Abstract
Vascular inflammation activated by pro-inflammatory cytokines is an inflammatory response that occurs in the early stages of atherosclerosis. Endothelial dysfunction in vascular inflammation begins with the expression of cell surface adhesion molecules by pro-inflammatory cytokines. The purpose of this study was to evaluate and verify the vascular inflammatory effects of isobavachalcone. In this study, we investigated the effects of isobavachalcone on inflammatory responses in vascular inflammation induced by the tumor necrosis factor-α (TNF-α) in human umbilical vein endothelial cells (HUVECs). TNF-α stimulation significantly increased the expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) proteins, and concentration-dependently decreased by isobavachalcone in HUVECs. Isobavachalcone suppressed TNF-α-induced ICAM-1 and VCAM-1 expression in HUVECs, thereby inhibiting TNF-α-induced increase in monocyte adhesion. In addition, isobavachalcone decreased the phosphorylation of the NF-κB (necrosis factor κB) p65 subunit. The findings of this study demonstrate that isobavachalcone prevents TNF-α-induced vascular inflammation and has the potential to protect against the early progression of atherosclerosis.
Similar content being viewed by others
Introduction
Inflammation is a process in which the system that protects the human body from infection and external stimuli is activated. In acute inflammation, the inflammatory reaction disappears within hours or several days and causes symptoms such as redness, swelling, pain, and fatigue, whereas chronic inflammation is a prolonged inflammatory state that can last several months or years. In the inflammatory process of blood vessels, acute inflammation correlates with vasodilation, increased vascular permeability, and the release of tumor necrosis factor-α (TNF-α), interleukins (ILs), and nitric oxide (NO), commonly known as inflammatory cytokines [1]. TNF-α is critical for increasing the expression of adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in endothelial cells, and activates macrophages and neutrophils [2,3,4]. In addition, TNF-α promotes edema by regulating vascular permeability [5, 6].
Isobavachalcone (IBC, Fig. 1) is an active molecule present in the medicinal plant Psoralea corylifolia and has been reported to have antioxidant, anti-inflammatory, antibacterial, and anticancer activities [7,8,9,10]. However, to our knowledge, studies on the protective effects of isobavachalcone on vascular inflammation have not been reported. In this study, we examined whether isobavachalcone decreased the expression of TNF-α-induced ICAM-1 and VCAM-1 in human umbilical vein endothelial cells (HUVECs), and the adhesion of monocytes to the vascular endothelium. We also investigated the signaling mechanism that mediates the suppressive effect of isobavachalcone on adhesion molecule expression in endothelial cells. Our findings suggest that isobavachalcone inhibits TNF-α-stimulated ICAM-1 and VCAM-1 protein expression in HUVECs by regulating the NF-κB signaling pathway.
Materials and methods
Materials and cell culture
Isobavachalcone was purchased from Sigma-Aldrich (St. Louis, MO, USA). The human umbilical vein endothelial cells (HUVECs) used in this study were purchased from Lonza (Walkersville, MD, USA). HUVECs were cultured in endothelial cell growth basal medium (EBM-2) supplemented with various growth factors at 37 °C and 5% CO2. The antibodies anti-ICAM-1, anti-VCAM-1 (Santa Cruz Biotechnology, Santa, CA, USA), anti-phospho-p65 (Ser536), anti-IκB-α, anti-phospho-ERK and anti-ERK (Cell Signaling Technology, Beverly, MA, USA), anti-p65 (Upstate Biotechnology, Lake Placid, NY, USA), and anti-β-actin (Sigma-Aldrich, St Louis, MO, USA) were used in this study.
Western blot analysis
Western blot analysis was performed as described previously [11]. Briefly, the protein samples were mixed with 5 × sample buffer, heated for 9 min, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred onto polyvinylidene difluoride membrane. Chemiluminescent detection was performed using enhanced chemiluminescence detection reagents and analyzed using a ChemiDoc XRS Imaging System (Bio-Rad Laboratories, Hercules, CA, USA). After stripping the membrane, it was confirmed that each group was loaded with the same amount of protein by blotting with an anti-β-actin antibody.
