Abstract
The Solanaceae family includes the largest flowering crops such as tomatoes, potatoes, and eggplants. Consumer demand has led to massive development of plants in the Solanum genus, and many different Solanum varieties are now available on the market. The recent advances in Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/CRISPR-associated protein 9 (Cas9)-based genome editing have allowed laboratories and smaller crop production companies to utilize the technology in various crops. The traditional transformation method in crops involves the use of Agrobacterium, which is considered the most efficient method for introducing exogenous genetic materials in target plants. The Agrobacterium-mediated transformation method has been also established in the Solanaceae family, enabling CRISPR/Cas-based genome editing in crops like tomatoes, potatoes, and eggplants. However, the Agrobacterium-mediated approach inevitably accompanies the insertion of exogenous DNA into the plant genome and often causes the formation of chimera that require further propagation steps. Alternatively, the CRISPR/Cas components can be introduced into protoplasts in the form of DNA for transient expression or a mixture of protein and RNA to avoid genomic insertion of foreign materials. The protoplast transformation approach involves processes including protoplast preparation, transfection, and regeneration, which require a comprehensive understanding and greater technical mastery of the tissue culture phase. Here we highlight the current research advances in protoplast transformation and discuss how to optimize the procedures of protoplast isolation, transfection, and regeneration for efficient and reproducible CRISPR/Cas-based genome editing in the genus Solanum.
Similar content being viewed by others
Introduction
The genus Solanum comprises a huge collection of more than 1500 species, including important economic plants such as tomatoes (Solanum lycopersicum), potatoes (Solanum tuberosum), and eggplants (Solanum melongena). The genome sizes of tomato diploid, potato diploid, and eggplant haploid are estimated to be 950 Mb, 844 Mb, and 1.21 Gb, respectively [1, 2]. These plants have a relatively compact gene size, which makes them attractive targets for genetic engineering. Tomatoes, in particular, have become a popular model organism for scientific research due to their many fundamental biological evens including abundant nutritional constituents both primary and secondary metabolites, abiotic and biotic stress responses, typical developmental growth of fruit vegetable, and the most importantly their relatively short life cycle [3]. Short life cycle of model plant allows phenotypic and genetic observation over multiple generations quickly, which is required for rapid experimental turnaround. The Agrobacterium-mediated transformation method has been extensively developed in plants and is well-established for tomatoes and potatoes. This technique has enabled the production of genetically engineered plants such as the pioneering Flavr Savr tomato with delayed softening and improved resistance to environmental stressors [4, 5]. In potatoes, genetic modification has led to the development of varieties with desirable traits, such as lower acrylamide content to ensure food safety and the development of firm-cooking potatoes known as Amflora, which contains pure amylopectin starch [6, 7]. In addition, recent studies have shown the potential of CRISPR/Cas9 technology in generating mutant tomato plants for crop improvement [8, 9]. Most transgenic Solanum reports including the application of CRISPR/Cas9 have been employed stable gene transfer method mediated by Agrobacterium tumefaciens.
In the era of genome editing, an alternative modality has been devised for the delivery of the CRISPR-Cas system. Specifically, CRISPR ribonucleoprotein (RNP) complexes or plasmids harboring the CRISPR-Cas system can be directly introduced into the protoplasts, which is the unicellular state achieved through the removal of the cell wall, utilizing a method known as transient transfection [10]. There are multiple advantages to genome editing using RNP with protoplasts, including the absence of transgene insertions (DNA-free approach), rapid degradation of RNPs leading to reduced off-targeting and lower mosaicisms, the lack of necessity for codon optimization in the target plants by using Cas9 protein, and the elimination of a high level of DNA construction steps with a binary vector to insert T-DNA for expressing Cas9 and gRNA in the target plants [11]. Especially, the absence of transgene insertions allows for mitigating concerns related to genetically modified organisms (GMOs). In the case of utilizing plasmid vectors instead of RNP for transfection with protoplast, we can still anticipate the absence of T-DNA insertions, and minimal mosaicisms by expressing Cas9 and gRNA transiently [12]. And the strong advantage of plasmid vector for Cas9 and gRNA expression is that it is the most accessible method, and most of researchers and crop developers are able to use the plasmid vector.
The RNP delivery method entails protoplast isolation, protoplast transfection, and protoplast regeneration into reproductive plants. The bottleneck in plant genome editing with RNP lies in the protoplast treatment and tissue culture processes, necessitating specialized expertise in tissue culture and being inherently time-consuming. Recent studies have demonstrated that efficient gene editing is achievable by employing an optimized protocol to introduce CRISPR/Cas9-RNP complexes into tomato or potato protoplasts [11, 13]. Although previous studies have reported high editing efficiency in protoplast transformation, the regeneration of shoots from RNP-transfected protoplasts remains a challenging technical bottleneck due to low survival rates until the generation of whole transgenic plants [11, 13]. Several previous reports have documented successful regeneration from the non-transformed protoplasts of cultured tomatoes [14, 15]. Moreover, recent studies have demonstrated protoplast regeneration in transformed tomatoes with CRISPR/Cas9-induced mutations [10, 16, 17]. However, genetic modifications of other important Solanum species such as potatoes, eggplants, and bell peppers are still challenging due to the low efficiency of the transfection and regeneration [13, 18]. Given the reported variability in regeneration and low mutation rates, an optimized protocol needs to be developed to achieve both high editing efficiency and high reproducibility in target plants.
