Abstract
Acne is a highly prevalent skin disease with a great psychological impact on patients as self-perception, self-confidence, and depression. This work aimed to develop an anti-acne preparation from active anti-bacterial medicinal plants to circumvent the severe side effects and drug resistance commonly reported with topical erythromycin anti-acne preparations. Essential oils: Salvia officinalis L. (sage), Rosmarinus officinalis L. (rosemary), Commiphora myrrha Nees Engl. (myrrh), Origanum majorana L. (marjoram), Pelargonium zonale L. L’Hér. ex Aiton (geranium) and Chrysanthemum morifolium Ramat. (chrysanthemum) were extracted by hydrodistillation and analyzed using gas chromatography/mass spectrometry (GC/MS). The anti-acne activities of the oils against Cutibacterium acnes ATCC 6919 were evaluated by microdilution methods to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). The most active essential oils were loaded in a film-forming nanogel prepared with chitosan, pluronic F127 and glycerol in the ratio of 3:1:1, prior to investigation in a murine acne in vivo model. Marjoram and chrysanthemum oils showed the highest antimicrobial activity against C. acnes (MIC = 0.156% v/v and 0.125% v/v, respectively). GC/MS of the actives showed that gamma-terpinene (26.46%) and terpinen-4-ol (22.24%) were the predominant constituents in marjoram, whereas chrysanthenone (32.79%) was the main component in chrysanthemum. The formulated essential oil-loaded film-forming nanogels of both oils exhibited significant anti-acne activity in mice via reducing the bacterial loads, activating the antioxidant nuclear factor erythroid 2–related factor 2 (Nrf2) pathway and inhibiting the inflammatory tumor necrosis factor-alpha (TNF-α) pathway. Further studies should be designed to evaluate the clinical evidence for the use of marjoram and chrysanthemum oil products in acne treatment.
Graphical Abstract

Similar content being viewed by others
Introduction
Acne vulgaris is a cutaneous chronic inflammatory condition with multifaceted pathogenesis, affecting 80% of the teenage population [1]. It is believed that 100% of the population endures from a more or less severe form of acne [1]. Despite of not being a life-threatening disorder, acne leads to psychosocial consequences, affecting the quality of the patient’s life such as social isolation, low self-confidence and depression [2, 3]. Several factors play vital roles in acne pathophysiology including hyperseborrhea, follicular hyperkeratinization, inflammation and increased colonization of Cutibacterium acnes (C. acnes) (the primary pathogen involved in acne, previously known as Propionibacterium acnes) [3, 4].
Common anti-acne agents include antibiotics (such as tetracyclines; doxycycline & minocycline, macrolides; erythromycin, and clindamycin), benzoyl peroxide, retinoids, azelaic acid and hormonal agents (e.g. combination of oral contraceptive pills, spironolactone and flutamide) [5]. Clinical trials highlighted the uncertain safety profile of minocycline, isotretinoin’s teratogenicity and liver function abnormalities upon the use of systemic anti-acne agents in addition to various forms of cutaneous irritations (e.g., skin dryness, erythema and peeling) with topical anti-acne treatments [6]. Despite the common use of antibiotics as topical and oral anti-acne agents, increasing bacterial resistance to acne-causing strains is a limitation rendering them less and less effective [5]. In this aspect, C. acnes resistance to several antibiotics has been clinically reported in the last decades with high ranking in Mediterranean countries predominantly due to antibiotic abuse. This would also lead to the spreading of antibiotic resistance to other skin commensal bacterial species such as Staphylococcus epidermidis and Staphylococcus aureus via genetic transfer [7].
Thus, phytotherapeutic approaches have provoked global concerns nowadays, to minimize the side effects and higher costs of synthetic drugs. In this context, plant essential oils have attracted more attention due to their potential as antibacterial, antiseptic and anti-inflammatory agents [2, 8]. Tea tree oil is widely marketed over the counter as anti-acne treatment, being the most commonly used after benzoyl peroxide [9, 10]. Its clinical anti-acne efficacy has been proved via anti-bacterial and anti-inflammatory actions [9], overcoming resistance development by bacterial species towards conventional drugs owing to its variety of constituents [11].
In the current study, six commonly known anti-bacterial essential oils including sage, rosemary, myrrh, marjoram, geranium, and chrysanthemum were investigated against C. acnes as possible anti-acne agents. These essential oils are composed of a mixture of constituents such as monoterpenes, sesquiterpenes and their alcohols which could contribute to their antimicrobial activities [2, 8, 11, 12]. To circumvent the skin irritation that usually accompanies the application of essential oils, a nano-sized hydrogel has been formulated for their delivery. Nanogels prepared from naturally occurring polysaccharides, such as chitosan seem appealing due to their biocompatibility and functionality as well as their low toxicity [13]. Recently, film-forming nanogels have become an attractive delivery system due to their high flexibility, resistance to wearing off and sustained drug permeation. The reason behind this is their ability to overcome the common drawbacks of conventional films such as brittleness, limited shape and size and skin irritation [14]. Therefore, this study aimed to develop a safe phytopharmaceutical formulation with anti-acne activity. Based on the in vitro data of the six tested essential oils, marjoram and chrysanthemum oils were formulated as film-forming nanogels. Their anti-bacterial, antioxidant and anti-inflammatory activities were further investigated in vivo via an acne mice model, as potential new anti-acne agents.
Materials and methods
Plant material
Commiphora myrrha Nees Engl. gum-resin (Myrrh) was purchased from the local market of herbs and spices in Egypt (Ahmed Abdelrahman EL Harauz). Leaves during the flowering stage of Salvia officinalis L (sage), Rosmarinus officinalis L. (rosemary), Origanum majorana L. (marjoram), Pelargonium zonale (L.) L’Hér. ex Aiton (geranium) and Chrysanthemum morifolium Ramat. (chrysanthemum) were obtained from the Egyptian German Co. for Agricultural Production. The plants were kindly authenticated by Dr. Mohamed El-Gebali, Senior Botanist at El- Orman Botanical Garden. Voucher specimens (No 25.02.2022, 23.02.2022 I, 23.02.2022 II, 23.02.2022 III, 26.02.2022, 24.02.2022 II) were kept at the Herbarium of Pharmacognosy Department, Faculty of Pharmacy, Cairo University.