Monocyte adhesion assay
THP-1 cells (American Type Culture Collection, Manassas, VA, USA) were incubated with the CellTracker dye (Invitrogen) for 15 min at 37 °C. Monocyte-endothelial adhesion was determined by fluorescence labeling of monocytes, as described previously [12]. HUVECs were pretreated with isobavachalcone (50 µM) for 30 min and stimulated with TNF-α (10 ng/mL) and isobavachalcone (50 µM) for 4 h. Fluorescence-labeled monocytes were added to the HUVEC monolayer and allowed to adhere for 2 h. The number of monocytes attached to the HUVECs was expressed as the perceived fluorescence intensity.
Statistical analysis
Data are expressed as mean ± SD. Mean comparisons between two groups were examined for significant differences using analysis of variance, followed by individual comparisons using Tukey’s post hoc test, with a P-value < 0.05 indicating a statistically significant difference.
Results
Cell viability of isobavachalcone on HUVECs
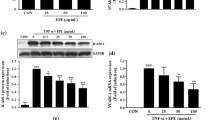
Cell viability was measured after treatment with varying concentrations of isobavachalcone, before determining its active concentration, to ensure the cells were non-toxic. The goal of this experiment was to determine the concentration of isobavachalcone that resulted in over 90% cell viability. HUVEC viability showed a slightly increasing trend with increasing concentrations of isobavachalcone from 0.1 to 50 µM (Fig. 2).
Cell viability assay of HUVECs. The HUVECs were exposed to concentrations of isobavachalcone from 0.1 to 50 µM for 24 h and viability was detected using the CCK-8 assay. Control cells (CB) received vehicle alone. All data are expressed as the mean ± SD of the three independent experiments. *, p < 0.05 vs. CB
Isobavachalcone attenuates the expression of TNF-α-induced ICAM-1 and VCAM-1 protein in HUVECs
We determined whether isobavachalcone affects the protein expression of ICAM-1 and VCAM-1. The HUVECs were pre-incubated with isobavachalcone in a dose-dependent manner (10, 20, and 50 µM) for 30 min, prior to incubation with or without TNF-α for 6 h. Protein expression of ICAM-1 and VCAM-1 increased with TNF-α alone treatment and was significantly downregulated by isobavachalcone (20 and 50 µM) (Fig. 3A and B).
Isobavachalcone reduces the expression of TNF-α-induced ICAM-1 and VCAM-1 proteins in HUVECs. Immunoblot analysis results are shown for ICAM-1 (A) and VCAM-1 (B) protein expression in TNF-α-stimulated HUVECs co-treated with isobavachalcone. β-Actin protein was used as an internal control. The bar graphs (bottom panel) represent the mean ± SD of three different experiments. ***, p < 0.001 vs. CB; ###, p < 0.001 vs. TNF-α
Isobavachalcone extract inhibits TNF-α-induced NF-κB p65 phosphorylation
TNF-α is a transcription factor in NF-κB signaling responsible for the expression of cell adhesion molecules that increase inflammatory responses [13, 14]. We measured the phosphorylation of p65 of NF-κB to investigate the effect of isobavachalcone on NF-κB activity. TNF-α-induced phosphorylation of the p65 subunit of NF-κB increased approximately 1.7-fold compared to CB alone, but phosphorylation of the p65 subunit of NF-κB was decreased by isobavachalcone (50 μM) (Fig. 4A). The results are expressed as relative ratios (Fig. 4B). IκB-α is known to inhibit NF-κB by masking its nuclear localization signal and sequestering it in an inactive state in the cytoplasm. IκB-α was reduced by TNF-α but recovered by co-treatment with isobavachalcone (50 μM) (Fig. 3C and D). These data demonstrate that isobavachalcone reduces the increase in ICAM-1 and VCAM-1 levels by inhibiting NF-κB phosphorylation and IκB-α degradation activated by TNF-α. Isobavachalcone also showed the effect of inhibiting phosphorylation of ERK, which is known as a representative MAPK pathway (Fig. 4E and F).