The establishment of a technique for transgene-free genome editing and proficient protoplast regeneration within the genus Solanum holds the potential to facilitate precise genome modifications. Consequently, a comprehensive examination of experimental parameters concerning protoplast isolation, protoplast transfection, and subsequent regeneration within the Solanum genus was undertaken to delineate the optimal conditions conducive to successful genome modifications.
Protoplast transformation for transgene-free CRISPR/Cas-based gene editing
The gene editing process involving protoplasts comprises three primary phases: Protoplast isolation, protoplast transfection, and protoplast regeneration. Protoplast isolation is a fundamental procedure wherein plant cells undergo enzymatic or mechanical treatment for the removal of cell walls. This process yields denuded cells utilized across diverse domains of plant research, encompassing genetics, biotechnology, and cell biology [19,20,21,22]. Protoplast transfection serves as a potent tool in plant genetic engineering, enabling the introduction of foreign genes, elucidation of gene function, and the generation of transgenic plants with desired traits [14, 23]. These techniques provide researchers with the means to investigate the functional aspects of specific genes and introduce new genetic materials into plant cells, thereby facilitating the development of improved and novel plant varieties [10, 16]. Protoplast regeneration encompasses the division of protoplasts, the synthesis of new cell walls, and their eventual development into fertile plants [14, 24]. This technique finds extensive application in the generation of transgenic plants, achieved either through particle bombardment of callus or Agrobacterium-mediated transformation. Overall, protoplast isolation and regeneration techniques play an important role not only for plant scientists and breeders seeking to improve plants and advance plant biology, but also in plant biotechnology for the production of genetically modified plants with desirable traits such as increased resistance to pests or diseases, improved flavor, or longer shelf life.
In addition to protoplast isolation and regeneration, the selection of the CRISPR-Cas delivery system serves another critical technical aspect for establishing an efficient crop genome editing procedure. The use of a pre-made CRISPR/Cas9 ribonucleic acid protein complex with protoplasts is considered the most advanced and efficient method for CRISPR-Cas system transfection [13, 25]. The utilization of a pre-made RNP complex eliminates the need for cells to transcribe and translate the Cas9 protein, thereby enabling faster and more efficient editing and enhancing editing efficiency [26]. Transient expression of CRISPR/Cas9 is important to avoid prolonged exposure and minimize the risk of unintended mutations [27]. Therefore, pre-made RNP can minimize off-target effects by mitigating continuous Cas9 protein expression, thereby reducing cellular stress and potential unintended effects on cell physiology. Additionally, it simplifies the experimental workflow by eliminating the need to clone Cas9 and guide RNA into a vector, saving time and resources in the experimental setup. These advantages contribute to the efficiency, precision, and broad applicability of the CRISPR/Cas9 system in various experimental settings [28].
Considerations for high-efficiency protoplast isolation
To enhance the efficiency of protoplast transfection and regeneration, it is crucial to generate healthy and viable protoplasts. Some essential considerations for the effective isolation of protoplasts are outlined in Table 1 and described below. Optimal isolation results are typically attained by utilizing fresh and actively growing tissues, such as young leaves or hypocotyls, as their cell walls are thinner and more susceptible to enzymatic digestion [12, 29].
In protoplast isolation methods, the efficiency of enzymatic cell wall digestion is crucial. Complete removal of the cell wall is necessary without compromising the viability of the protoplast. To efficiently separate protoplasts, the dissolution conditions of fresh plant tissue, enzyme solution (e.g., cellulase, pectinase, macerozyme), mannitol, and calcium chloride play a major role. Cellulase is an enzyme that hydrolyzes cellulose, the primary component of plant cell walls. The recommended concentration is typically 1.5–2% (w/v), but higher concentrations of cellulase may be required to efficiently digest cellulose-rich cell walls in tomato and eggplant tissues. The concentration range of cellulase can be optimized for the specific cell wall composition of these plant species [12, 30,31,32,33]. Macerozyme is a complex enzyme mixture containing cellulase, hemicellulase, and pectinase activities. Hemicellulase and pectin lyase specifically target hemicellulose and pectin, respectively, which are components of plant cell walls. The inclusion of these enzymes, along with cellulases, helps break down a wide range of cell wall components, ensuring thorough digestion and release of protoplasts [34, 35]. Osmotic equilibrium is imperative for maintaining the structural and functional integrity of protoplasts. Mannitol or sorbitol is employed to sustain osmotic balance as an osmotic substance, thereby preventing osmotic shock to the protoplasts [36]. Consequently, these osmotic substances are consistently utilized in all subsequent procedures, encompassing the washing of protoplasts after enzymatic digestion, transfection, and regeneration, until the formation of callus. Calcium ions play a pivotal role in stabilizing the plasma membrane of protoplasts, thereby contributing to the facilitation of the fusion process [37]. Calcium chloride is also utilized to induce the fusion of isolated protoplasts. In this context, calcium ions aid in the establishment of bridges between adjacent protoplasts, thereby facilitating their fusion. Optimal execution of the cell wall lysis step necessitates precise adjustment of enzyme concentrations, incubation time, mannitol concentration, and shaking speed, tailored to the characteristics of the specific plant species and tissue types, ensuring optimal outcomes. This optimization process aims to maximize both the yield and viability of the protoplasts. Through careful optimization of these parameters, the attainment of a high yield of viable protoplasts becomes feasible for subsequent analysis and manipulation [24].
