Abstract
The present study was planned to investigate the possible therapeutic effects of silver/hydroxyapatite nanocomposite (nAg/HAp) on neurotoxicity induced by cadmium chloride (CdCl2) in albino rats. The nanocomposite has been formulated by a chemical route and characterized by scanning electron microscope (SEM), Transmission Electron Microscopy (TEM), and energy-dispersive X-ray Analysis spectroscopy (EDAX). A population of rats was randomly assorted into three groups; the animals were subjected to intraperitoneal CdCl2 administration every 2 days at a dose level of 1.0 mg/kg b.wt. for 3 months while the treatment with nAg/HAp was performed via intravenous injection at a dose level of 50 mg/kg b,wt. once a week for 4 weeks. Quantitative DNA fragmentation and biochemical analysis including the content of γ-aminobutyric acid (GABA), noradrenaline (NA), dopamine (DA), caspase-3, calmodulin (CaM), calcium adenosine 5′-triphosphatase (Ca++ATPase), tau protein, glutathione (GSH) and malondialdehyde (MDA) were measured in brain tissue. The results revealed the potent efficacy of nAg/HAp in attenuating DNA fragmentation and partially recovering most of the investigated parameters manifested by a significant elevation in GABA, NA, DA, Ca++ATPase, and GSH levels and a decrease in tau protein, caspase-3, CaM and MDA tissue content in comparison with Cd—intoxicated groups. Accordingly, the synthesized nAg/HAp at the selected dose can be used as a biosafe intravenous injection in neurodegenerative diseases.
Similar content being viewed by others
Introduction
Nano-silver (nAg) is one of the most important nanoparticles in biomedical applications because it has distinctive physicochemical properties [/]. Nevertheless, the clinical use of nAg is limited due to the cytotoxic effect as demonstrated previously in in-vitro studies [2]. Most in-vitro investigations are based on cellular short-term animal experiments that are drastically different from in vivo conditions and the concentration of nAg used is not relevant for real-life situations and does not exceed the toxic level [3]. In biological media, the surface of nAg is oxidized and releases Ag+ ions which have a strong affinity to interact with sulfur-containing macromolecules and induce apoptosis mediated ROS and mitochondrial pathway [4]. To avoid this disadvantage, the nAg formulation must be supported on the surface of substrates [5]. Thus, research efforts focused on the preparation of nAg by various methods to obtain nanocomposite with new physical and chemical strategies appropriate for practical use [6,7,8,9]. However, nanocomposite of Ag and HAp (nAg/HAp) have potential medical applications because nHAp is an inorganic component of hard tissue and has better bioactivity and nAg has antimicrobial properties [10, 11].
The brain is the most important and complex organ in the human body that controls, regulates and coordinates actions and reactions. The brain sends and receives and also interprets the chemical and electrical signals throughout the body that control different processes. The injury of the brain may lead to disrupt a particular step of the vital activity. Brain contains the highest quantity and diversity of plasma membrane Ca2+ATPase isoforms, which involves in many neuronal functions of Ca2+ and preserve its homeostasis to avoid cell damage [12]. Calcium (Ca2+) is a vital element in the process of neurotransmitter release that helps to transmit depolarization status and synaptic activity to the biochemical machinery of a neuron. The process of Ca2+ synaptic activity implicates key protein effectors such as calmodulin. Calmodulin is a Ca2+-binding protein and has a presynaptic modulator of synaptic transmission function [13]. Under conditions, Synaptic vesicle and synaptic membrane interactions were mediated by Ca2+ and calmodulin that simultaneously stimulated neurotransmitter release [14].
Cadmium (Cd) is one of the most health-hazardous elements among the toxic heavy metals and has been classified as a human carcinogen [15]. There are several sources of human exposure to Cd, including employment in the primary metal industries, production of certain batteries, some electroplating processes, and consumption of tobacco products [16]. The biological half-life of Cd reaches to 30 years [17]. After Cd absorption, about 30% deposits in the liver (half time ranged between 4 and 19 years) and 30% in the kidneys (half time ranged between 6 and 38 years), while the rest distributes throughout the body [18]. However, one of the most dangerous properties of Cd is its ability to penetrate the blood–brain barrier (BBB) and interfere with the conformation of the functional and structural neural cells resulting in degeneration of neurons, impairment of the synaptic transmission, and behavioral changes. The toxic effect of Cd is ascribed the induction of lipid peroxidation consequent by the generation of various types of free radicals and disability of cellular antioxidant defense mechanism in the brain [15].
To address the safety issues of nAg/HAp in vivo applications based nano-medicine, the present work aimed to formulate a bio-safe Ag/HAp nanocomposite by the simple method to inspect the efficacy of its intravenous injection against cadmium exposure induced neurotoxicity in albino rats. To achieve this aim, DNA damage (Comet assay) and biochemical analyses related to brain function and oxidant/antioxidant status were performed in the brain tissues.
Materials and methods
Chemicals
The preparation of Ag/HAp nanocomposite was performed using the following pure chemicals and reagents: silver nitrate (AgNO3, Mwt 169.88 g/mole, Johnson Matthey), ammonium hydroxide (NH4OH, Mwt. 35.5 g/mole, May & Baker, England), polyvinyl alcohol (PVAL) (Mwt≈160,000 g/mole), anhydrous diammonium hydrogen orthophosphate (NH4)2HPO4, 132.06 g/mole, S.D. Fine Chem. Ltd. Mumbai), calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, Mwt. 236.15 g/mole, Merk, Germany), polyvinylpyrrolidone (PVP), and sodium hydroxide (NaOH). Deionized water was used in preparing the solutions. Lead nitrate was used as a solution in 0.9% saline. All chemicals as mentioned from its source purchased from Alpha Aromatic Company as a chemical supplier in Cairo Egypt.