Isobavachalcone decreases phosphorylation of NF-κB p65 in TNF-α-stimulated HUVECs. Immunoblot analysis of phosphorylated NF-κB p65 (A) and degradation of IκB-α (C) in total cell extract were conducted. HUVECs were pretreated with 50 µM isobavachalcone for 30 min and exposed to TNF-α (10 ng/mL) for 4 h (p-p65) and 1 h (IκB-α). Total cell extracts were prepared and analyzed for phosphorylation of p65, total p65, IκB-α and β-Actin. Densitometric analyses results are presented as the relative ratios of phospho-p65 to p65, and IκB-α to β-Actin (B and D). E Expression of phospho-ERK and ERK in TNF-α-stimulated HUVECs co-treated with isobavachalcone for 15 min. (F) Densitometric analyses results are presented as the relative ratios of phospho-ERK to ERK. The bars represent the mean ± SD of three different experiments. ***, p < 0.001 vs. CB; ###, p < 0.001 vs. TNF-α
Isobavachalcone disturbs the adhesion of monocytes to HUVEC monolayers
We investigated whether isobavachalcone reduced monocyte adhesion to HUVECs through TNF-α-stimulated ICAM-1 and VCAM-1 expression. Monocytes attached to HUVECs were identified through fluorescent labeling of the monocytes using a cell tracker dye. HUVECs stimulated with TNF-α (10 ng/mL) showed increased monocyte adhesion (approximately 4.8-fold) compared to those treated with CB, and the group co-treated with isobavachalcone (50 μM) showed a 54% decrease in monocyte adhesion (Fig. 5A and B).
Isobavachalcone disturbs adhesion of monocytes to HUVEC monolayers. A Monocyte adherence to HUVEC monolayers is represented in the images. HUVECs were incubated with the indicated isobavachalcone (50 µM) and TNF-α (10 ng/mL) for 4 h. Monocytes labeled with a cell tracker were added to the HUVEC monolayer and the degree of adhesion was measured. B Monocyte adhesion to HUVECs was quantified. Bars represent the mean ± SD of three experiments. ***, p < 0.001 vs. CB; ##, p < 0.01 vs. TNF-α
Discussion
Despite recent advances in the understanding of the pathogenesis of vascular diseases, there is limited information on the preventive measures against the development of these diseases. Isobavachalcone is a chalcone isolated from the multipurpose medicinal plant Psoralea corylifolia, which is known to possess antioxidant, antiplatelet, antimicrobial, anti-inflammatory, anti-tumor, and neuroprotective properties. It is a report that isobavachalcone exhibits an anti-inflammatory effect by reducing ICAM-1 in the inflammatory response induced by LPS [15]. In this study, human venous endothelial cells were directly stimulated with TNF-α, which has a single mechanism, rather than LPS, to induce an inflammatory response. We also demonstrated the effect of regulating VCAM-1 involved in adhesion of lymphocytes, monocytes, eosinophils, and basophils to the endothelium [16] as well as ICAM-1 limited to leukocyte-endothelium adhesion. Taken together, we demonstrated that isobavachalcone suppresses TNF-α-induced ICAM-1 and VCAM-1 expression by regulating the NF-κB signaling pathway in cultured HUVECs. These data suggest that isobavachalcone has an anti-vascular inflammatory effect that is mediated by the downregulation of adhesion molecule expression.
Leukocyte adhesion to activated endothelial cells is a key process in the inflammatory response and the development of atherosclerosis. NF-κB is functionally important for the expression of pro-inflammatory genes, including vascular cell adhesion molecules [17, 18]. Isobavachalcone inhibits the adherence of leukocytes to adjacent endothelial cells by reducing the expression of ICAM-1 and VCAM-1. These data suggest that isobavachalcone regulates the rate of leukocyte recruitment at inflammatory sites.
In conclusion, our findings suggest that isobavachalcone suppresses TNF-α-induced ICAM-1 and VCAM-1 protein expression in HUVECs by regulating the NF-κB signaling pathway. These results demonstrated that isobavachalcone prevents TNF-α-induced vascular inflammation and has the potential to protect against inflammatory diseases. Isobavachalcone, is believed to impart beneficial effects and play a pivotal role in vascular diseases, such as hypertension, systemic inflammation, and oxidative stress. In addition, isobavachalcone is expected to prevent the early progression of atherosclerosis.
Availability of data and materials
The datasets used in this study are available from the corresponding authors upon request.