The procedure commences with the excision of fresh plant tissue, which is then finely cut into small pieces, approximately 0.5 to 1 mm in size (Fig. 1A). Subsequently, these tissue fragments are immersed in an enzyme solution designed to facilitate the digestion of cell walls, leading to the subsequent release of protoplasts (Fig. 1B, C). The enzyme solution employed for efficient protoplast isolation from tomatoes typically comprises 0.75% (w/v) Macerozyme R-10, 1.5% (w/v) Cellulase Onozuka R-10, 0.6 M mannitol, 10 mM CaCl2, 0.1% (w/v) BSA, 10 mM MES, with the pH adjusted to 5.8 [11, 16, 23]. The tomato tissue is fully immersed in the enzyme solution and subsequently incubated at room temperature in the dark for 3 to 5 h with gentle shaking at 50 to 70 rpm. It is noteworthy that the incubation time may vary depending on the specific tissue type and the combination of enzymes employed. The selection of enzymes for protoplast isolation is contingent upon the plant species, as detailed in Table 1. For the extraction of protoplasts from tomato and eggplant, it is generally advisable to utilize cellulase at a concentration of 1.5 to 2% (w/v), in conjunction with additional cell wall lysis enzymes such as macerozyme or pectin lyase [38,39,40,41,42]. 1% (w/v) cellulase was shown to be effective for extracting protoplasts from leaves and tubers in potatoes [43,44,45]. The isolation buffer for tomato protoplasts needs to be supplemented with 0.6 M sorbitol or mannitol to reduce osmotic shock during the isolation process, as suggested before [11, 23, 46]. Proper control of enzyme concentrations and treatment durations is crucial to minimize damage to the protoplasts and maximize the yield of viable cells. Following cell wall lysis, the reaction mixture is filtered through a 40-μm nylon mesh to eliminate undigested tissue and large debris (Fig. 1D). Subsequently, the filtrates are subjected to low-speed centrifugation (e.g., 100 × g) for 5 to 10 min, forming a pellet consisting of protoplasts. Protoplasts, being denser than the buffer solution, can be easily separated in this manner. The isolated protoplasts are washed in a stabilizing solution containing 0.6 M sorbitol or mannitol at least twice to remove residual enzymes (Fig. 1E) and examined under microscope (Fig. 1F). As the isolation process can cause stress in the cells, it is imperative to minimize mechanical stress to avoid damage to the protoplasts. During the filtering step, gentle pipetting is highly recommended to mitigate excessive mechanical stress in the cells.
Schematic diagram of protoplast isolation from plant cotyledons. A Cotyledon preparation by slicing into thin strips (0.5 to 1 mm), B Incubation of sliced cotyledons in the digestion solution for 16 h, C Confirmation of the release of protoplasts in the digestion solution, D Filtration using a 40 µm mesh to remove cell debris, E Removal of the enzyme solution by washing with the stabilizing solution twice, F Microscopic observation to count protoplasts. Scale bar indicates 50 μm
Protoplast transfection for transgene-free gene editing
Protoplast transfection is a well-known scientific method employed for the introduction of exogenous DNAs into plant cells, enabling their transient expression with no need for genomic integration [11]. During the process, protoplasts are exposed to foreign DNA and subsequently incubated under controlled conditions to facilitate DNA uptake [47]. The success of transfection relies on the species of target plants and the types of foreign genetic materials used, requiring optimization of factors such as DNA or RNA concentration, incubation time, and temperature.
The efficacy of protoplast transfection depends on the selected approach and shows variability. Numerous transfection conditions have been extensively documented, each of which is associated with distinct efficiencies [24]. Among the widely embraced methods for introducing foreign DNA into eukaryotic cells is transfection through polyethylene glycol (PEG) [11]. PEG, a water-soluble polymer, forms complexes with DNA, thereby enhancing its cellular uptake [48]. The success rates of the PEG-mediated protoplast transfection typically fall within the range of 30 to 50% [23]. According to the Wang et al. [48], PEG-mediated transfection system of eggplant protoplasts, the transformation efficiency was increased until the PEG concentration was reached to 40% and then slightly decreased. An optimal transfection efficiency of approximately 53% was observed at 40% of PEG concentration in the eggplant [48].
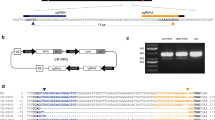
A common protocol for PEG-based transfection with nucleic acids is described below (Fig. 2A). The DNA or RNA intended for transfection is purified and subsequently dissolved in an appropriate buffer solution (40% PEG, 0.2 M Mannitol, 100 mM CaCl2). Protoplasts extracted from fresh plant tissues as described earlier (Fig. 1) are resuspended in a solution containing PEG and the purified nucleic acids and gently mixed (Fig. 2A). The mixture of protoplasts-PEG-DNA is incubated at room temperature with swirling for 10 to 15 min. The transfected protoplasts are washed twice with a buffer solution (5 M NaCl, 1 M CaCl2, 1 M KCl, 1 M MES) to mitigate the potential toxicity of PEG to the cells, and then plated onto tissue culture plates or dishes containing complete media, facilitating their growth and expansion. Introduction of foreign genetic materials can be confirmed by visualizing of marker proteins such as fluorescent proteins (Fig. 2A). We found that the concentration of PEG affects the viability of transfected protoplasts. As the concentration of PEG increases from 10 to 40%, the fraction of viable cells tends to decrease (Fig. 2B). The vitality was assessed using 0.01% fluorescein diacetate (FDA) staining and visualization under a fluorescence microscope. As the PEG concentration increased from 10 to 40%, there was a tendency for the fraction of viable cells to decrease (Fig. 2B). Notably, at a 40% PEG concentration, approximately 20% of healthy protoplasts were observed in tomato protoplasts under the previously described transfection conditions. Therefore, it is crucial to carefully consider and determine the optimal PEG concentration for both protoplast transfection efficiency and cell viability.