Materials for formulation
High molecular weight chitosan and pluronic F127 were procured from Sigma Aldrich Chemical Co. (St. Louis, USA). Glycerol was bought from El-Nasr Pharmaceutical Chemicals Co. (Cairo, Egypt).
Essential oils extraction
Different Samples (1 kg, each) were cut off and separately subjected to hydrodistillation for 2 h using a Clevenger-type apparatus and myrrh for 4 h. Oil samples were collected and dried over anhydrous sodium sulphate. The percentage yields were then calculated, and the samples were finally stored at 4 ℃ in completely sealed vials until subjected to further analysis. The percentage yield was calculated on a fresh weight basis (v/w).
Gas chromatography/mass spectrometry (GC/MS) analysis
The analysis was performed at the Department of Medicinal and Aromatic Plants Research, National Research Center (Giza, Egypt) on gas chromatography–mass spectrometry instrument with the following specifications: a TRACE GC Ultra Gas Chromatographs (THERMO Scientific Corp., USA), coupled with a thermo mass spectrometer detector (ISQ Single Quadrupole Mass Spectrometer). GC–MS system was equipped with a Tr-5MS column (30 m × 0.32 mm i.d., 0.25 μm film thickness). Analyses were carried out using helium as a carrier gas at a flow rate of 1.0 mL/min and a split ratio of 1:10 using the following temperature program: 60 ℃ for 2 min; rising at 4.0 ℃/min to 240 ℃ and held for 5 min. The injector and detector were held at 210 ℃. Diluted samples (1:10 hexane, v/v) of 1 μL of the mixtures were always injected. Mass spectra were obtained by electron ionization (EI) at 70 eV, using a spectral range of m/z 35–500.
Volatiles identification
Volatile components were identified by comparing their retention indices (RI) relative to a series of n-alkanes (C8-C28), mass spectrum matching to the National Institute of Standards and Technology (NIST) mass spectral library, Wiley Registry of Mass Spectral Data 8th edition and with standards whenever available and confirmed by reported literature data [15]. Peaks were first deconvoluted using AMDIS software (www.amdis.net) prior to mass spectral matching. All reagents and controls used for GLC analysis were obtained from Sigma (Sigma Aldrich GmbH, Sternheim, Germany) [16]. Literature survey of the chemical compositions of the different analyzed plants were compared with our profiles.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The antibacterial activity against Cutibacterium acnes ATCC 6919 was investigated. The minimum inhibitory concentration (MIC) was determined by the broth microdilution method according to the guidelines of the Clinical and Laboratory Standards Institute [17, 18]. 200 μL of double-strength reinforced clostridial medium (RCM) was pipetted into each well of a sterile 96-well microplate and 200 μL of the tested oils (64% v/v in DMSO) were then added separately to the first well of each column. Two-fold serial dilutions (32–0.0625% v/v) were done across the microplates, after that, each well was inoculated with 20 μL of bacterial suspension (1X107 CFU/mL). One column was used as a sterility control (neither bacterial suspension nor tested oil was added) and another column was used as a growth control (inoculated with bacterial suspension along with DMSO only but without the tested oil). The microplates were incubated under anaerobic conditions at 37 ℃ for 48 h and then the MIC was recorded. The MIC was defined as the lowest concentration with no detectable bacterial growth (no turbidity). All wells were spotted onto RCM agar plates and then incubated anaerobically at 37 ℃ for 48 h. The minimum bactericidal concentration (MBC) was defined as the lowest concentration of the tested oil with no detectable colonies of C. acnes. The experiment was repeated four independent times.
Preparation of film-forming nanogel
Essential oil-loaded film-forming nanogel was formulated as per the method stated by Xu et al. (2019) [19]. In detail, chitosan was dissolved in 1% (v/v) acetic acid to form chitosan solution at 1.5% (w/v) and pluronic F127 was dissolved in cold distilled water at a concentration of 3% (w/v) through magnetic stirring. Chitosan and pluronic F127 solutions along with glycerol were mixed in a ratio of 3:1:1, respectively. The mixture was kept on a magnetic stirrer for 30 min. Essential oil-loaded film-forming nanogel was prepared through the slow injection of either marjoram oil or chrysanthemum oil by a syringe in the film-forming nanogel solution, while kept on a magnetic stirrer for an additional 30 min, to reach a final concentration of 1.5% (v/v) in the preparation. The formulated film-forming nanogels were kept in tightly closed vials at 4 ℃ until use.
Characterization of film-forming nanogels
Measurement of particle size (PS), polydispersity index (PDI) and zeta potential (ZP)
The formed nanogels were characterized by Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) to determine the particle size (PS), particle size distribution (PDI) and zeta potential (ZP). All determinations were performed in triplicates and values were calculated as mean ± standard deviation (SD).
pH and viscosity measurements
The pH value of the formulated nanogels was determined via JENWAY model 350 (JENWAY Ltd., UK) at 30 ℃. The viscosity measurements of the formulated nanogels were carried out by a cone and plate rheometer (Brookfield DV3THB cone/plate rheometer, spindle CPE- 40, and RheocalcT software, v 1.1.13). A sample (1 g) of the formulated nanogels was added to the plate of the apparatus and the temperature was maintained constant at 25 ± 2 ℃. The formulation was exposed to continuous change in the shear rate and the shear stress, and the values were documented. The measurements were conducted in triplicates and values were calculated as mean ± standard deviation (SD).