Preparation of nAg/HAp
Nano-silver is synthesized in nHAp structure to inhibit its toxicity by controlling the release of Ag ions. Nano Ag was prepared chelated by nHAp in the polymeric matrix route. HAp stands out because it is similar in structure and chemical composition to the mineral of hard tissue in the body. Other factors such as ionic strength, pH, and the presence/absence of other salts were utilized to produce an advanced structure of nAg supported on the surface of nAg/Hap with developed characteristics. Polyvinyl alcohol was dissolved in 500 ml of warm deionized water at 70 °C a free complete evolved. Calcium nitrate was added to 0.005 gm of silver nitrate (1/5 LD50), then ammonium hydrogen ortho phosphate was added with molar ratio 1.67 to calcium nitrate under PH control. After the addition of ammonium orthophosphate, the crystal structure of HAp was formed and trapped Ag ions. The formed gel was filtered and dried at 80 °C for 24 h.
Characterization of nAg/HAp
Sample of the nAg/HAp product characterized by using Energy-dispersive X-ray analysis spectroscopy (EDAX) using EDAX (Ametek), High-resolution transmission electron microscope (TEM) JEOL2100 and Scanning Electron Microscopy (SEM) on Philips XL30 instrument made in Holland.
Pharmacological study of acute toxicity
Determination of acute toxicity for intravenous treatment with nAg/HAp was carried out using the method previously published by Lorke [19]. Eighteen rats that weighted 140–150 gm were equally divided into six groups. They were injected intravenously with different doses of nAg/HAp (10, 30, 50, 90,120, and 150 mg/kg b.wt.). Mortality was recorded for 24 h and the final LD50 value was determined from the minimum concentration (full death) and maximum concentration (no death) of the dose according to the coming relation:
where: M0 = Highest dose of substance at which no mortality, M1 = Lowest dose of substance at which mortality.
A preliminary study using nano-HAp to treat brain damage
Several experiments were carried out to evaluate the pathophysiological features of the brain in rats intoxicated by CdCl2 before and after treatment with nAg/HAp. The selected dose of nAg/HAp was examined by the intravenous injection at different time intervals in either a single dose or a fractionated dose to detect the optimal therapeutic results. The structural and functional changes in the brain were investigated including DNA fragmentation, neurotransmitters, and oxidation status. Based on the obtained results and consistent with the previous study by Abdel-Gawad et al. [11], the divided dose of 200 mg/kg b.w. nAg/HAp was applied in the present experiment.
Animals and treatment schedule
Twenty-four male albino rats (140 ± 20 g body weight) were housed in standard laboratory conditions (12 h. dark/light cycle), a temperature of 25 °C and suitable humidity. Animals were provided with standard food and water ad libitum for at least one week before the experiment. Rats were assorted into three groups (8 rats/each). Group 1: normal control rats. Group2: rats were received intraperitoneal (i.p.) injection of CdCl2 solution at a dose level of 1.0 mg/kg b.wt. 3 times a week for three months [20]. Group3: rats were received i.p. injection of CdCl2 with the same conditions as those of group 2, and then injected intravenously with nAg/HAp at a dose level of 50 mg/Kg bwt. once a week for 4 weeks [11] on the next day of the last dose of CdCl2.
On the next day of the last injection of nAg/HAp, the animals were euthanized by diethyl ether and sacrificed by cervical decapitation. The brains were separated carefully by making a midline incision to view the skull. A small incision from the caudal part of the parietal bone and a firm cut in the anterior part of the frontal bone was made to remove the brain more easily. The isolated brain tissues were immediately taken out and washed with ice-cold saline to remove the excess blood and they were stored at − 80 °C, until later analysis.
Preparation of brain homogenate
The whole brain was homogenized in ice-cold phosphate buffer solution (PBS; pH 7.4). The volume of the buffer was depended on the weight of the tissue and usually kept at 10% (brain mass: the buffer volume). The homogenate was centrifuged at 4000 rpm for 20 min at 4 °C. The clear supernatant was separated for biochemical assays. All the processes were carried out in cold conditions.
DNA fragmentation
Brain DNA damage was determined by a single-cell gel electrophoresis (comet) assay according to the method previously published by Singh et al. [21]. A 0.5 g of crushed brain sample was transferred to 1 mL ice-cold phosphate buffer saline (PBS). The suspension was stirred for 5 min then filtered. Cell suspension (100 μL) was mixed with 600 μL of low-melting agarose (0.8% in PBS). 100 μL of this mixture was spread on pre-coated slides, which were immersed in lyses buffer (0.045 M TBE, pH 8.4, containing 2.5% SDS) for 15 min. The slides were placed in an electrophoresis chamber containing the same TBE buffer, but devoid of SDS. The electrophoresis conditions were 2 V/cm and 100 mA for 2 min. Staining was made with Ethidium bromide (EtBr) 20 μg/mL at 4 °C. The observation was reported while the samples are still humid, the DNA fragment migration patterns of 100 cells for each dose level were evaluated with a fluorescence microscope (With excitation filter 420–490 nm (issue 510 nm). For visualization of DNA damage, observations were made of EtBr-stained DNA using a 40 × objective on a fluorescent microscope. The comets tails lengths were measured from the middle of the nucleus to the end of the tail.