PEG-based protoplast transfection for gene edition. A An overview of the PEG-based protoplast transfection procedure. Transfection can be confirmed by visualizing a marker protein, GFP. B The viability of tomato protoplasts was observed under a fluorescence microscope. Protoplasts with green fluorescence were viable. Scale bars indicate 50 μm. Protoplasts exhibiting a perfect round shape were counted as viable protoplasts (n = 6; *p < 0.05). C Comparison of PEG-mediated protoplast transfection and Agrobacterium-mediated transfection targeting the ALS2 gene in tomato cells using the CRISPR/Cas9 system. The target sequences are in bold, and the edited regions are in red. The black underline indicates the gRNA sequences, and the red letters indicate the PAM sequence
The DNA-free genome editing method employing CRISPR/Cas9 RNPs has emerged as a viable alternative to conventional DNA-based approaches [11]. Protoplasts can be transfected in a completely DNA-free manner by introducing a complex of protein and RNA [16]. This strategy involves the direct delivery of preassembled Cas9 protein and gRNA to the target cells, circumventing the possibility of DNA integration into the genome [26, 49]. Furthermore, the RNP complex delivered into the cell is less stable and subsequently degrades by cellular enzymes after inducing mutations in the target gene. This degradation helps prevent off-target mutagenesis, resulting in low off-target rates [50]. To create a pre-made CRISPR/Cas-gRNA RNP complex, both components can be easily obtained by ordering commercial supplements. The Cas protein can be obtained from bacterial expression, and the gRNA can be transcribed in vitro [51, 52]. The gRNA synthesized in vitro can be modified and used for versatile purposes [53, 54].
Notably, the PEG-mediated transfection has been attempted to deliver CRISPR/Cas9 RNPs for genome editing in Solanum genus, including potatoes (S. tuberosum) and tomatoes (S. lycopersicum) [13, 23]. In their study, Andersson et al. [13] performed the PEG-mediated transfection on potato protoplasts to introduce CRISPR/Cas9 RNPs targeting the granule-bound starch synthase (GBSS) gene. The CRISPR/Cas9 RNPs successfully induced mutations in the GBSS gene, yielding transgene-free edited plants with altered starch contents [13]. In their study, Naing et al. [17] also utilized PEG-mediated delivery to introduce CRISPR/Cas9 RNPs targeting the phytoene desaturase (PDS) gene into tomato protoplasts. This resulted in the production of albino plants with reduced pigment contents [17]. Despite the limited number of cases studied, the utilization of PEG-mediated transfection as a delivery technique for CRISPR/Cas9 RNPs presents numerous benefits including exceptional efficiency, minimal toxicity, and ease of use, as described before in this report. In our previous study, a highly efficient gRNA was selected to edit the herbicide-related gene ALS2 in tomatoes [55, 56]. Using CRISPR-P 2.0 (http://crispr.hzau.edu.cn/CRISPR2/), a sequence of 20 bp in the 5 direction from the protospacer adjacent motif (PAM, 5ʹ-AGG) sequence in the target gene of the tomato genome was selected as 5ʹ-CTATTACAGGTCAAGTGCCA-3ʹ. According to previous research Yu et al. [56], the corresponding guide RNA (gRNA) targets the allele to produce the ALS2 protein with reduced susceptibility to herbicides by correcting the amino acid at position P197 [56]. With the same gRNA, we found that the PEG-mediated protoplast transfection is approximately twelve times more efficient than the Agrobacterium-mediated transfection, supporting the superior editing efficiency of this method (Fig. 2C). The experimental results of ALS2 gene transfection via PEG-mediated protoplasts revealed a maximum value of 8 and an average value of 4.8. The value depicted in Fig. 2C is a measurement of 5.25 within the box plot range, displaying diverse patterns. In contrast, the experimental results from the Agrobacterium-mediated transfection method displayed a maximum value of 1.3 and an average value of 0.7. Various editing patterns were observed within the box plot range, with a value of 0.44. These findings suggest that the protoplast transfection method might exhibit higher efficiency in gene editing compared to the Agrobacterium-mediated transfection method.
Electroporation is another widely employed technique for protoplast transfection with exogenous DNA. In this method, a transient electric field is applied to the protoplasts to temporarily induce pores in the cell membrane, allowing the entry of foreign DNA [57, 58]. Jones et al. employed electroporation to investigate the factors influencing transient gene expression in protoplasts derived from various potato tissues, such as leaves, tubers, and suspension cells. They suggested that the most favorable field strength depends on the protoplast size, and the optimal field strength varies in response to the application of electrical pulses, ranging from 150 to 250 V/cm, highlighting the roles of each parameter in successful electro-transfection [58]. As the effects of these factors may also vary depending on the plant species, the quality of the starting protoplasts, and the specific conditions used in the transfection, the optimization of the electroporation process is crucial for maximizing transfection efficiency while minimizing cell damage [59, 60].