Transmission electron microscopy
Transmission electron microscopy (TEM; JEM-1230, Jeol, Tokyo, Japan) was applied to visualize the morphology of the inspected formulations. A metallic grid was loaded with a drop of the tested oil samples and the surplus was withdrawn by filter paper, then the grid was left in the air to dry. Finally, the grid was examined via TEM at 80 kV.
Film-forming time
To determine the in vitro film-forming time, the formulations were placed onto a microscope slide in a defined area (10 mg/cm2), with a thickness of 1 mm. The samples were kept at 25 ℃. The film formation was monitored visually, and the film-drying time was documented.
To determine the in vivo film-forming time, the test was performed using rat skin, where skin samples of 1 mm thick and 1 × 1 cm2 area were excised from decapitated rats. The skin was wiped with absolute ethanol to allow the drying of the skin surface. The skin samples were then sited on a glass petri dish and incubated at 37 ℃. Finally, the formulation (10 mg) was spread over 1 cm2 of the skin sample, and the film-forming time on the skin was determined.
In vivo evaluation of the anti-acne activity of the tested oils
All the animal care and procedures in this study were approved by the Research Ethics Committee of the Faculty of Pharmacy, Cairo University (Approval no. MP 2790) following the “Guide for the Care and Use of Laboratory Animals” published by the Institute of Laboratory Animal Research (Washington, DC, USA). The in vivo acne murine model was done as described before with slight modifications [8, 20]. Thirty-five male BALB/C mice (7 weeks old) were obtained from the Modern Veterinary Office for Laboratory Animals, Cairo, Egypt. Standard commercial food and tap water ad libitum were supplied to the mice and they were allowed to acclimate for 5 days (25 ± 2 ℃, 12:12 h light–dark regime) before starting the experiment. All the mice’s ears were initially examined and only animals without any signs of ear inflammation were included in the study. Infection with Cutibacterium acnes was induced by intradermal injection of the left ear of each mouse with 20 μL of the bacterial suspension (3 × 108 CFU) in phosphate-buffered saline (PBS). A 30-gauge needle was used in the intradermal injection and a bulge containing the injected inoculum was formed post-injection. The right ear of each mouse was intradermally injected with 20 μL PBS and served as the healthy normal control (group 1) during the experiment. The mice were observed for 48 h post-infection and the appearance of microcomedones, erythema and oedema was considered an indicator of acne induction. After the infection, the infected mice were randomly divided into five groups (seven mice per group, n = 7). Group 2 didn’t receive any treatment and served as the negative control, while group 3 received the plain formula. Group 4 was treated with a 2% erythromycin solution and served as the positive control, whereas groups 5 and 6 were treated respectively with 1.5% (v/v) marjoram oil formula and 1.5% (v/v) chrysanthemum oil formula. Moreover, for the infected mice ears, treatments were applied epicutaneous for 3 days. At the end of the experiment, mice ears were excised, and each ear was homogenized in 0.5 mL PBS (homogenizer, DAIHAN-scientific-pacificlab). Samples were 10 folds diluted and plated on RCM agar plates for an anaerobic viable count. After anaerobic incubation at 37 ℃ for 72 h, the colony-forming units (CFU) were counted, and the results of the treated groups were compared to that of the plain formula (vehicle control) and negative control groups.
Histopathology
Skin tissue samples from each experimental group were collected and kept in 10% neutral buffered formalin, followed by processing protocol in alcohols, xylenes, and paraffin wax. Tissue sections were cut and stained with hematoxylin and eosin (H&E) for light microscopy [21]. Tissue slides were examined using Leica DM4 B light microscope (Leica, Germany) and images were captured using Leica DMC 4500 digital camera (Leica, Germany).
Immunohistochemistry
Tissue sections were cut on positively charged slides and subjected to heat retrieval step in microwave, followed by peroxidase blocking and incubated with primary anti-tumor necrosis factor-alpha (TNF-α) (Santa Cruz, Biotechnology, Inc.) and anti-nuclear factor erythroid 2–related factor 2 (Nrf2) (Protein tech, Germany) for 1 h at a dilution of 1:100. After washing, universal HRP detection kit (Bio SB, USA) was used to develop the color. The escape of primary antibody was done to obtain negative control slides. The positive reaction was quantified as area percentage using LAS-X (Leica software, Germany).
Statistical analysis
All experiments were carried out in triplicate. Statistical evaluation was carried out in GraphPad Prism, version 8. One-way ANOVA followed by multiple comparisons post-tests were used to determine significance. Specific tests used in comparisons were described in Figure legends.
Results and discussion
Acne is a chronic inflammatory disease, where its pathogenesis is very complex and multifactorial [1]. Anti-acne agents include drugs with anti-bacterial, anti-inflammatory, anti-seborrheic and anticomadogenic actions [2, 9]. Oxidative stress-initiated inflammation within the pilosebaceous unit, evident in acne vulgaris suggests also the efficacy of antioxidants in acne treatment [22]. Subsequently, the most effective treatments should tackle several pathogenetic mechanisms via combination therapy for agents with different pharmacological actions.
Due to the increasing antibiotic resistance of acne-causing strains, complementary and alternative medicines such as essential oils and their terpenes are the most popular choice with potent antimicrobial, anti-inflammatory and antioxidant properties [2]. Tea tree, eucalyptus, lemon, cinnamon, rosemary, myrtle, oregano and clove oils are used due to their antiseptic effects as well as basil, bergamot, thyme and lavender oils which are used for their antibacterial and anti-inflammatory actions. Essential oil components, present in these plants, as terpinen-4-ol, limonene, thymol, α and β-pinene, linalool, carvacrol and 1,8-cineole are responsible for those actions [2, 23]. In our study, we investigated the effectiveness of six antibacterial essential oils against C. acnes, followed by assessing the anti-acne efficacy of the potential candidates in vivo for their antibacterial, antioxidant and anti-inflammatory properties in relation to their chemical compositions.
