et al (2020) IL-10 protects mice from the lung infection of Acinetobacter baumannii and contributes to bacterial clearance by regulating STAT3-mediated MARCO expression in macrophages. Front Immunol 11:270
Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M et al (2021) MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res 49:W388–W396
Adebajo AC, Olugbade TA, Elujoba AA, Aladesanmi AJ, Reisch J (1997) 2", 3"-Epoxyindicolactone from Murraya koenigii. Niger J Nat Prod Med 1:21–24
Adebajo AC, Reisch J (2000) Minor furocoumarins of Murraya koenigii. Fitoterapia 71:334–337
Hemavathy J (1991) Lipid composition of murraya koenigii seed. J Am Oil Chem Soc 68:651–652
Al Harbi H, Irfan UM, Ali S (2016) The antibacterial effect of curry leaves (Murraya Koenigii). EJPMR 3:382–387
Tamene D, Endale M (2019) Antibacterial activity of coumarins and carbazole alkaloid from roots of clausena anisata. Adv Pharmacol Pharm Sci. https://doi.org/10.1155/2019/5419854
Maneerat W, Ritthiwigrom T, Cheenpracha S, Promgool T, Yossathera K, Deachathai S et al (2012) Bioactive carbazole alkaloids from Clausena wallichii roots. J Nat Prod 75:741–746
de Breij A, Eveillard M, Dijkshoorn L, Van Den Broek PJ, Nibbering PH, Joly-Guillou M-L (2012) Differences in Acinetobacter baumannii strains and host innate immune response determine morbidity and mortality in experimental pneumonia. PLoS ONE 7:e30673
Lee H, Krishnan M, Kim M, Yoon YK, Kim Y (2022) Rhamnetin, a natural flavonoid, ameliorates organ damage in a mouse model of carbapenem-resistant Acinetobacter baumannii-induced sepsis. Int J Mol Sci 23:12895
Khan MA, Allemailem KS, Maswadeh H, Younus H (2022) Safety and prophylactic efficacy of liposome-based vaccine against the drug-resistant Acinetobacter baumannii in Mice. Pharmaceutics 14:1357
Sepahvand S, Madani M, Sepahvand H, Davarpanah MA (2022) Comparative assessment of the mouse immune responses to colistin-resistant and colistin-sensitive isolates of Acinetobacter baumannii. Microb Pathog 173:105834
Sitarek P, Merecz-Sadowska A, Kowalczyk T, Wieczfinska J, Zajdel R, Śliwiński T (2020) Potential synergistic action of bioactive compounds from plant extracts against skin infecting microorganisms. Int J Mol Sci 21:5105
Chen W (2020) Host innate immune responses to Acinetobacter baumannii infection. Front Cell Infect Microbiol 10:486
Mouterde LM, Peru ALA, Mention MM, Brunissen F, Allais F (2020) Sustainable straightforward synthesis and evaluation of the antioxidant and antimicrobial activity of sinapine and analogues. J Agric Food Chem 68:6998–7004
Han Jie L, Jantan I, Yusoff SD, Jalil J, Husain K (2021) Sinensetin: an insight on its pharmacological activities, mechanisms of action and toxicity. Front Pharmacol 11:553404
Yassin AS, Abu El Wafa SA, El Menofy NG, Elmerigy AH, Marzouk M (2023) HPLC quantitative analysis of two major flavonoids and antimicrobial effectiveness against multi-drug resistant bacteria of different parts of Khaya senegalensis. Azhar Int J Pharm Med Sci. https://doi.org/10.21608/aijpms.2023.196073.1196
Pinheiro PG, Santiago GMP, da Silva FEF, de Araújo ACJ, de Oliveira CRT, Freitas PR et al (2022) Ferulic acid derivatives inhibiting Staphylococcus aureus tetK and MsrA efflux pumps. Biotechnol Rep 34:e00717
Lee K, Chae SW, Xia Y, Kim NH, Kim HJ, Rhie S et al (2014) Effect of coumarin derivative-mediated inhibition of P-glycoprotein on oral bioavailability and therapeutic efficacy of paclitaxel. Eur J Pharmacol 723:381–388
Ahmad S, Raza S, Uddin R, Azam SS (2017) Binding mode analysis, dynamic simulation and binding free energy calculations of the MurF ligase from Acinetobacter baumannii. J Mol Graph Model 77:72–85