Electroporation has been successfully employed for delivering CRISPR/Cas9 components into protoplasts, with a demonstrated transfection efficiency ranging from 20 to 30% [61]. A protoplast electro-transfection protocol for the Solanum genus is outlined as follows [58, 62]. The purified DNA to be transfected is dissolved in a buffer solution (1 M Mannitol, 0.3 M MgCl2, 1 M MES) that is compatible with the protoplasts. Subsequently, 10 to 20 µg/ml (final concentration) of the DNA is mixed with the isolated protoplasts (approximately 2 × 105 cells/ml) in a tube or cuvette. Electrical DC pulses of 50 µs duration at 500 to 800 V/cm are applied to the mixture of protoplasts and DNA using an electroporator [62]. The transfected protoplasts are then washed twice with a buffer solution (5 M NaCl, 1 M CaCl2, 1 M KCl, 1 M MES) and plated onto tissue culture dishes containing complete media for cell growth and expansion. Commonly used protoplast culture media are described later in the protoplast regeneration section.
Plant regeneration from transfected protoplasts
Protoplast regeneration is the process of reconstructing plant cells from isolated protoplasts (Fig. 3). The mechanism of regeneration varies depending on the tissue types or plant species [24]. In certain cases, regeneration involves the activation of stem cells or the reprogramming of existing cells into a less differentiated state, facilitating tissue repair and regeneration [63]. Regeneration is commonly achieved by sequentially growing protoplasts in two distinct media before initiating the general plant callus growth and regeneration process: the protoplast culture medium and the callus-inducing medium. Specialized media known as protoplast culture medium and callus-inducing medium are formulated with essential nutrients and growth factors that promote cell division and cell wall reconstitution [14, 23]. Similar to other plant tissue culture media, the Murashige and Skoog (MS) medium or Kao and Michayluk (KM) medium, a balanced salt solution, is commonly employed to constitute the both medium. MS medium has been employed for providing essential inorganic nutrients including nitrogen, phosphorus, potassium, and micronutrients [24]. Additionally, vitamins such as thiamine, pyridoxine, and nicotinic acid are introduced to support plant tissue growth and development. Carbohydrates, such as sucrose or glucose, are included as an energy source in most cases, and glutamine, asparagine, and proline may be added to serve as additional nitrogen sources for protein synthesis. The pH of medium is typically maintained around 5.5 to 6.5, with buffering agents like MES or MOPS added to stabilize the pH.
Plant regeneration from the tomato protoplasts. A Tomato protoplasts freshly isolated from cotyledons, B Protoplasts during first cell divisions in 7 days after isolation in the protoplast culture medium, C Protoplasts during second division after 14 days in the protoplast culture medium, D Cell aggregate formation after 3 weeks in the protoplast culture medium, E Callus formation after 1 months on the callus induction medium, F Callus growth for 2 months on the callus growth medium, G Shoot regenerated on the shoot induction medium for 2 months. Scale bars, 50 μm A–D and 1 cm E–G
The first distinct medium for protoplast regeneration, the protoplast culture medium, may contain additional components such as 0.3 to 0.8 M of mannitol to provide osmotic support, maintain cell integrity, and promote cell division [39, 46]. Plant growth regulators are also included to facilitate cell division and regeneration. Among the most commonly used plant growth regulators are Indole-3-acetic acid (IAA) or 1-Naphthaleneacetic acid (NAA) as auxins, and zeatin as cytokinins, added to the medium at a concentration of 0.1 to 1.0 μM to promote cell division and differentiation [24, 64, 65]. The protoplast regeneration process is challenging and requires careful manipulation of various factors, including nutrient concentrations, plant growth regulators, and environmental conditions [24]. Additionally, the composition of the media needs to be optimized for specific plant species or cell types, as presented in Table 2 [23, 24, 66]. The solution used for efficient protoplast regeneration from tomatoes usually consists of 3.62 g/L KM medium, 3% sucrose, 0.5 M mannitol, 2 mg/L 2,4-D, 0.5 mg/L BAP, and 10% KM vitamin solution, with the pH adjusted to 5.8.
Once the protoplasts are successfully cultured and maintained in the culture medium, they are transferred to the callus-inducing medium, which supports cell proliferation and development of pluripotent callus. For callus induction of the protoplasts from tomato leaves, the MS medium is typically supplemented with 1 or 2 mg/L of 2,4-Dichlorophenoxyacetic acid (2,4-D), 0.5 mg/L of IAA. The optimal concentration of 2,4-D may vary depending on the Solanum species (Table 2). For eggplants, add 0.2 or 1.0 mg/L of 2,4-D and 1 mg/L of NAA to the medium. Additionally, 1 mg/L of kinetin or 0.5 mg/L of benzyladenine (BA) may be included to promote callus induction [67, 68]. The concentration of cytokinin is typically lower than that of auxins. The medium is typically solidified with 0.8% (w/v) of agar and maintained at pH 5.7. Other additives such as sucrose, vitamins, and amino acids can also be incorporated into the medium to support the growth and differentiation of the callus. The composition of the media used during the leaf-to-callus transition in three different Solanum species—tomatoes, potatoes, and eggplants—is similar (Table 2). The regenerated cells are examined to confirm the presence of transfected DNA by PCR and sequencing.