Gas chromatography/mass spectrometry (GC/MS) analysis
The percentage yield of the investigated essential oils and identification of different components using GC/MS analysis are presented in Table 1. The percentage yield of the oils were calculated as 8.5%, 9%, 5%, 11%, 2%, and 4% (v/w) for sage, rosemary, myrrh, marjoram, geranium, and chrysanthemum, respectively. In regard to the chemical profiles of the investigated oils, the results revealed that the main components of sage oil were camphor and α-thujone, representing about 50% of the total essential oil composition, in accordance with Jakovljević and coworkers [24]. Whereas, the bicyclic monoterpene; α-pinene and oxygenated monoterpenes; camphor and borneol constituted more than 53% of the total essential oil of rosemary, in accordance with previously published data [25]. Furthermore, myrrh was rich in curzerene, furanoeudesma-1,3-diene and β-elemene altogether representing more than 60% of the total essential oil composition [26]. The volatile oil of marjoram was rich in monocyclic monoterpenes; γ-terpinene (26.46%) and α-terpinene (16.62%) and monoterpene alcohol; terpinen-4-ol (22.24%) [27, 28]. For geranium oil, monoterpene hydrocarbons was the predominant class, detected as limonene [29] (42.63%) and myrcene (19.74%), followed by the sesquiterpene hydrocarbons namely α-humulene (12.02%) and caryophyllene (6.66%). In contrast, a previous report on geranium leaves essential oil from Egypt stated β-caryophyllene and α-humulene as the major constituents [30]. Finally, chrysanthenone was the main constituent of chrysanthemum, representing about 32.79% of the total essential oil composition. In this context, previous researches on the essential oils of C. morifolium flowerheads showed that oxygenated monoterpenes were the predominant components, namely as chrysanthenone [31, 32]. This is the first report on the chemical composition of the essential oil of Chrysanthemum morifolium leaves from Egypt. Furthermore, GC–MS analysis of sage, rosemary, myrrh, marjoram, geranium, and chrysanthemum revealed the identification of 81 compounds, representing 99.99%, 99.94%, 95.45%, 99.65%, 98.06%, and 97.50%, respectively. Different classes contributed to the composition of the tested oils, such as mono and sesquiterpene hydrocarbons, as well as oxygenated mono and sesquiterpenes and other aliphatic alcohols, aldehydes, and esters constituents. Monoterpene hydrocarbons are the predominant class in bioactive marjoram oil, with a percentage of 69.60%, while oxygenated monoterpenes (73.52%) represent the major class in chrysanthemum oil.
Determination of MIC & MBC
Essential oils under investigation were tested for their in vitro antibacterial activity against C. acnes ATCC 6919. Among tested oils, marjoram and chrysanthemum oils exhibited the highest anti-bacterial activity against C. acnes (MIC < 0.2% v/v) (Table 2).
Meanwhile, sage, rosemary and myrrh oils revealed an intermediate anti-bacterial effect (MIC > 0.2% v/v) against the tested bacteria, whereas geranium oil showed no anti-bacterial activity (Table 2). In accordance with MIC results, sage, rosemary, and myrrh oils showed the highest MBC values (16–20% v/v). On the other hand, MBC results further confirmed the efficacy of marjoram and chrysanthemum oils as bactericidal at concentrations of 1.78 and 1.65% v/v, respectively (Table 2). No significant difference was found between the antibacterial activity (MIC / MBC) of marjoram and chrysanthemum oils (One way ANOVA, Tukey’s post-test, P < 0.05). Subsequently, these oils were selected for developing pharmaceutical formulations prior to further investigating their anti-acne activities in vivo.
Preparation and characterization of film-forming nanogels
The essential oils of marjoram and chrysanthemum were incorporated into a stable topical formulation aiming to develop a naturally derived pharmaceutical product for the treatment of acne. Chitosan and pluronic F127 are reliable gel forming polymers that act as highly applicable drug delivery carriers as per their nature, biocompatibility, biodegradability, and ability to encapsulate, carry and release the drug to the desired target flexibly. Figure 1 displays the charts of PS and ZP analysis of the plain nanogel, marjoram and chrysanthemum oils-loaded nanogels, where the measured PS were 550.5 ± 26.09, 632.5 ± 40.95, and 611.6 ± 32.67 nm, respectively.
The incorporation of oils led to a substantial rise in the PS (P < 0.05) due to their hydrophobic nature. PDI values of all formulations were less than 0.5 indicating homogenous PS distribution [36]. ZP indicates the overall surface charges, and correlates directly with the stability of the formulation; where the formulation is considered stable if the ZP value is around ± 30 mV as per the repulsion forces in between the particles [37]. The acquired ZP values were 43.4 ± 3.26, 46.3 ± 0.98 and 44.00 ± 1.01 mV, respectively. The observed high positive ZP values are due to the presence of positively charged chitosan and these large values indicate higher stability of the formulations. A highly acidic or highly basic pH value of formulations could alter the skin environment, which in turn can cause skin irritation. The pH values of the plain nanogel, marjoram and chrysanthemum oil-loaded nanogels were found to be 4.86 ± 0.02, 4.89 ± 0.02 and 4.85 ± 0.03, respectively.
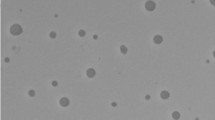
The pH value of all formulations was close to the normal pH of the skin, indicating the lack of potential skin irritation due to the difference in pH [38]. The measured viscosities of the plain nanogel, marjoram and chrysanthemum oils-loaded nanogels were 2,069 ± 53, 2,045 ± 61 and 1,997 ± 76 cp, respectively. These nanogels showed pseudoplastic flow (share thinning), which is optimum for the topical application of formulations. TEM micrograph revealed spherically shaped particles with a size smaller than that obtained from Zetasizer Nano ZS (Fig. 2).