Comet capture and analysis
A total of 100 randomly captured comets from each slide were examined at 40 ×magnification using a fluorescence microscope connected to a CCD camera using an image analysis system [Comet 5 image analysis software developed by Kinetic Imaging Ltd. Liverpool, UK]. A computerized image analysis system acquires images, computes the integrated intensity profiles for each cell, estimates the comet cell components, and then evaluates the range of derived parameters. To quantify the DNA damage, the tail length (TL), the percentage of migrated DNA (tail DNA %), and tail moment (TM) were evaluated. TL (length of DNA migration) is related directly to the DNA fragment size and is presented in micrometers. It was calculated from the center of the cell. Finally, the program calculates TM.
The DNA damage was quantified by measuring the displacement between the genetic material of the nucleus (Comet head) and the resulting (tail).
Biochemical analyses
Calmodulin and Ca+ + ATPase were determined in tissues according to the method of Vig et al. [22]. The activities of ATPase enzyme in tissue were expressed as μmol of inorganic phosphate liberated/min/mg protein. Tau protein was measured by Rat Tau Protein ELISA Kit Catalog # MBS725098 from MyBiosource, Inc, Southern California, San Diego (USA) but caspase-3 was assessed using sandwich ELISA kits. NA was measured using ELISA kits, while GABA and DA were estimated according to the method of Zagrodzka et al. [23]. Assessment of oxidant/antioxidant status in brain tissues was performed by measuring the activities of GSH and MDA as the product of lipid peroxidation according to the colorimetric method of Paglia and Valentine [24] and Erdelmeier et al. [25]. The inorganic phosphate was measured according to Schulz et al. [26] and the protein content was estimated by the method of Lowry et al. [27].
Statistical analysis
The values presented are the mean ± SE. Data were analyzed using a one-way analysis of variance (ANOVA): post Hoc Multiple Comparison, Duncan’s multiple range test. The level of significance between mean values was set at p ≤ 0.05. All statistical analyses were performed using SPSS software (version 20.0).
Results
Characterization of the formed nAg/HAp
The formed nanocomposite was analyzed using, SEM, TEM, and EDAX. It was clearly shown that silver synthesized in nano range (75–80 nm) dispersed successfully in the nHAp carrier (Fig. 1). While the EDAX analysis showed the presence of nAg after filtration and drying with its percentage, as the analysis of the filtered solution did not detect the silver or calcium ions (Fig. 2). While TEM showed the distribution of agglomerated nAg on the nHAp as shown in Fig. 3a, b. The characteristic XRD pattern showed the formation of Ca/P with molar ratio 1.67. It was noticed that the presence of nAg did not change the crystal structure of nHAp.
Detection of DNA damage (comet assay)
The effect of nAg/HAp on CdCl2 induced DNA damage in rat brains has been investigated using the comet assay. This assay facilitates the detection of various types of DNA injury such as double-strand breaks, single-strand breaks, alkali-labile sites, incomplete repair sites, and cross-links. The migration length of DNA is directly proportional to its damage.
Brain cells of control rats showed no tails (Table 1 and Fig. 4a). Tail length was substantially long in CdCl2 intoxicated group (Table 1 and Fig. 4b) as compared to corresponding controls. However, the extent of damage has a notable decrease in rats after treatment with nAg/HAp as shown in Table 1 and Fig. 4c.
Biochemical results
Table. 2 and Fig. 5 showed that exposure to CdCl2 induced augmentation in brain tau protein and caspase-3 concentration (P < 0.05) as compared to control animals. Intravenous injection with nAg/HAp decreased significantly (P < 0.05) the levels of Tau protein and caspase-3 as compared to the group that received CdCl2, but did not reach the control level.
Table. 3 and Fig. 6 showed that exposure to CdCl2 was the detrimental factor to the redox status as evidenced by a significant rise (p < 0.05) in MDA level and significant depletion (p < 0.05) in GSH activity related to the controls. As compared to the group administered CdCl2, the group treated with the nAg/HAp showed a significant increase (p < 0.05) in the activity of GSH and a significant decrease (p < 0.05) in the level of MDA.
Concerning the brain neurotransmitters (Table 4 and Fig. 7, there was a significant increase (p < 0.05) in GABA and a significant decrease (p < 0.05) in NA and DA concentrations in CdCl2–intoxicated rats. The injection of nAg/HAp counteracted the levels of these neurotransmitters manifested by augmentation in GABA, NA, and DA activity.
As shown in Table. 5 and Fig. 8, administration of CdCl2 to rats caused alteration in brain tissue which was manifested by a significant increase (p < 0.05) in CaM level and a significant decrease (p < 0.05) in Ca++ATPase activity as compared to controls. While the intravenous treatment with nAg/HAp ameliorated the activity of CaM and Ca++ ATPase as compared to CdCl2 group but did not reach to control level.