References
Sprague AH, Khalil RA (2009) Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 78:539–552. https://doi.org/10.1016/j.bcp.2009.04.029
McHale JF, Harari OA, Marshall D, Haskard DO (1999) Vascular endothelial cell expression of ICAM-1 and VCAM-1 at the onset of eliciting contact hypersensitivity in mice: evidence for a dominant role of TNF-alpha. J Immunol 162:1648–1655
Chan SC, Shum DK, Tipoe GL, Mak JC, Leung ET, Ip MS (2008) Upregulation of ICAM-1 expression in bronchial epithelial cells by airway secretions in bronchiectasis. Respir Med 102:287–298. https://doi.org/10.1016/j.rmed.2007.08.013
Abraham E, Singer M (2007) Mechanisms of sepsis-induced organ dysfunction. Crit Care Med 35:2408–2416. https://doi.org/10.1097/01.ccm.0000282072.56245.91
Duque GA, Descoteaux A (2014) Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5:491. https://doi.org/10.3389/fimmu.2014.00491
Hofmann S, Grasberger H, Jung P, Bidlingmaier M, Vlotides J, Janssen OE, Landgraf R (2002) The tumour necrosis factor-alpha induced vascular permeability is associated with a reduction of VE-cadherin expression. Eur J Med Res 7:171–176
Palko-Łabuz A, Błaszczyk M, Środa-Pomianek K, Wesołowska O (2021) Isobavachalcone as an active membrane perturbing agent and inhibitor of ABCB1 multidrug transporter. Molecules 26:4637. https://doi.org/10.3390/molecules26154637
He H, Wang C, Liu G, Ma H, Jiang M, Li P, Lu Q, Li L, Qi H (2021) Isobavachalcone inhibits acute myeloid leukemia: potential role for ROS-dependent mitochondrial apoptosis and differentiation. Phytother Res 35:3337–3350. https://doi.org/10.1002/ptr.7054
Wang M, Lin L, Lu JJ, Chen X (2021) Pharmacological review of isobavachalcone, a naturally occurring chalcone. Pharmacol Res 165:105483. https://doi.org/10.1016/j.phrs.2021
Gao D, Liu F, Li Z, Guan Y (2019) Isobavachalcone attenuates Sephadex-induced lung injury via activation of A20 and NRF2/HO-1 in rats. Eur J Pharmacol 848:49–54. https://doi.org/10.1016/j.ejphar.2019.01.034
Ahn SY, Cho CH, Park KG, Lee HJ, Lee S, Park SK, Lee IK, Koh GY (2004) Tumor necrosis factor-alpha induces fractalkine expression preferentially in arterial endothelial cells and mithramycin A suppresses TNF-alpha-induced fractalkine expression. Am J Pathol 164:1663–1672. https://doi.org/10.1016/s0002-9440(10)63725-x
Moon SO, Kim W, Sung MJ, Lee S, Kang KP, Kim DH, Lee SY, So NJ, Park SK (2006) Resveratrol suppresses tumor necrosis factor-alpha-induced fractalkine expression in endothelial cells. Mol Pharmacol 70:112–119. https://doi.org/10.1124/mol.106.022392
Liu T, Zhang L, Joo DH, Sun SC (2017) NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2:e17023. https://doi.org/10.1038/sigtrans.2017.23
Zhong L, Simard MJ, Huot J (2018) Endothelial microRNAs regulating the NF-kB pathway and cell adhesion molecules during inflammation. FASEB J 32:4070–4084. https://doi.org/10.1096/fj.201701536R
Lee KM, Kim JM, Baik EJ, Ryu JH, Lee SH (2015) Isobavachalcone attenuates lipopolysaccharide-induced ICAM-1 expression in brain endothelial cells through blockade of toll-like receptor 4 signaling pathways. Eur J Pharmacol 5(754):11–18
Laurelee O, Catherine H, Richard T, Cornelia V, Stefan L, Chi-R G, Roy L (1989) Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 59(6):1203–1211
De Martin R, Hoeth M, Hofer-Warbinek R, Schmid JA (2000) The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol 20:E83–E88. https://doi.org/10.1161/01.atv.20.11.e83
Zao S, Liang M, Wang Y, Hu J, Zhong Y, Li J, Huang K, Li Y (2019) Chrysin suppresses vascular endothelial inflammation via inhibiting the NF-κB signaling pathway. J Cardiovasc Pharmacol Ther 24:278–287
Acknowledgements
Not applicable.
Funding
This study was supported by a research grant from the Korea Food Research Institute (Grant Number E0210300).
Author information
Authors and Affiliations
Contributions
Experimental Research: ASL; Data Analysis: ASL, JYH; Manuscript Writing: ASL, SYC; Study Design and Supervision: SYC. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, A.S., Hur, J. & Choi, S.Y. Isobavachalcone attenuates TNF-α-induced ICAM-1 and VCAM-1 expression in human umbilical vein endothelial cells by regulating the NF-κB signaling pathway. Appl Biol Chem 65, 45 (2022). https://doi.org/10.1186/s13765-022-00717-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-022-00717-7