The lesser size of the formulation from TEM microphotography could be accredited to the collapse of the nanogel during the processing of the TEM sample. The film-forming time of the plain nanogel, marjoram and chrysanthemum oils-loaded nanogels were 8.10 ± 0.80, 7.20 ± 0.50 and 7.10 ± 0.40 min (in vitro), and 7.30 ± 0.50, 6.40 ± 0.30 and 6.20 ± 0.20 min (ex vivo), respectively. In this situation, the formulation helps to overcome the undesirable effects related to skin irritation and allergic reaction regarding the topical use of the oils and to reduce the volatility as well. Due to the hydrophobicity of essential oils, the application of aqueous delivery systems is a patient-preferred alternative and also beneficial for anti-acnes activity since this bacterium colonizes the pilosebaceous units [39]. Consequently, our prepared film-forming nanogel, containing chitosan and pluronic F127, is considered a suitable water-based formulation for the incorporation of oils. Both chitosan and pluronic F127 are hydrophilic, biocompatible and biodegradable gel-forming materials with bio-adhesive capacity to support the drug retaining in the skin [39], and thus appear to be a promising approach for topical acne treatment. The concentration of polymers is crucial as it controls the drying time, viscosity, and visual appearance of the formed films. Higher polymer concentrations form a denser matrix and prolong the film-forming time, while lower concentrations failed to form films. Optimum polymer concentration gives a suitable viscosity that allows suitable formation time. Moreover, glycerol as a plasticizer led to the formation of a flexible dry film due to a lower glass transition temperature (Tg) and boosted mobility of polymer chains in the matrix [40]. The presence of essential oils resulted in a significantly (P < 0.05) shorter film-forming time, and this could be attributed to the hydrophobicity and volatility of essential oils, which allowed faster evaporation of the solvent and hence, faster film formation, in addition to the measured lower viscosities.
In vivo evaluation of the anti-acne activity
To assess the anti-acne efficacies of marjoram and chrysanthemum oils-loaded nanogels, their effect on bacterial load and histopathological analysis of skin tissues along with their antioxidant and anti-inflammatory properties were evaluated in a murine acne infection model.
Topical treatment with formulations containing either marjoram oil or chrysanthemum oil significantly reduced the bacterial load of C. acnes, compared to the negative control group and the plain formula group (P < 0.0001) (Fig. 3).
Efficacy of the tested oils in in vivo murine acne infection model. Thirty-five BALB/C mice divided into five groups (n = 7), four treated groups; groups 3–6 (plain formula, erythromycin, chrysanthemum oil formula and marjoram oil formula) and the fifth group; group 2 remained untreated as the negative control. A Photo image of the ears of the mice at the end of the experiment. B The box plot shows the bacterial load in murine acne infection model at the end of the experiment. **** Indicates that the difference is significant at P < 0.0001 (One Way ANOVA, Bonferroni's post-test)
No significant difference was observed between the bacterial load recovered from the vehicle control and the plain formula groups (P < 0.0001). The bacterial count recovered from mice ears topically treated with marjoram or chrysanthemum oils- loaded nanogels were 1.817 and 2.566 logs lower than that of the plain formula group as well as 2.398 and 3.147 logs lower than that of the negative control group, respectively. Interestingly, the tested nanogels revealed no significant difference from the positive control group (erythromycin) in reducing the bacterial load in acne-infected mice ears. Chrysanthemum formulation exhibited the most reduction in bacterial counts, followed by erythromycin and marjoram, respectively. The in vivo anti-acne activity of both investigated oils against Cutibacterium acnes ATCC 6919 was reported herein for the first time. Although testing the antimicrobial activity of the oils in vitro is essential for screening their possible biological applications, it is not enough to predict the actual in vivo activity. The in vivo model is a dynamic and multifactorial process where many factors contribute to the net oil activity not just the physicochemical properties of the applied formula. These biological factors include skin permeability, body temperature, physiology, and biochemical skin structure. Hence conducting an in vivo model and reporting the actual in vivo activity of the tested oils is an essential and crucial step before adopting clinical applications.
Marjoram oil has a wide spectrum of antimicrobial activity, where terpinen-4-ol, γ-terpinene, α-terpinene and α-terpineol are the major constituents, and those mainly responsible for its antimicrobial and anti-acne activities [41, 42]. It is worth highlighting that our profile of marjoram oil showed a certain similarity with the major constituents in tea tree oil, and this clarifies the promising activity of the oil as an anti-acne [39].
Regarding chrysanthemum, the essential oil of C. morifolium flowers exhibited potent antibacterial activity against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella enteritis and Bacillus subtilis [43]. Moreover, a recent report documented the bacteriostatic effect of the essential oils from C. morifolium different parts (flower heads, stems-leaves and roots) against Propionibacterium acnes, evaluated by the microdilution method [44]. To note, α-pinene, camphor, terpinen-4-ol, borneol, caryophyllene, α-humulene and δ-cadinene, present in the currently investigated oil, were found to be active against P. acnes [23, 45, 46]. In addition, borneol, detected at high percentage (15%) in our sample, enhanced the healing of acne via its anti-inflammatory effects [47].
Histopathological examination of skin sections of normal control group revealed normal structure of epidermis and dermis, appearing free from inflammation. Photographic pictures of the negative control group showed marked expansion of dermis with inflammatory edema. Numerous inflammatory cells occupied the dermis especially neutrophils together with many inflammatory cells invading both adnexa and the epidermis, whereas the skin surface was covered by sero-cellular crust. Similarly, many inflammatory cells infiltrated the dermis in infected mice receiving the plain formula. Erythromycin-treated mice exhibited moderate improvement where inflammation was greatly reduced. A marked improvement was noticed in acne-infected mice treated with chrysanthemum and marjoram essential oils-loaded nanogels, where most of the examined sections were apparently normal with existence of few inflammatory cells’ infiltration in some instances (Fig. 4).