Mondal P, Natesh J, Penta D, Meeran SM (2022) Extract of Murraya koenigii selectively causes genomic instability by altering redox-status via targeting PI3K/AKT/Nrf2/caspase-3 signaling pathway in human non-small cell lung cancer. Phytomedicine 104:154272
Thiyam-Holländer U, Aladedunye F, Logan A, Yang H, Diehl BW (2014) Identification and quantification of canolol and related sinapate precursors in Indian mustard oils and Canadian mustard products. Eur J Lipid Sci Technol 116:1664–1674
Thiyam U, Claudia P, Jan U, Alfred B (2009) De-oiled rapeseed and a protein isolate: characterization of sinapic acid derivatives by HPLC–DAD and LC–MS. Eur Food Res Technol 229:825–831
Singh AP, Wang Y, Olson RM, Luthria D, Banuelos GS, Pasakdee S et al (2017) LC-MS-MS analysis and the antioxidant activity of flavonoids from eggplant skins grown in organic and conventional environments. Food Nutr Sci 8:873
Scigelova M, Hornshaw M, Giannakopulos A, Makarov A (2011) Fourier transform mass spectrometry. Mol Cell Proteomics. https://doi.org/10.1074/mcp.O111.009431
Barreca D, Gattuso G, Laganà G, Leuzzi U, Bellocco E (2016) C-and O-glycosyl flavonoids in Sanguinello and Tarocco blood orange (Citrus sinensis (L.) Osbeck) juice: identification and influence on antioxidant properties and acetylcholinesterase activity. Food Chem 196:619–627
Rivera-Pastrana DM, Yahia EM, González-Aguilar GA (2010) Phenolic and carotenoid profiles of papaya fruit (Carica papaya L.) and their contents under low temperature storage. J Sci Food Agric 90:2358–2365
Aziz S, Sukari M, Rahmani M, Kitajima M, Aimi N, Ahpandi N (2010) Coumarins from Murraya paniculata (Rutaceae). Malay J Anal Sci 14:1–5
Wang Y, Zang W, Ji S, Cao J, Sun C (2019) Three polymethoxyflavones purified from Ougan (Citrus reticulata Cv. Suavissima) inhibited LPS-induced NO elevation in the neuroglia BV-2 cell line via the JAK2/STAT3 pathway. Nutrients 11:791
Wang D, Wang J, Huang X, Tu Y, Ni K (2007) Identification of polymethoxylated flavones from green tangerine peel (Pericarpium Citri Reticulatae Viride) by chromatographic and spectroscopic techniques. J Pharm Biomed Anal 44:63–69
Negi N, Abou-Dough AM, Kurosawa M, Kitaji Y, Saito K, Ochi A et al (2015) Coumarins from Murraya exotica collected in Egypt. Nat Prod Commun 10:1934578X1501000420
Kinoshita T, Wu J-B, Ho F-C (1996) The isolation of a prenylcoumarin of chemotaxonomic significance from Murraya paniculata var. omphalocarpa. Phytochemistry 43:125–128
Dai J, Ma D, Fu C, Ma S (2015) Gram scale total synthesis of 2-hydroxy-3-methylcarbazole, Pyrano [3, 2-a] carbazole and prenylcarbazole alkaloids. Eur J Org Chem 2015:5655–5662
Wang X, Liang H, Zeng K, Zhao M, Tu P, Li J et al (2019) Panitins AG: coumarin derivatives from Murraya paniculata from Guangxi Province, China show variable NO inhibitory activity. Phytochemistry 162:224–231
He S-D, Yang X-T, Yan C-C, Jiang Z, Yu S-H, Zhou Y-Y et al (2017) Promising compounds from murraya exotica for cancer metastasis chemoprevention. Integr Cancer Ther 16:556–562
Tripathi Y, Anjum N, Rana A (2018) Chemical composition and in vitro antifungal and antioxidant activities of essential oil from Murraya koenigii (L.) Spreng. Leaves. Asian J Biomed Pharm Sci 8:6–13
Furukawa H, Wu T, Ohta T, Kuoh C (1985) Chemical constituents of Murraya euchrestifolia Hayata. Structures of novel carbazolequinones and other new carbazole alkaloids. Chem Pharm Bull 33:4132–4138
Rao GV, Rao KS, Annamalai T, Mukhopadhyay T (2009) New coumarin diol from the plant, Chloroxylon swietenia DC. ChemInform. https://doi.org/10.1002/chin.200947189
Olennikov DN, Chirikova NK, Kashchenko NI, Nikolaev VM, Kim S-W, Vennos C (2018) Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front Pharmacol 9:756