Discussion
The current study dealt to modify the manufacture of nAg supported on nHAp in order to examine the efficacy of this nanocomposite in facing CdCl2 induced neurotoxicity in rats. The Nanocomposite contains low content of Ag nanoparticles (1/5 LD50) and has been obtained by the chemical route and subsequent chemical neutralization process. In this method, Polyvinyl alcohol (PVA) is used to act as a polymeric matrix where Ag ions are distributed on it to form a crystalline structure in nano-rang as the same as Ca ions without agglomeration.
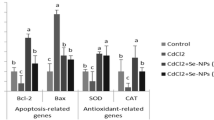
The brain is a highly complex tissue that regulates an array of biological metabolic events that render it consumes a large amount of oxygen. However, the brain tissues are prone to noxious oxidative stress attacks due to the presence of high concentrations of peroxidizable unsaturated fatty acids and a poorly developed antioxidant defense system [28, 29]. The cardinal reason for CdCl2 induced neurotoxicity is the ability to penetrate blood brain-barrier and collocate in the brain, which ultimately hinders the vital cellular processes mediated by induction of lipid peroxidation and competition with essential metals of cellular enzyme and DNA repair system [30]. As detected by comet assay, there was an increase in DNA fragmentation and the number of comets observed in CdCl2 intoxicated rats as compared to controls. Comet assay is widely used to detect genotoxicity and cellular DNA lesions [31]. CdCl2-induced alterations in DNA methylation metabolism through interaction with methyl transferase DNA binding domain, leading to initiation of gene-specific DNA hypo- or hyper-methylation and possibly aberrant gene expression [32]. On the other hand, it cannot be excluding the involvement of caspase-3 from DNA damage processes. Since, caspase-3 (executioner caspase), is activated during apoptotic pathways (intrinsic and extrinsic) and plays a dominant role in the coordination of the demolition phase of neural apoptosis by cleaving a diverse array of protein substrates [33, 34]. Previous studies display that exposure to Cd activates the extrinsic receptor-mediated pathway via Fas/FasL-mediated activation of procaspase-3, leading to neuronal apoptosis [34]. Additionally, the adverse effect of Cd on mitochondria (intrinsic pathway) is associated with up- regulation of apoptotic mediators (Bax, p53 and p21) and down-regulation of Bcl-2/Bcl-2 associated X protein (Bax) ratio, triggering the downstream apoptotic pathway [34, 35]. However, the elevation of caspase-3 level in the CdCl2-intoxicated group reflected a harmful effect of this metal on the molecular components of the cells.
Oxidative stress and rampant generation of free radicals are the hallmark events of Cd that motivate neurotoxicity. Cd-induced lipid peroxidation may be through the production of ROS or a decrease in the activities of antioxidant enzymes and/or metal complex de-compartmentalization [36]. The performance of CdCl2 in aggravating oxidation was manifested in the present study by a significant decrease in brain GSH and elevation of MDA. Thereby, there is a serious bearing on the functional development of the central nervous system such as reduction of axonal mitochondria turnover, disruption of Golgi, and reduction of synaptic vesicles [37]. The event cascade of lipid peroxidation is mediated by the over generation of superoxide radical to form a toxic product, MDA [38]. On the other, Cd targets the cysteine residues of GSH and forms an inactive mercapeptide complex, and conjugates with free or protein-bound –SH groups of metallothionein [38]. This structural modification hinders the antioxidant potential of GSH and thus makes the brain vulnerable to oxidative attack [39]. The lipid peroxidation process is a governor of the neurochemistry in the brain by destroying the brain cells membrane and/or producing carbonyl products, a mediator of neurotoxicity [40]. Consequently, the neuronal function disorder leads to inhibition of the catecholamine uptake in brain synaptosomes [41]. Importantly, the interrelation between oxidative stress and neural death is mediated by releasing of pro-apoptotic factors into the cytoplasm via activation of the Jun amino-terminal kinases (JNK) pathway and/or by activation of nuclear factor (NF-κB) accompanied by a marked inhibition of anti-apoptotic protein like Bcl-2 [42]. JNK activation induced neuronal death mediated by caspase-3 and specific nuclease, which cuts the genomic DNA between nucleosomes leading to apoptotic chromatin condensation and DNA fragmentation [43].
The obtained results elicited that the brain GABA and the catecholamine (NA, and DA) concentrations were significantly decreased in Cd-intoxicated rats. Boost of GABA release is due to inhibition of the voltage-dependent calcium channels with cadmium [32] resulting in an alteration in the degree and balance of excitation–inhibition in synaptic neurotransmission [44]. The decrease in NA and DA transmission activity is mediated by impairment in the intracellular calcium metabolism and function, as a second messenger in the CNS. In addition, overload production of calcium inhibits the Ca++ATPase activity in the cell membrane and modulates the intracellular calcium homeostasis leading to the alteration’s neurotransmitter functions [29]. CaM is a binding Ca+2 protein molecule and has a crucial role in the neurodynamic process. To clarify, CaM acts as an enhancement factor for releasing the neurotransmitters from the neural vesicles and activates Ca+2ATPase to reduce the free Ca+2 [45]. The elevation of CaM level possibility that one of Cd manifestation toxicity may be through activation of CaM upsetting its normal regulation by a cellular flux of Ca2+ [32]. The present study revealed that Cd administration increased Tau protein content when compared to control. Tau protein is expressed mainly in neurons and its activity is ruled by phosphorylation. Cd exposure caused Tau hyper-phosphorylation indirectly by activating the glycogen synthase kinase-3beta (GSK-3β) and cyclin-dependent kinase 5 (CDK5) [46]. As a result, Tau self-aggregates to form neurofibrillary structure subsequent to a cascade of events such as disruption in axonal transport and synaptic disconnection with ultimate dissipation of brain cytoskeleton [47, 48].