Concluding remarks
In the era of CRISPR/Cas9-based genome editing, protoplast transformation plays a key role in efficient crop engineering and breeding. Given the significant market shares of tomatoes, potatoes, and eggplants in the vegetable industry, it becomes essential to develop comprehensive protocols for protoplast isolation, transfection, and regeneration, customized to the specific requirements of the Solanum genus. The success of crop breeding greatly depends on technical proficiency, and understanding the processes of protoplast transformation and fine-tuning the parameters affecting editing efficiency would help achieve desired outcomes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALS:
-
Acetolactate synthase
- CRISPR:
-
Clustered regular interspaced short palindromic repeats
- Cas9:
-
CRISPR-associated protein 9
- gRNA:
-
Guide RNA
- PAM:
-
Protospacer adjacent motif
- NGS:
-
Next-generation sequencing
- MS:
-
Murashige and Skoog basal medium
- IAA:
-
Indole-3-acetic acid
- NAA:
-
1-Naphthaleneacetic acid
- PEG:
-
Polyethylene glycol
References
Sato S, Tabata S, Hirakawa H, Asamizu E, Shirasawa K, Isobe S et al (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485(7400):635–641. https://doi.org/10.1038/nature11119
Barchi L, Pietrella M, Venturini L, Minio A, Toppino L, Acquadro A et al (2019) A chromosome-anchored eggplant genome sequence reveals key events in Solanaceae evolution. Sci Rep 9(1):11769. https://doi.org/10.1038/s41598-019-47985-w
Liu W, Liu K, Chen D, Zhang Z, Li B, El-Mogy MM et al (2022) Solanum lycopersicum, a model plant for the studies in developmental biology, stress biology and food science. Foods 11(16):2402
Martineau B (2001) First fruit: the creation of the Flavr savr tomato and the birth of genetically engineered food, vol xvi. McGraw-Hill, New York, p 269
Foolad MR. 2007. Current Status of Breeding Tomatoes For Salt And Drought Tolerance. In: MA Jenks, PM Hasegawa, S Mohan Jain (Eds). Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops. Springer: Dordrecht
Ly DNP, Iqbal S, Fosu-Nyarko J, Milroy S, Jones MGK (2023) Multiplex CRISPR-Cas9 gene-editing can deliver potato cultivars with reduced browning and acrylamide. Plants-Basel 12(2):379. https://doi.org/10.3390/plants12020379
Tilocca MG, Serratrice G, Oggiano MA, Mancuso MR, Mascia I, Marongiu E et al (2014) Monitoring the presence of genetically modified potato EH92-527-1 (BPS-25271-9) in commercial processed food. Ital J Food Saf 3(1):57–59
Wang T, Zhang HY, Zhu HL (2019) CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Hortic Res-England. 6:77. https://doi.org/10.1038/s41438-019-0159-x
Nekrasov V, Wang CM, Win J, Lanz C, Weigel D, Kamoun S (2017) Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci Rep-Uk. 7:482 https://doi.org/10.1038/s41598-017-00578-x
Lin CS, Hsu CT, Yang LH, Lee LY, Fu JY, Cheng QW et al (2018) Application of protoplast technology to CRISPR/Cas9 mutagenesis: from single-cell mutation detection to mutant plant regeneration. Plant Biotechnol J 16(7):1295–1310. https://doi.org/10.1111/pbi.12870
Nicolia A, Andersson M, Hofvander P, Festa G, Cardi T (2021) Tomato protoplasts as cell target for ribonucleoprotein (RNP)-mediated multiplexed genome editing. Plant Cell Tiss Org 144(2):463–467. https://doi.org/10.1007/s11240-020-01954-8
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2(7):1565–1572. https://doi.org/10.1038/nprot.2007.199
Andersson M, Turesson H, Olsson N, Falt AS, Ohlsson P, Gonzalez MN et al (2018) Genome editing in potato via CRISPR-Cas9 ribonucleoprotein delivery. Physiol Plant 164(4):378–384. https://doi.org/10.1111/ppl.12731
Tan ML, Rietveld EM, van Marrewijk GA, Kool AJ (1987) Regeneration of leaf mesophyll protoplasts of tomato cultivars (L. esculentum): factors important for efficient protoplast culture and plant regeneration. Plant Cell Rep 6(3):172–175. https://doi.org/10.1007/BF00268470
Morgan A, Cocking EC (1982) Plant Regeneration from Protoplasts of Lycopersicon esculentum Mill. Z Pflanzenphysiol 106(2):97–104
Lin CS, Hsu CT, Yuan YH, Zheng PX, Wu FH, Cheng QW et al (2022) DNA-free CRISPR-Cas9 gene editing of wild tetraploid tomato Solanum peruvianum using protoplast regeneration. Plant Physiol 188(4):1917–1930. https://doi.org/10.1093/plphys/kiac022
Naing AH, Kyu SY, Pe PPW, Park KI, Lee JM, Lim KB et al (2019) Silencing of the phytoene desaturase (PDS) gene affects the expression of fruit-ripening genes in tomatoes. Plant Methods 15:110. https://doi.org/10.1186/s13007-019-0491-z
Yu Y, Ye W, He L, Cai X, Liu T, Liu J (2013) Introgression of bacterial wilt resistance from eggplant to potato via protoplast fusion and genome components of the hybrids. Plant Cell Rep 32(11):1687–1701. https://doi.org/10.1007/s00299-013-1480-8
Xu Y, Li R, Luo H, Wang Z, Li MW, Lam HM et al (2022) Protoplasts: small cells with big roles in plant biology. Trends Plant Sci 27(8):828–829. https://doi.org/10.1016/j.tplants.2022.03.010