Photomicrograph of skin (H&E). a Group 1 (Normal control) showing normal skin, b group 2 (Negative control): exhibiting marked inflammatory reaction (black arrows) and sero-cellular crust (red arrow), c group 3 (Plain Formula): intense inflammation in the dermis (arrow), d group 4 (Erythromycin): apparently normal skin, e group 5 (Chrysanthemum oil formula): apparently normal skin, f group 6 (Marjoram oil formula): mild focal inflammatory cells aggregation (black star)
Being a multifactorial disease, several factors affect each other in the acne process. In this aspect, excessive sebum together with increased keratinization create a favorable media for P. acnes colonization, which in turn releases chemotactic substances that induce the generation of ROS, finally leading to inflammation marked by redness, swelling and pain [48]. The oxidative stress also creates a perfect environment for more colonization of such bacterium species [22]. As documented via several studies, acne patients are under increased oxidative stress, compared to healthy individuals [22]. Additionally P. acnes induces the production of pro-inflammatory cytokines such as TNF-α, IL-12 and IL-8 via triggering toll-like receptors; TLR2 and TLR4 on monocytes and keratinocytes, thus further contributing to the inflammatory reactions [49, 50].
NRF2 is a basic leucine zipper (bZIP) protein, which is released in response to oxidative stress leading to increased expressions of antioxidant genes and other several target genes that control cell proliferation, differentiation and inflammation [51]. Consequently, natural compounds that induce Nrf2 could be excellent candidates in reducing oxidative stress in patients with acne [22]. Following P. acnes stimulation (groups 2 and 3), Nrf2 expression was increased in response to oxidative stress, compared to normal mice group (Figs. 5 and 6).
Photomicrograph of skin (Immune staining- Nrf2) a group 1 (Normal control): mild expression, b group 2 (Negative control) and c group 3 (Plain Formula) showing increased positive staining, d group 4 (Erythromycin)and e group 5 showing marked elevation in Nrf2, f group 6: intense positive expression. Group 1 (normal control), group 2 (negative control), group 3 (plain formula), group 4 (positive control, erythromycin), group 5 (chrysanthemum oil formula) and group 6 (marjoram oil formula)
Chart represents Nrf2 expression as area percentage; data are presented as mean ± SEM. Group 1 (normal control), group 2 (negative control), group 3 (plain formula), group 4 (positive control, erythromycin), group 5 (chrysanthemum oil formula) and group 6 (marjoram oil formula). (One way ANOVA, Tukey’s post-test, P < 0.05)
These increments were significantly enhanced by the different used treatments, in agreement with previous report [52]. Thus, the formulated oils alleviated P. acnes-induced oxidative stress via Nrf2 pathway [52] and exhibited more significant effects than the positive control group (erythromycin). Moreover, marked increase in TNF-α expression was noticed in acne-infected group, compared to other experimental groups (Figs. 7 and 8).
Photomicrograph of skin (Immune staining- TNF-α) a group 1: mild limited expression, b group 2 and c group 3 showing increased positive staining, d group 4 and e group 5 showing marked reduction in TNF-α, f group 6: moderate positive expression. Group 1 (normal control), group 2 (negative control), group 3 (plain formula), group 4 (positive control, erythromycin), group 5 (chrysanthemum oil formula) and group 6 (marjoram oil formula)
Chart represents TNF-α expression as area percentage; data are presented as mean ± SEM. Group 1 (normal control), group 2 (negative control), group 3 (plain formula), group 4 (positive control, erythromycin), group 5 (chrysanthemum oil formula) and group 6 (marjoram oil formula). (One way ANOVA, Tukey’s post-test, P < 0.05)
All treated groups showed significant decline in TNF-α expression, compared to acne-infected mice, highlighting their significant anti-inflammatory effect. The most reduction was observed in erythromycin group, followed by marjoram and chrysanthemum oil-loaded nanogels groups. These findings match with the previously reported anti-inflammatory effect of erythromycin [53].
In this context, the antimicrobial, anti-inflammatory and antioxidant effects of α-terpineol [54,55,56], γ-terpinene [57, 58], α-terpinene [56, 59] and terpinen-4-ol [56, 60], predominant constituents in marjoram oil, were previously reported explaining the effectiveness of marjoram oil application for acne treatment. Furthermore, chrysanthemum essential oil demonstrated anti-inflammatory activity via reducing the pro-inflammatory cytokine; IL-1β in P. acnes-induced THP-1 cells [44], in addition to its potent antioxidant activity [32]. In our investigated sample, chrysanthenone was the predominant constituent, yet nothing was traced on its potential as antiacne agent, thus its exploration is worth our attention.
Collectively, the topical application of marjoram or chrysanthemum nanogels attenuated Cutibacterium acnes via multiple pharmacological actions viz., antibacterial, antioxidant and anti-inflammatory, compared to erythromycin. Therefore, these oils might be outstanding complementary treatments for acne after further clinical studies.
Data availability
All data are available through the manuscript or its supplementary file.
References
Fox L, Csongradi C, Aucamp M, Du Plessis J, Gerber M (2016) Treatment modalities for acne. Molecules 21:1063
Nurzyńska-Wierdak R, Pietrasik D, Walasek-Janusz M (2022) Essential oils in the treatment of various types of acne—a review. Plants 12:90
Vedamurthy M. Topical anti-acne agents. Essentials for aesthetic dermatology in ethnic skin: practice and procedure. 2023.