The obtained data showed the efficiency of nAg/HAp in amelioration the neural dysfunction associated with CdCl2 exposure and such findings were evidenced in improvement the oxidant/antioxidant status with subsequent repair of fragmented DNA and susceptibility to apoptotic cell death as well as improvement of neurotransmitters within the experimental period. Numerous studies have a special interest in the various methods of nAg/HAp preparation to be more qualified for usage in the sterilization field [2, 10, 49] or recently as an anticancer agent [50]. However, the literature concerning with improvement of nAg preparation to be suitable for in vivo usage as a therapeutic agent is not available and the data explained the mechanism of the effect of nAg/HAp on neurotoxicity induced Cd noxiousness. Therefore, the explanation of the present results was dependent upon the properties of nAg that proven in other fields. Elsewhere, the studies by Sondi and Salopek-Sondi [51]; Mo et al. [52], and Cameron et al. [53] interesting with the toxicity of nAg which proved that low concentration of nAg is non-toxic for mammalian cells. The confirmatory study conducted by Shayesteh et al. [54], detected that nAg is safe when administered in a range from 5 to 50 mg/kg/day for 4 weeks. In in vitro study of Gonzalez-Carter et al. [49] examined the chemical and morphological transformation of nAg as well as neurotoxic-related issues in mice. They reported that just nAg internalized into microglial cells, Ag+ ions released within it, sulphided and sequestered more efficiently in comparison to neurons. The formation of intracellular Ag2S, resulting from cystathionine γ-lyase-mediated H2S production in microglia, sequesters Ag+ ions released from nAg. The anti-inflammatory effect of Ag2S controlled the microglia-mediated neurotoxicity because the insoluble Ag2S complexes around nAg particles may act as Ag+-sequestering and inhibit the toxicity mechanism. The lack of cystathionine γ-lyase enzyme in certain neuronal cells associated with decreasing H2S levels may be one of the reasons for the restriction of the ability of some neural cells in preventing detoxification of Ag+ [55]. Thereby, highly controlled targeting of nAg into microglia could decrease brain inflammation locally by inhibiting microglia reactivity consequent by reduction in inflammatory injury to neighboring neuronal cells and serves a neuroprotective role [56]. On the other hand, the studies were conducted on drug delivery field proved that hybrid molecular unit of nAg particularly is a suitable carrier of anti-inflammatory [57], anti-oxidant [58] and anticancer [59] therapeutic molecules due to their exceptional biocompatibility and viable features for nanoscale-derived therapeutic settings [60].
However, there is no consensus on nAg toxicity because in vivo studies were carried out with short-term experiments or in in-vitro studies, which cannot apply to the living system [61]. There are some studies challenged that nAg induced ROS production and based their results on the difference in experimental conditions such as treatment duration, cell types, the method used for ROS detection as well as factors that contributed to nAg synthesis and the physiochemical characteristic of the final product [1, 62]. Moreover, Inder and Kumar [2] suggested that it is important to standardize the formulation of nAg to avoid the potential toxicity, and with each combination with another particle; the composite is being utilized to produce nanoparticles with unique properties with controlled size and shape of particles.
The prevailing view is that oxidative stress accompanied by ROS has complete responsibility for any damage at the cellular or systemic level that occurs from either extrinsic or intrinsic conditions [63]. The current results showed inhibition of lipid peroxidation products represented by a decrease of brain MDA level and activation of GSH antioxidant enzyme. Thus, the extent of brain physiological response to i.v. nAg/HAp against Cd neurotoxicity may be attributed to an antioxidant property, scavenging activity, and chelating power as reported in in vivo [54, 64] and in vitro [65] studies. The antioxidant potential of nAg might be augmented due to the adhering functional group, which originated from nHAp. Because oxidative stress is the supervisor controlling the internal and/or external environments induced cellular adverse effect, the inhibition of damaged DNA and caspase-3 content observed upon the injection with nAg/HAp is logical and considered great indicators for minimizing the neurodegeneration symptoms. Safari et al. [66] hypothesized that although the deposit of nAg has been identified in the cutaneous nerves as well as astrocytes tend to reside for a considerable time within the CNS than in other organs, nAg was not a cause of neurotoxic damage neither by the acute exposure to nAg nor by the chronic presence of large amounts of accumulated.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files), and are available from the corresponding author on reasonable request.