Fournier D, Lejeune F, Tourte Y (1995) Cytological events during the initiation of meristematic nodules in calli derived from eggplant protoplasts. Biol Cell 85(1):93–100. https://doi.org/10.1016/0248-4900(96)89131-3
Lee J, Oh N, Yun JY, Choi HS, Seo JK, Kang JH et al (2023) Application of CRISPR-Based C-to-G Base editing in rice protoplasts. Appl Biol Chem 66:18. https://doi.org/10.1186/s13765-023-00775-5
Kikkert M, van Poelwijk F, Storms M, Kassies W, Bloksma H, van Lent J et al (1997) A protoplast system for studying tomato spotted wilt virus infection. J Gen Virol 78(Pt 7):1755–1763. https://doi.org/10.1099/0022-1317-78-7-1755
Liu Y, Andersson M, Granell A, Cardi T, Hofvander P, Nicolia A (2022) Establishment of a DNA-free genome editing and protoplast regeneration method in cultivated tomato (Solanum lycopersicum). Plant Cell Rep 41(9):1843–1852. https://doi.org/10.1007/s00299-022-02893-8
Reed KM, Bargmann BOR (2021) Protoplast regeneration and its use in new plant breeding technologies. Front Genome Ed 3:734951. https://doi.org/10.3389/fgeed.2021.734951
Liang Z, Chen K, Zhang Y, Liu J, Yin K, Qiu JL et al (2018) Genome editing of bread wheat using biolistic delivery of CRISPR/Cas9 in vitro transcripts or ribonucleoproteins. Nat Protoc 13(3):413–430. https://doi.org/10.1038/nprot.2017.145
Woo JW, Kim J, Kwon SI, Corvalán C, Cho SW, Kim H et al (2015) DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol 33(11):1162–1164
Farooq R, Hussain K, Nazir S, Javed MR, Masood N (2018) CRISPR/Cas9; A robust technology for producing genetically engineered plants. Cell Mol Biol 64(14):31–38
Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B et al (2014) A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol 14:327. https://doi.org/10.1186/s12870-014-0327-y
Horvát E (2009) Protoplast isolation from Solanum lycopersicum L. leaf tissues and their response to short-term NaCl treatment. Acta Biol Szegediensis 53(2):83–86
Nagata T, Takebe I (1971) Plating of isolated tobacco mesophyll protoplasts on agar medium. Planta 99(1):12–20
Kanai R, Edwards G (1973) Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol 51(6):1133–1137
Sheen J (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol 127(4):1466–1475
Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, WANG GL (2006) A highly efficient transient protoplast system for analyzing defence gene expression and protein–protein interactions in rice. Mol Plant Pathol 7(5):417–427
Cocking EC (1960) A method for the isolation of plant protoplasts and vacuoles. Nature 187(4741):962–963
Cocking EC (1972) Plant cell protoplasts-isolation and development. Annu Rev Plant Physiol 23(1):29–50
Sinha A, Wetten AC, Caligari PDS (2003) Optimisation of protoplast production in white lupin. Biol Plantarum 47(1):21–25
Grimes HD, Boss WF (1985) Intracellular calcium and calmodulin involvement in protoplast fusion. Plant Physiol 79(1):253–258. https://doi.org/10.1104/pp.79.1.253
NISHIO T, SaKT T (1987) Efficient plant regeneration from hypocotyl protoplasts in eggplant (Solanum melongena L. and Solanum insanum L.). Japan J Breed 37:389–396
Niedz RP, Rutter SM, Handley LW, Sink KC (1985) Plant regeneration from leaf protoplasts of six tomato cultivars. Plant Sci 39(3):199–204
Shin DS, Han MW, Kim YK (2004) Isolation of protoplasts from tomato root by two-step osmotic treatment. Appl Biol Chem 47(2):192–196
Shao Y, Mu D, Pan L, Wilson IW, Zheng Y, Zhu L et al (2023) Optimization of Isolation and Transformation of Protoplasts from Uncaria rhynchophylla and Its application to transient gene expression analysis. Int J Mol Sci 24(4):3633. https://doi.org/10.3390/ijms24043633
Rueda A, Rojas M, Lobo M, Urrea A, Restrepo C, Botero C et al (2011) Stress responses of tomato protoplasts to copper and paraquat. Trop Plant Pathol 36(2):81–88. https://doi.org/10.1590/S1982-56762011000200003. PMID: WOS:000291883100003
Laimbeer FPE, Holt SH, Makris M, Hardigan MA, Buell CR, Veilleux RE (2017) Protoplast isolation prior to flow cytometry reveals clear patterns of endoreduplication in potato tubers, related species, and some starchy root crops. Plant Methods 13:27. https://doi.org/10.1186/s13007-017-0177-3
Konovalova LN, Strelnikova SR, Zlobin NE, Kharchenko PN, Komakhin RA (2021) Efficiency of transient expression in protoplasts of various potato cultivars. Appl Biochem Microbiol 57(0003–6838):800–807
Jones H, Karp A, Jones MG (1989) Isolation, culture, and regeneration of plants from potato protoplasts. Plant Cell Rep 8(5):307–311. https://doi.org/10.1007/BF00274137
Shahin EA (1985) Totipotency of tomato protoplasts. Theor Appl Genet 69(3):235–240. https://doi.org/10.1007/BF00662431. PubMed PMID: 24253814
Pan C, Ye L, Qin L, Liu X, He Y, Wang J et al (2016) CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Sci Rep 6:24765. https://doi.org/10.1038/srep24765