Chakraborty D, Bhattacharyya P, Roy S, Bhattacharyya S, Biswas A (1978) Structure and synthesis of mukonine, a new carbazole alkaloid from Murraya koenigii. Phytochemistry 17:834–835
Liu B-Y, Zhang C, Zeng K-W, Li J, Guo X-Y, Zhao M-B et al (2018) Anti-inflammatory prenylated phenylpropenols and coumarin derivatives from Murraya exotica. J Nat Prod 81:22–33
Kinoshita T, Wu J-B, Ho F-C (1996) Prenylcoumarins from Murraya paniculata var omphalocarpa (Rutaceae): the absolute configuration of sibiricin, mexoticin and omphamurin. Chem Pharm Bull 44:1208–1211
You C-X, Yang K, Wang C-F, Zhang W-J, Wang Y, Han J et al (2014) Cytotoxic compounds isolated from Murraya tetramera Huang. Molecules 19:13225–13234
Lv H-N, Zeng K-W, Liu B-Y, Zhang Y, Tu P-F, Jiang Y (2015) Biological activity and chemical constituents of essential oil and extracts of Murraya microphylla. Nat Prod Commun 10:1934578X1501000935
Wu T-S (1988) Coumarins from the leaves of Murraya paniculata. Phytochemistry 27:2357–2358
Furukawa H, Wu T, Kuoh C (1985) Structures of murrafoline-B and-C, new binary carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull 33:2611–2613
Ito C, Furukawa H (1987) Constituents of Murraya exotica L. structure elucidation of new coumarins. Chem Pharm Bull 35:4277–4285
Wang Y-S, He H-P, Shen Y-M, Hong X, Hao X-J (2003) Two new carbazole alkaloids from Murraya k Oenigii. J Nat Prod 66:416–418
Yang H, Zhou Q, Peng C, Liu L, Xie X, Xiong L et al (2014) Coumarins from Leonurus japonicus and their anti-platelet aggregative activity. Zhongguo Zhong yao za zhi = Zhongguo Zhongyao Zazhi= China J Chin Mater Med 39:4356–4359
Kureel S, Kapil R, Popli S (1969) Terpenoid alkaloids from Murraya koenigii spreng.-II.: the constitution of cyclomahanimbine, bicyclomahanimbine, and mahanimbidine. Tetrahedron Lett 10:3857–3862
Murray RD, Zeghdi S (1989) Synthesis of the natural coumarins, murraol (CM-c2), trans-dehydroosthol and swietenocoumarin G. Phytochemistry 28:227–230
Ochung’ AA, Manguro LAO, Owuor PO, Jondiko IO, Nyunja RA, Akala H et al (2015) Bioactive carbazole alkaloids from Alysicarpus ovalifolius (Schumach). J Korean Soc Appl Biol Chem 58:839–846
Schuster C, Börger C, Julich-Gruner KK, Hesse R, Jäger A, Kaufmann G et al (2014) Synthesis of 2-hydroxy-7-methylcarbazole, glycozolicine, mukoline, mukolidine, sansoakamine, clausine-H, and clausine-K and structural revision of clausine-TY. Eur J Org Chem 2014:4741–4752
Furukawa H, Wu T, Ohta T (1983) Bismurrayafoline-A and-B, Two Novel" Dimeric" Carbazole Alkaloids from Murraya euchrestifolia. Chem Pharm Bull 31:4202–4205
Tan S-P, Ali AM, Nafiah MA, Awang K, Ahmad K (2015) Isolation and cytotoxic investigation of new carbazole alkaloids from Murraya koenigii (Linn.) Spreng. Tetrahedron 71:3946–3953
Joshi B, Kamat V, Gawad D (1970) On the structures of girinimbine, mahanimbine, isomahanimbine, koenimbidine and murrayacine. Tetrahedron 26:1475–1482
Hesse R, Gruner KK, Kataeva O, Schmidt AW, Knölker HJ (2013) Efficient construction of Pyrano [3, 2-a] carbazoles: application to a biomimetic total synthesis of cyclized monoterpenoid Pyrano [3, 2-a] carbazole alkaloids. Chem A Eur J 19:14098–14111
McPhail AT, Wu T-S, Ohta T, Furukawa H (1983) Structure of (±)-murrafoline, a novel biscarbazole alkaloid from Murraya euchrestifolia. Tetrahedron Lett 24:5377–5380
Wu T-S (1991) Murrayamine-A,-B,-C and (+)-mahanine, carbazole alkaloids from Murraya euchrestifolia. Phytochemistry 30:1048–1051
Ito C, Thoyama Y, Omura M, Kajiura I, Furukawa H (1993) Alkaloidal constituents of Murraya koenigii. Isolation and structural elucidation of novel binary carbazolequinones and carbazole alkaloids. Chem Pharm Bull 41:2096–2100