References
Verma P, Maheshwari SK (2019) Applications of silver nanoparticles in diverse sectors. Int J Nano Dimens 10(1):18–36. https://doi.org/10.22034/ijnd.2018.87646.1609
Inder D, Kumar P (2018) The Scope of nano-silver in medicine: a systematic review. Int J Pharmacogn Chinese 2(2):000134
Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17(9):1534. https://doi.org/10.3390/ijms17091534
McShan D, Ray PC, Yu H (2014) Molecular toxicity mechanism of nanosilver. J Food Drug Anal 22(1):116–127
Oliveira MM, Ugarte D, Zanchet D, Zarbin AJG (2005) Influence of synthetic parameters on the size, structure, and stability of dodecanethiol-stabilized silver nanoparticles. J Colloid Interface Sci 292(2):429–435
Chung RJ, Hsieh MF, Huang KC, Perng LH, Chou FI, Chin TS (2005) Anti-microbial hydroxyapatite particles synthesized by a sol-gel route. J Solgel Sci Technol 33(2):229–239. https://doi.org/10.1007/s10971-005-5618-1
Chen W, Oh S, Ong AP, Oh N, Liu Y, Courtney HS, Appleford M, Ong JL (2007) Antibacterial and osteogenic properties of silver-containing hydroxyapatite coatings produced using a sol gel process. J Biomed Mater Res A Part A 82(4):899–906. https://doi.org/10.1002/jbm.a.31197
Rameshbabu N, Kumar TSS, Prabhakar TG, Sastry VS, Murty KVGK, Rao KP (2007) Antibacterial nanosized silver substituted hydroxyapatite: synthesis and characterization. J Biomed Mater Res A Part A 8(3):581–591. https://doi.org/10.1002/jbm.a.30958
Han IH, Lee IS, Song JH et al (2007) Characterization of a silver-incorporated calcium phosphate film by RBS and its antimicrobial effects. Biomed Mater 2(3):S91–S94. https://doi.org/10.1088/1748-6041/2/3/S01
Díaz M, Barba M F, Miranda M, Guitián F, Torrecillas R, Moya JS (2009) Synthesis and antimicrobial activity of a silver-hydroxyapatite nanocomposite. J Nanomater 2009:498505. https://doi.org/10.1155/2009/498505
Abdel-Gawad EI, El-Sherbiny EM, Awwad SA (2021) New synthesis for nano-silver formulated as a bio-safe composite structure to employ against hepatotoxicity in rats. Front Drug Chem Clin Res 4:1–8
Mata AM, Sepulveda MR (2010) Plasma membrane Ca2+ ATPases in the nervous system during development and ageing. World J Biol Chem 1(7):229–234
Sprenger J, Trifan A, Patel N, Vanderbeck A, Bredfelt J, Tajkhorshid E, Rowlett R, Leggio L, Åkerfeldt KS, Linse S (2021) Calmodulin complexes with brain and muscle creatine kinase peptides. Curr Res Struct Biol 3:121–132
Liang CQ, Zhang G, Zhang L, Chen SY, Wang JN, Zhang TT, Singer JH, ke JB (2020) Calmodulin bidirectionally regulates evoked and spontaneous neurotransmitter release at retinal ribbon synapses research article. eNeuro 8. https://doi.org/10.1523/ENEURO.0257-20.2020
Braga MM, Dick T, de Oliveira DL, Scopel-Guerra A, Mussulini BH, de Souza DO, da Rocha JBT (2015) Evaluation of zinc effect on cadmium action in lipid peroxidation and metallothionein levels in the brain. Toxicol Rep 2:858–863
Brender J, Suarez L, Felkner M, Gilani Z (2006) Maternal exposure to arsenic, cadmium, lead, and mercury and neural tube defects in offspring. Environ Res 101(1):132–139. https://doi.org/10.1016/j.envres.2005.08.003
Satarug S, Garrett SH, Sens MA, Sens DA (2010) Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 118(2):182–190. https://doi.org/10.1289/ehp.0901234
Jarup L, Rogenfelt A, Nogawa ECG, K and Kjellström T, (1983) Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand J Work Environ Health 9(4):327–331
Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54(4):275–287. https://doi.org/10.1007/BF01234480
Lamtai M, Chaibat J, Ouakki S, Berkiks I, Rifi E, El Hessni A, Mesfioui A, Hbibi AT, Ahyayauch H, Essamri A, Ouichou A (2018) Effect of Chronic administration of cadmium on anxiety-like, depression-like and memory deficits in male and female rats: possible involvement of oxidative stress mechanism. J Behav Brain Sci 8:240–268. https://doi.org/10.4236/jbbs.2018.85016
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Vig PJ, Pentyala SN, Chetty CS, Rajanna B, Desaiah D (1994) Lead alters inositol polyphosphate receptor activities: protection by ATP. Pharmacol Toxicol 75:17–22
Zagrodzka J, Romaniuk A, Wieczorek M, Boguszewski P (2000) Bicuculline administration into ventromedial hypothalamus: effects on fear and regional brain monoamines and GABA concentrations in rats. Acta Neurobiol Exp 60(3):333–343
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Erdelmeier I, Gerard-Mounnier D, Yadan J, Chaudiere D (1998) Reactions of N-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals mechanistic aspects of the colorimetric assay of lipid peroxidation. Chem Res Toxicol 11(10):1184–1194. https://doi.org/10.1021/tx970180z
Schulz DW, Passonneau JV, Lowry OH (1967) An enzymic method for the measurement of inorganic phosphate. Anal Biochem 19(2):300–314
Lowry OH, Rosebrough NJ, Farr AL, Ranell RJ (1951) Protein measurement with the Follin phenol reagent. J Biol Chem 193(1):265–275