Wang YY, Zhang Y, Dong YX, Li DL, Shi SL, Li SH et al (2022) A highly efficient mesophyll protoplast isolation and PEG-mediated transient expression system in eggplant. Sci Hortic-Amsterdam 304:111303. https://doi.org/10.1016/j.scienta.2022.111303
Demirer GS, Silva TN, Jackson CT, Thomas JB, Ehrhardt DW, Rhee SY et al (2021) Nanotechnology to advance CRISPR–Cas genetic engineering of plants. Nat Nanotechnol 16(3):243–250
Hahn F, Nekrasov V (2019) CRISPR/Cas precision: do we need to worry about off-targeting in plants? Plant Cell Rep 38(4):437–441
Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC (1987) Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res 15(21):8783–8798
Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB et al (2017) Enhanced proofreading governs CRISPR–Cas9 targeting accuracy. Nature 550(7676):407–410
Zhang T, Gao Y, Wang R, Zhao Y (2017) Production of guide RNAs in vitro and in vivo for CRISPR using ribozymes and RNA polymerase II promoters. Bio-Protoc 7(4):e2148-e
Kelley ML, Strezoska Ž, He K, Vermeulen A, van Brabant SA (2016) Versatility of chemically synthesized guide RNAs for CRISPR-Cas9 genome editing. J Biotechnol 233:74–83
Yang SH, Kim E, Park H, Koo Y (2022) Selection of the high efficient sgRNA for CRISPR-Cas9 to edit herbicide related genes, PDS, ALS, and EPSPS in tomato. Appl Biol Chem 65:13. https://doi.org/10.1186/s13765-022-00679-w
Yu Q, Han HP, Powles SB (2008) Mutations of the ALS gene endowing resistance to ALS-inhibiting herbicides in populations. Pest Manag Sci 64(12):1229–1236. https://doi.org/10.1002/ps.1624
Bates GW (1999) Plant transformation via protoplast electroporation. Methods Mol Biol 111:359–366. https://doi.org/10.1385/1-59259-583-9:359
Jones H, Ooms G, Jones MG (1989) Transient gene expression in electroporated Solanum protoplasts. Plant Mol Biol 13(5):503–511. https://doi.org/10.1007/BF00027310
Widholm JM, Dhir SK, Dhir S (1992) Production of transformed soybean plants by electroporation of protoplasts. Physiol Plantarum 85(2):357–361
Walker M, Dhir SK (2005) Electroporation mediated gene transfer in Stevia rebaudiana protoplasts. In Vitro Cell Dev-An 41:45a-a
Fish N, Karp A, Jones MG (1988) Production of somatic hybrids by electrofusion in Solanum. Theor Appl Genet 76(2):260–266. https://doi.org/10.1007/BF00257854
Matsunaga R, Sawamura K, De Kok M, Makino T, Miki K, Kojima M et al (1992) Electrotransfection of protoplasts from tomato, wild tomato, barley and chrysanthemum with tobacco mosaic virus RNA. J Gen Virol 73(Pt 4):763–766. https://doi.org/10.1099/0022-1317-73-4-763
Jeong YY, Lee HY, Kim SW, Noh YS, Seo PJ (2021) Optimization of protoplast regeneration in the model plant Arabidopsis thaliana. Plant Methods 17(1):21. https://doi.org/10.1186/s13007-021-00720-x
Shin SY, Choi Y, Kim SG, Park SJ, Park JS, Moon KB et al (2022) Submergence promotes auxin-induced callus formation through ethylene-mediated post-transcriptional control of auxin receptors. Mol Plant 15(12):1947–1961. https://doi.org/10.1016/j.molp.2022.11.001
Schaller GE, Bishopp A, Kieber JJ (2015) The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell 27(1):44–63. https://doi.org/10.1105/tpc.114.133595
Qin YL, Shu XC, Zhuang WB, Peng F, Wang Z (2017) High efficiency callus induction and regeneration of solanum torvum plants. HortScience 52(12):1755–1758. https://doi.org/10.21273/Hortsci12232-17
Moon KB, Park JS, Park SJ, Lee HJ, Cho HS, Min SR et al (2021) A more accessible, time-saving, and efficient method for in vitro plant regeneration from potato protoplasts. Plants 10(4):1–19. https://doi.org/10.3390/plants10040781
Sihachakr D, Ducreux G (1987) Cultural behavior of protoplasts from different organs of eggplant (Solanum melongena L.), and plant regeneration. Plant Cell, Tissue Organ Cult 11:179–188
Acknowledgements
Not applicable.
Funding
YK received funding from “Cooperative Research Program for Agriculture Science and Technology Development” of Rural Development Administration of Korea (Project No. PJ016902) and from Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry(IPET) through Agriculture, Food and Rural Affairs Convergence Technologies Program for Educating Creative Global Leader Program funded by Ministry of Agriculture, Food and Rural Affairs(MAFRA)(no.321001-03).
Author information
Authors and Affiliations
Contributions
Conducting experiment, SHY, SEK; Writing, SHY, SL, YK; Investigation, YK. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, S.H., Kim, S.W., Lee, S. et al. Optimized protocols for protoplast isolation, transfection, and regeneration in the Solanum genus for the CRISPR/Cas-mediated transgene-free genome editing. Appl Biol Chem 67, 21 (2024). https://doi.org/10.1186/s13765-024-00870-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-024-00870-1