Reisch J, Aladesanmi AJ (1994) Two carbazole alkaloids from Murraya koenigii. Phytochemistry 36(4):1073–1076. https://doi.org/10.1016/S0031-9422(00)90494-1
Chakrabarty M, Nath AC, Khasnobis S, Chakrabarty M, Konda Y, Harigaya Y et al (1997) Carbazole alkaloids from Murraya koenigii. Phytochemistry 46:751–755
Steck W (1972) Paniculatin, a new coumarin from Murraya paniculata (L.) Jack. Can J Chem 50:443–445
Ashokkumar K, Selvaraj K, Km SD (2013) Reverse phase-high performance liquid chromatography-diode array detector (RP-HPLC-DAD) analysis of flavonoids profile from curry leaf (Murraya koenigii L.). J Med Plants Res 7:3393–3399
Fiebig M, Pezzuto JM, Soejarto DD, Kinghorn AD (1985) Koenoline, a further cytotoxic carbazole alkaloid from Murraya koenigii. Phytochemistry 24:3041–3043
Bernal P, Tamariz J (2007) Total synthesis of murrayanine involving 4, 5-dimethyleneoxazolidin-2-ones and a palladium (0)-catalyzed diaryl insertion. Helv Chim Acta 90:1449–1454
Ruangrungsi N, Ariyaprayoon J, Lange GL, Organ MG (1990) Three new carbazole alkaloids isolated from Murraya siamensis. J Nat Prod 53:946–952
Chakraborty D (1993) Chemistry and biology of carbazole alkaloids. Alkaloids Chem Pharmacol 44:257–364
Avila-Melo JL, Benavides A, Fuentes-Gutiérrez A, Tamariz J, Jiménez-Vázquez HA (2021) Total synthesis of the natural carbazoles O-demethylmurrayanine and Murrastanine A, and of a C4, C4′ symmetric murrastanine A dimer from N-phenyl-4, 5-dimethylene-1, 3-oxazolidin-2-one. Synthesis 53:2201–2211
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Riham A. El-Shiekh: Methodology, data analysis, investigation, writing-original draft preparation, Rana Elshimy: Methodology, data analysis, and writing-original draft preparation, Asmaa A. Mandour: Methodology, data analysis, and writing-original draft preparation, Hanaa A. H. Kassem: Conceptualization, and writing—review and editing, Amal E. Khaleel: Conceptualization, and writing—review and editing, Saleh Alseekh: Methodology, data analysis, and writing—review and editing, Alisdair R. Fernie: Conceptualization, and writing—review and editing, Mohamed A. Salem: Methodology, data analysis, investigation, writing—original draft preparation, and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by the Animal Experiment Ethics Committee of the National Hepatology & Tropical Medicine Research Institute (NHTMRI) for Research Ethics Committee (REC) (#NHTMRI REC A5-2023).
Competing interests
There is no conflict of interest among authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Tigecycline (TGC 30 µg), amikacin (30 µg), Trimethoprim/sulfamethoxazole (SXT 25µg), cefoxitin (FOX 30µg), Levofloxacin (LVX 5µg), gentamicin (CN 10 µg). Fig. S2. A. baumannii on LB (Luria-Bertani) agar upon examining pulmonary bacterial loads. Fig. S3. Identification of a flavonoid compound (Quercitrin). Fig. S4. Identification of a phenolic compound (Chlorogenic acid). Fig. S5. Identification of a carbazole alkaloid compound (Murrayacinine). Fig. S6. Identification of a carbazole alkaloid compound (Murrayamine A). Fig. S7. Inhibition diameter zones obtained by well diffusion method for A. baumnii against M. koenigii seed extracts and M. koenigii fruit extracts. 1. M. koenigii seed extracts, 2. M. koenigii fruit extracts and 3. DMSO: negative control. Table S1. Parameters for MZmine processing of UHPLC-MS/MS data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Shiekh, R.A., Elshimy, R., Mandour, A.A. et al. Murraya koenigii (L.) Sprengel seeds and pericarps in relation to their chemical profiles: new approach for multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Appl Biol Chem 67, 35 (2024). https://doi.org/10.1186/s13765-024-00886-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-024-00886-7