Bondy SC (1997) Free-radical-mediated toxic injury to the nervous system. In: Wallace KB (ed) In: Free Radical Toxicology. Taylor and Francis, Oxford
Vijaya P, Sharma S (2018) Protective effects of natural antioxidant supplementation on cadmium induced toxicity in albino mice. JIPBS 5(2):16–21
Mohamed EG, Masoud MA, Ebtehal Mohammad F, Georgy GS (2018) Evaluation of low and high daily intakes of cinnamon against cadmium-induced liver and brain toxicity in rats. Pharm Chem J 5(4):81–96
Uslu H, Uslu GA, Özen H, Karaman M (2018) Effects of different doses of Prunus laurocerasus L leaf extract on oxidative stress, hyperglycaemia and hyperlipidaemia induced by type I diabetes. Indian J Tradit Knowl. 17(3):43–436
Wang B, Du Y (2013) Cadmium and Its neurotoxic effects. Oxid Med Cell Longev 2013:898034. https://doi.org/10.1155/2013/898034
Liu X, Zhang Y, Wang Y, Yan Y, Wang J, Gu J, Chun B, Liu Z (2016) Investigation of cadmium-induced apoptosis and the protective effect of N-acetylcysteine in BRL 3A cells. Mol Med Rep 14:373–379. https://doi.org/10.3892/mmr.2016.5218
Yuan Y, Zhang Y, Zhao S, Chen J, Yang J, Wang T, Zou H, Wang Y, Gu J, Liu X, Bian J, Liu Z (2018) Cadmium-induced apoptosis in neuronal cells is mediated by Fas/FasL-mediated mitochondrial apoptotic signaling pathway. Sci Rep. https://doi.org/10.1038/s41598-018-27106-9
Zhang R, Kang KA, Piao MJ, Maeng YH, Lee KH, Chang WY, You HJ, Kim JS, Kang SS, Hyun JW (2009) Cellular protection of morin against the oxidative stress induced by hydrogen peroxide. Chem Biol Interact 177:21–27. https://doi.org/10.1016/j.cbi.2008.08.009
Jarup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208. https://doi.org/10.1016/j.taap.2009.04.020
Singh S, Singh R, Kushwah AS, Gupta G (2014) Neuroprotective role of antioxidant and pyranocarboxylic acid derivative against AlCl3 induced Alzheimer’s disease in rats. J Coast Life Med 2:571–578. https://doi.org/10.12980/JCLM.2.2014J58
Ma S, Zhang L, Jiang Q (2017) Protective effect of bioflavonoid morin on cadmium induced oxidative neuropathy. Biomed Res 28(3):1148–1154
Hansen JM, Zhang H, Jones DP (2006) Differential oxidation of thioredoxin 1, thiore-doxin 2 and glutathione by metal ions. Free Radic Biol Med 40:138–145. https://doi.org/10.1016/j.freeradbiomed.2005.09.023
Adefegha SA, Omojokun OS, Oboh G, Fasakin O, Ogunsuyi O (2015) Modulatory effects of ferulic acid on cadmium-induced brain damage. J Evid Based Complementary Altern Med 21(4):56–61. https://doi.org/10.1177/2156587215621726
Alam RTM, Hendawi MY (2015) Protective efficacy of spirulinaplatensis against cadmium induced neurotoxicity in rats. Glob Vet 14(4):490–499. https://doi.org/10.5829/idosi.gv.2015.14.04.93239
Mohamd EM, Ahmed HH, Estefan SF, Farrag AE, Salah RS (2011) Windows into estradiol effects in Alzheimer’s disease therapy. Eur Rev Med Pharmacol Sci 15(10):1131–1140
Kumar V, Gill KD (2009) Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol 83:965–978. https://doi.org/10.1007/s00204-009-0455-6
Lavoie J, Illiano P, Sotnikova TD, Gainetdinov RR, Beaulieu JM, Hébert M (2014) The electroretinogram as a biomarker of central dopamine and serotonin: potential relevance to psychiatric disorders. Biol Psychiatry 75(6):479–486. https://doi.org/10.1016/j.biopsych.2012.11.024
Hsiang J, Díaz E (2011) Lead and developmental neurotoxicity of the central nervous system. Curr Neurobiol 2(1):35–42
Kim AC, Lim S, Kim YK (2018) Metal Ion effects on Aβ and tau aggregation. Int J Mol Sci 19(1):128. https://doi.org/10.3390/ijms19010128
Issa GI, Abbas OA, Abdel-Gawad EI (2018) Potential impact of strawberry leaves extract on neurotoxicity in rats. IJISRT 3(11):370–376
Alonso AD, Cohen LS, Corbo C, Morozova V, ElIdrissi A, Phillips G, Kleiman FE (2018) Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability. Front Cell Neurosci 12:338. https://doi.org/10.3389/fncel.2018.00338
Gonzalez-Carter DA, Leo BF, Ruenraroengsak P, Chen S, Goode AE, Theodorou IG, Chung KF, Carzaniga R, Shaffer MS, Dexter DT, Ryan MP, Porter AE (2017) Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes. Sci Rep 7:42871. https://doi.org/10.1038/srep42871
Buttacavoli M, Albanese NN, Di Cara G, Alduina R, Faleri C, Gallo M, Pizzolanti G, Gallo G, Feo S, Baldi F, Cancemi P (2018) Anticancer activity of biogenerated silver nanoparticles: an integrated proteomic investigation. Oncotarget 9(11):9685–9705. https://doi.org/10.18632/oncotarget.23859
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent a case study on E coli as a model for gram-negative bacteria. J Colloid Interface Sci 275:177
Mo A, Liao J, Xu W, Xian S, Li Y, Bai S (2008) Preparation and antibacterial effect of silver–hydroxyapatite/titania nanocomposite thin film on titanium. Appl Surf Sci 255:435
Cameron SJ, Hosseinian F, Willmore WG (2018) A current overview of the biological and cellular effects of nanosilver. Int J Mol Sci 19(7):2030. https://doi.org/10.3390/ijms19072030