Cong T-X, Hao D, Wen X, Li X-H, He G, Jiang X (2019) From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res 311:337–349
Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS et al (2016) Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 74(945–73):e33
Tripathi SV, Gustafson CJ, Huang KE, Feldman SR (2013) Side effects of common acne treatments. Expert Opin Drug Saf 12:39–51
Alkhawaja E, Hammadi S, Abdelmalek M, Mahasneh N, Alkhawaja B, Abdelmalek SM (2020) Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol 20:1–9
Taleb MH, Abdeltawab NF, Shamma RN, Abdelgayed SS, Mohamed SS, Farag MA et al (2018) Origanum vulgare L. essential oil as a potential anti-acne topical nanoemulsion—In vitro and in vivo study. Molecules 23:2164
Hammer K (2015) Treatment of acne with tea tree oil (melaleuca) products: a review of efficacy, tolerability and potential modes of action. Int J Antimicrob Agents 45:106–110
Kairey L, Agnew T, Bowles EJ, Barkla BJ, Wardle J, Lauche R (2023) Efficacy and safety of Melaleuca alternifolia (tea tree) oil for human health—A systematic review of randomized controlled trials. Front Pharmacol 14:1116077
Yadav E, Kumar S, Mahant S, Khatkar S, Rao R (2017) Tea tree oil: a promising essential oil. J Essent Oil Res 29:201–213
Nasser WS, Mikhail MW, Issa MY, Abdel-Sattar E (2022) Effect of selected essential oils against Zoonoses and their epidemiological survey among domestic rodents in Egypt. Bull Fac Pharm Cairo Univ 60:4
Luckanagul JA, Pitakchatwong C, Bhuket PRN, Muangnoi C, Rojsitthisak P, Chirachanchai S et al (2018) Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohyd Polym 181:1119–1127
Kim DW, Kim KS, Seo YG, Lee B-J, Park YJ, Youn YS et al (2015) Novel sodium fusidate-loaded film-forming hydrogel with easy application and excellent wound healing. Int J Pharm 495:67–74
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation, Carol Stream
Farag MA, Ramadan NS, Shorbagi M, Farag N, Gad HA (2022) Profiling of primary metabolites and volatiles in apricot (Prunus armeniaca L.) seed kernels and fruits in the context of its different cultivars and soil type as analyzed using chemometric tools. Foods 11:1339
Humphries RM, Ambler J, Mitchell SL, Castanheira M, Dingle T, Hindler JA et al (2018) CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol 56:e01934-e2017
Salem MA, El-Shiekh RA, Hashem RA, Hassan M (2021) In vivo antibacterial activity of star anise (Illicium verum Hook.) extract using murine MRSA skin infection model in relation to its metabolite profile. Infect Drug Res 14:33
Xu T, Gao C, Feng X, Huang M, Yang Y, Shen X et al (2019) Cinnamon and clove essential oils to improve physical, thermal and antimicrobial properties of chitosan-gum arabic polyelectrolyte complexed films. Carbohyd Polym 217:116–125
Luo H, Lv X-D, Wang G-E, Li Y-F, Kurihara H, He R-R (2014) Anti-inflammatory effects of anthocyanins-rich extract from bilberry (Vaccinium myrtillus L.) on croton oil-induced ear edema and Propionibacterium acnes plus LPS-induced liver damage in mice. Int J Food Sci Nutr 65:594–601
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier Health Sciences, Amsterdam
Wong A, Zhang B, Jiang M, Gong E, Zhang Y, Lee S (2016) Oxidative stress in acne vulgaris. J Clin Dermatol Ther 3:1–6
Kubo I, Muroi H, Kubo A (1994) Naturally occurring antiacne agents. J Nat Prod 57:9–17
Jakovljević M, Jokić S, Molnar M, Jašić M, Babić J, Jukić H et al (2019) Bioactive profile of various Salvia officinalis L. preparations. Plants 8:55
Jordán MJ, Lax V, Rota MC, Lorán S, Sotomayor JA (2013) Effect of bioclimatic area on the essential oil composition and antibacterial activity of Rosmarinus officinalis L. Food Control 30:463–468
Hanuš LO, Řezanka T, Dembitsky VM, Moussaieff A (2005) Myrrh-commiphora chemistry. Biomed Pap 149:3–28
Lis A, Piter S, Gora J (2007) A comparative study on the content and chemical composition of essential oils in commercial aromatic seasonings. Herba polonica 53:21–26
Mossa A, Nawwar G (2011) Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Human Exp Toxicol 30:1501–1513
Mohammed S, Babeanu N, Cornea CP, Radu N (2022) Limonene—A biomolecule with potential applications in regenerative medicine. Sci Bulletin Ser F Biotechnol 26:139–148
Koheil M, Khalek SA, El-Hefnawy H, El-Deen AS, Haleem MA (2012) Composition and antimicrobial activity of the essential oil of Pelargonium zonale L. from Egypt. J Biol Act Prod Nat 2:178–185
Hodaei M, Rahimmalek M, Arzani A (2017) Variation in morphological characters, chemical composition, and anthocyanin content of different Chrysanthemum morifolium cultivars from Iran. Biochem Syst Ecol 74:1–10
Luong T-h, Trinh T-n, Han V-c, Jung W-j (2022) Chemical properties and antioxidant activity of essential oils of Chrysanthemum morifolium Ramat. and Chrysanthemum indicum L. in Vietnam. J Appl Biol Chem 65:367–374
Adams RP (2017) Identification of essential oil components by gas chromatography/mass spectrometry, 5th edn. Texensis Publishing, Gruver
NIST Standard Reference Data. https://webbook.nist.gov/chemistry/. Accessed 15 Jul 2023.