Shayesteh TH, Khajavi F, Ghasemi H, Zijoud SMH, Ranjbar A (2014) Effects of silver nanoparticle (Ag NP) on oxidative stress, liver function in rat: hepatotoxic or hepatoprotective? Biol Sci Pharm Res 2(5):040–044
Miyamoto R, Otsuguro KI, Yamaguchi S, Ito S (2014) Contribution of cysteine aminotransferase and mercaptopyruvate sulfur transferase to hydrogen sulfide production in peripheral neurons. J Neurochem 130:29–40. https://doi.org/10.1111/jnc.12698
Mosher K, Wyss-Coray T (2014) Microglial dysfunction in brain aging and Alzheimer’s dsiease. Biochem Pharmacol 88:11
Jiang Q, Yu S, Li X, Ma C, Li A (2018) Evaluation of local anesthetic effects of lidocaine-ibuprofen ionic liquid stabilized silver nanoparticles in male swiss mice. J Photochem Photobiol B Biol 178:367–370. https://doi.org/10.1016/j.jphotobiol.2017.11.028
Arumai Selvan D, Mahendiran D, Senthil Kumar R, Kalilur Rahiman A (2018) Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: phytochemical, antioxidant and in vitro cytotoxicity studies. J Photochem Photobiol B Biol 2018(180):243–252. https://doi.org/10.1016/j.jphotobiol.02.014
Petrov PD, Yoncheva K, Gancheva V, Konstantinov S, Trzebicka B (2016) Multifunctional block copolymer nanocarriers for co-delivery of silver nanoparticles and curcumin: synthesis and enhanced efficacy against tumor cells. Eur Polym J 81:24–33. https://doi.org/10.1016/j.eurpolymj.2016.05.010
KJ P, (2017) Multi-functional silver nanoparticles for drug delivery: a review. Int J Curr Pharm Rev Res 9:1–5
Ge L, Li Q, Wang M, Ouyang J, Li X, Xing MMQ (2014) Nanosilver particles in medical applications synthesis, performance and toxicity. Int J Nanomed 9:2399–2407
Pereira LC, Pazin M, Franco-Bernardes MF, da Cunha A, Martins GR, Barcelos M, Pereira MC, Mesquita JP, Rodrigues JL, Barbosa F, Dorta DJ (2018) A perspective of mitochondrial dysfunction in rats treated with silver and titanium nanoparticles (AgNPs and TiNPs). J Trace Elem Med Biol 47(63):69
Kim HK, Trepanier CI, Elmi N, Rapoport SI, Andreazza AC (2016) Mitochondrial dysfunction and lipid peroxidation in rat frontal cortex by chronic NMDA administration can be partially prevented by lithium treatment. J Psychiatr Res 76:59–65. https://doi.org/10.1016/j.jpsychires.2016.02.001
Shanmugasundaram T, Radhakrishnanc M, Gopikrishnanc V, Kadirvelub K, Balagurunathana R (2017) Biocompatible silver, gold and silver/gold alloy nanoparticles for enhanced cancer therapy: an in vitro and in vivo perspectives. Nanoscale 9(43):16773–16790. https://doi.org/10.1039/c7nr04979j
Bhakya S, Muthukrishnan S, Sukumaran M, Muthukumar M (2016) Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl Nanosci 6(5):755–766. https://doi.org/10.1007/s13204-015-0473-z
Safari M, Bidgoli A, Rezayat SM (2016) Differential neurotoxic effects of silver nanoparticles: a review with special emphasis on potential biomarkers. Nanomed J 3(2):83–94
Acknowledgements
The authors wish to thank Dr. Sameh A. Awwad, chemical engineering, Department of Higher Institute of Engineering & Technology, New Damietta, Egypt, for his effort in preparation and characterization nAg/HAp composite used in this investigation.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors did not receive any fund from any organization.
Author information
Authors and Affiliations
Contributions
EIAG designed the study, collected the data, drafted the manuscript, and provided necessary support, in addition to final approval of the version to be published. EMElS designed the study, collected the data, drafted the manuscript, and analyzed the data. HFO drafted the manuscript, and directed implementation and data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval consent to participate
The study was conducted in accordance with the guidelines set by the CIOHS & ICLAS International Guiding Principles for Biomedical Research involving animals (2012), which accordance with the Guide for the Care and Use of Laboratory Animals (Eighth Edition, 2011, published by The National Academies Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA). This guide was approved by the Ethical Committee at National Center for Radiation Research, Egyptian Atomic Energy Authority, Cairo, Egypt (NCRR- EAEA).
Consent to participate
The current article is including albino rats as experimental animals not humans.
Consent for publications
The current article is including albino rats as experimental animals not humans, and the authors are responsible for correctness of the statements provided in the manuscript.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sherbiny, E.M., Abdel-Gawad, E.I. & Osman, H.F. Impact of nano silver composite structure on cadmium neurotoxicity in albino rats. Appl Biol Chem 65, 70 (2022). https://doi.org/10.1186/s13765-022-00738-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-022-00738-2