Chang R, de Morais SA, Napolitano DR, Duarte KC, Guzman VB, Nascimento EAD (2011) A new approach for quantifying furanodiene and curzerene: a case study on the essential oils of Eugenia uniflora L., Myrtaceae (pitangueira) leaves. Rev Bras 21:392–396
Rehman S, Nabi B, Baboota S, Ali J (2021) Tailoring lipid nanoconstructs for the oral delivery of paliperidone: Formulation, optimization and in vitro evaluation. Chem Phys Lipid 234:105005
Naguib MJ, Hassan YR, Abd-Elsalam WH (2021) 3D printed ocusert laden with ultra-fluidic glycerosomes of ganciclovir for the management of ocular cytomegalovirus retinitis. Int J Pharm 607:121010
Das B, Nayak AK, Nanda U (2013) Topical gels of lidocaine HCl using cashew gum and Carbopol 940: preparation and in vitro skin permeation. Int J Biol Macromol 62:514–517
da Silva NP, Pereira EdCRL, Duarte LM, de Oliveira Freitas JC, de Almeida CG, da Silva TP et al (2020) Improved anti-Cutibacterium acnes activity of tea tree oil-loaded chitosan-poly (ε-caprolactone) core-shell nanocapsules. Coll Surf B: Biointerfaces 196:111371
Ngo HV, Tran PH, Lee B-J, Tran TT (2019) Development of film-forming gel containing nanoparticles for transdermal drug delivery. Nanotechnology 30:415102
Sellami IH, Maamouri E, Chahed T, Wannes WA, Kchouk ME, Marzouk B (2009) Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind Crops Prod 30:395–402
Raouafi K, Nefzi H, Esghaier B, Sadfi N, Abderrabba M, Sameh AS (2021) Biological activity and characterization of essential oil of areal part from Origanum majorana L: first report of antifungal activity against Fusarium oxysporum and against his biofilm. J Mater Environ Sci 12:746–756
Kuang C-l, Lv D, Shen G-H, Li S-S, Luo Q-Y, Zhang Z-Q (2018) Chemical composition and antimicrobial activities of volatile oil extracted from Chrysanthemum morifolium Ramat. J Food Sci Technol 55:2786–2794
Liu X-J, Li Y, Su S-L, Wei D-D, Yan H, Guo S et al (2022) Comparative analysis of chemical composition and antibacterial and anti-inflammatory activities of the essential oils from chrysanthemum morifolium of different flowering stages and different parts. Evid-Based Complement Altern Med 2022: 5954963.
Sinha P, Srivastava S, Mishra N, Yadav NP (2014) New perspectives on antiacne plant drugs: contribution to modern therapeutics. BioMed Res int 2014:301304.
Zhu J, Lower-Nedza AD, Hong M, Jiec S, Wang Z, Yingmao D et al (2013) Chemical composition and antimicrobial activity of three essential oils from Curcuma wenyujin. Nat Prod Commun 8:1934578X1300800430
Ji J, Zhang R, Li H, Zhu J, Pan Y, Guo Q (2020) Analgesic and anti-inflammatory effects and mechanism of action of borneol on photodynamic therapy of acne. Environ Toxicol Pharmacol 75:103329
Jaffri JM (2023) Reactive oxygen species and antioxidant system in selected skin disorders. Malays J Med Sci MJMS 30:7
Pretsch A, Nagl M, Schwendinger K, Kreiseder B, Wiederstein M, Pretsch D et al (2014) Antimicrobial and anti-inflammatory activities of endophytic fungi Talaromyces wortmannii extracts against acne-inducing bacteria. PLoS ONE 9:e97929
Soleymani S, Farzaei MH, Zargaran A, Niknam S, Rahimi R (2020) Promising plant-derived secondary metabolites for treatment of acne vulgaris: a mechanistic review. Arch Dermatol Res 312:5–23
Kaspar JW, Niture SK, Jaiswal AK (2009) Nrf 2: INrf2 (Keap1) signaling in oxidative stress. Free Radical Biol Med 47:1304–1309
Zhu T, Fang F, Sun D, Yang S, Zhang X, Yu X et al (2020) Piceatannol inhibits P. acnes–induced keratinocyte proliferation and migration by downregulating oxidative stress and the inflammatory response. Inflammation 43:347–357
Erythromycin BW, Factor ITN (1998) Erythromycin inhibits tumor necrosis factor. Antimicrob Agents Chemother 42:1605
Held S, Schieberle P, Somoza V (2007) Characterization of α-terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells. J Agric Food Chem 55:8040–8046
Bicas J, Neri-Numa I, Ruiz A, De Carvalho J, Pastore G (2011) Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem Toxicol 49:1610–1615
Lee C-J, Chen L-W, Chen L-G, Chang T-L, Huang C-W, Huang M-C et al (2013) Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J Food Drug Anal 21:169–176
Ramalho TR, Filgueiras LR, de Oliveira MTP, de Araujo Lima AL, Bezerra-Santos CR, Jancar S et al (2016) Gamma-terpinene modulation of LPS-stimulated macrophages is dependent on the PGE2/IL-10 axis. Planta Med 82:1341–1345
Guo Y, Baschieri A, Amorati R, Valgimigli L (2021) Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chem 345:128468
Rudbäck J, Bergström MA, Börje A, Nilsson U, Karlberg A-T (2012) α-Terpinene, an antioxidant in tea tree oil, autoxidizes rapidly to skin allergens on air exposure. Chem Res Toxicol 25:713–721
Badr MM, Taktak NE, Badawy ME (2022) Comparison of the antimicrobial and antioxidant activities of tea tree (Melaleuca alternifolia) oil and its main component terpinen-4-ol with their nanoemulsions. Egypt J Chem 6:111–120. https://doi.org/10.21608/ejchem.2022.131758.5808
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
EAK; methodology, data analysis, and writing-original draft preparation. RAE; data analysis, investigation, and writing-original draft preparation. MH; data analysis, investigation, methodology, and writing review and editing. WHA; data analysis, investigation, and writing-original draft preparation. NE; Conceptualization and writing review and editing. ASE; Conceptualization, methodology, data analysis, investigation, writing-original draft preparation, and review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The protocol for this study was approved by the Research Ethics Committee of the Faculty of Pharmacy, Cairo University (Approval no. MP 2790) following the “Guide for the Care and Use of Laboratory Animals” published by the Institute of Laboratory Animal Research (Washington, DC, USA).
Competing interests
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kotb, E.A., El-Shiekh, R.A., Hassan, M. et al. Potential anti-acne loaded nanogel formulations of Origanum majorana L. and Chrysanthemum morifolium Ramat. essential oils. Appl Biol Chem 67, 9 (2024). https://doi.org/10.1186/s13765-024-00859-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-024-00859-w