Abstract
Isoacteoside is a caffeoyl phenylethanoide glycoside found in various plant parts, such as the flower of Magnolia denudata. In particular, magnolia has been studied for its anti-obesity, anticancer, and anti-inflammatory effects. However, isoacteoside has not been extensively studied, except for its anti-inflammatory effects. In this study, the anti-obesity effects of isoacteoside were investigated in 3T3-L1 mouse pre-adipocytes. Isoacteoside treatment did not induce cytotoxicity in 3T3-L1 cells up to a concentration of 1000 μM. The anti-obesity effects on 3T3-L1 cells were confirmed using oil red O staining. In addition, the expression of obesity-related proteins and genes, such as peroxisome proliferator-activated acceptor gamma (PPARγ), CCAAT/enhancer-binding protein alpha (C/EBPα), and perilipin (PLIN1), was determined by western blotting and qRT-PCR assays to confirm the anti-obesity effects of isoacteoside. The results of this study suggest that isoacteoside, a natural substance isolated from plant extracts, is not highly toxic to normal cells, such as pre-adipocytes, and displays anti-obesity effects in vitro.
Similar content being viewed by others
Introduction
Obesity is a disease that is prevalent worldwide, and as of 2016, 39% of the world’s population is overweight and 12% is obese. The extent of obesity increased by three times compared to that in 1975 [1]. Obesity is a dangerous disease because it can cause chronic inflammation and lead to cancer and cardiovascular disease [2]. Therefore, studies investigating obesity in many models are being conducted.
Our previous studies have shown that the extract of Magnolia danudata flower exerts anti-obesity effects. Consequently, the chemical composition of the magnolia flower extract has been investigated and isoacteoside was identified as one of the 16 main compounds [3, 4]. Other studies have also reported that several plant extracts that exhibit anti-obesity effects contain isoacteoside [5, 6]. However, there is a lack of scientific evidence establishing that isoacteoside is responsible for these anti-obesity effects. In this study, we hypothesized that isoacteoside exerts anti-obesity effects. We used 3T3-L1 mouse pre-adipocytes to observe the inhibitory effects of isoacteoside on lipid accumulation in vitro and to study the expression of anti-obesity-related proteins following isoacteoside treatment.
Materials and methods
Materials
The 3T3-L1 pre-adipocytes were obtained from the Korean Cell Line Bank (Seoul, Korea). Isoacteoside was purchased from MedChemExpress Co. (#HY-N0022, purity > 99.27%; NJ, USA). Differentiation reagents such as 3-isobutyl-1-methylxanthine (IBMX, #I5879), dexamethasone (DEX, #D4902), and insulin (INS, #I6634) were purchased from Sigma-Aldrich Chemicals (MO, USA).
Cell culture and differentiation
The 3T3-L1 pre-adipocytes were incubated at 37 °C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM, #LM001-01, Welgene, Gyeongsan, Korea) containing 10% bovine calf serum (BCS, #S003-01, Welgene), 100U/mL penicillin, and 100 μg/mL streptomycin (#LS202-02, Welgene). The cell culture was passaged with 104 cells/mL every 72 h. To differentiate 3T3-L1 pre-adipocytes into adipocytes, 1 × 105 cells per well were added into a 6-well plate and incubated for a week to reach confluency. Then, the medium was replaced with DMEM containing 10% fetal bovine serum (FBS, #S001-01, Welgene) with 0.05 mM IBMX, 1 μM DEX, and 20 μg/μL INS to initiate differentiation. The differentiation medium was replaced every 2 days with DMEM containing 20 μg/μL INS.
Cell viability
The 3T3-L1 pre-adipocytes (density: 5 × 104 cells per well) were cultured in 96-well cell culture plates (#30096, SPL Life Science, Pocheon, Korea) and incubated in a humidified atmosphere of 5% CO2 at 37 °C. Isoacteoside was added to the cells after incubation for 24 h. After 24 and 48 h of incubation, the cells were washed twice with Dulbecco’s phosphate-buffered saline (DPBS, #LB001-02, Welgene), and 10 μL of water-soluble tetrazolium-1 (WST-1, #MK-400, Takara Bio, Shiga, Japan) solution was added to each well. Absorbance was measured at 450 nm using a microplate reader (EL800, Bio-Tek, MA, USA).
Oil red O staining
Nine days after differentiation, the adipocytes were stained with oil red O (#O0625, Sigma-Aldrich Chemicals) to measure the fat content of the adipocytes. The cells were washed twice with phosphate buffered saline (PBS) and fixed with 10% formalin (#252549, Sigma-Aldrich Chemical) for 1 h. Then, the cells were washed with distilled water and treated with oil red O dissolved in 60% isopropanol (#W292907, Sigma-Aldrich Chemical) for 30 min. The absorbance of the stained fat was measured at a wavelength of 490 nm after extraction of oil red O from the cell using 100% isopropanol.
Quantitative RT-PCR (qRT-PCR)
The cells were harvested 9 days after isoacteoside treatment, i.e., when the cells differentiated. TRIzol reagent (#15596026, Invitrogen, CA, USA) was used to isolate total RNA from the 3T3-L1 cells. The first-strand synthesis kit (GeneAll, Seoul, Korea) was used to synthesize cDNA, and the synthesized cDNA was quantified at 280 nm using a Nanodrop spectrophotometer (Coliri LB 915, JC Bio, Seoul, Korea). BrightGreen 2 × qPCR MasterMix (#MasterMix-S-XL, ABM, Vancouver, Canada) was used for 45 cycles of qPCR performed in a CFX Connect Real-Time PCR machine (Bio-Rad, CA, USA). Primer sequences are listed in Table 1. β-Actin gene was used as the housekeeping gene. Gene expression was calculated using the 2−ΔΔCt method.
Western blotting
Protein was extracted from the cells 9 days, at which protein expression was the highest, after differentiation using radioimmunoprecipitation assay buffer (RIPA, #RC2002-050-00, Biosesang, Sungnam, Korea) containing a halt protease and phosphatase inhibitor cocktail (#78429, Thermo Fisher Scientific, MA, USA). The extracted proteins were quantified using a BCA protein assay kit (#23225, Thermo Fisher Scientific). The protein was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (0.45 μm, EMD Millipore, MA, USA). The membranes were blocked for 1 h with 5% skim milk or 5% BSA solution. The membranes were then incubated overnight at 4 °C with primary antibodies diluted to 1/1000. Primary antibodies against PPARγ (#2435), C/EBPα (#8178), and PLIN1 (perilipin, #9349) were purchased from Cell Signaling Technology (MA, USA). The membranes were washed twice with Tris-buffered saline (TBS) containing 0.1% Tween-20, and incubated for 2 h with horseradish peroxidase (HRP)-conjugated secondary antibodies (#7074, Cell Signaling Technology) at room temperature. Finally, the membranes were washed twice with 0.1% Tween-20 in TBS and visualized using ECL western blotting detection reagents (Thermo Fisher Scientific). Luminescence was analyzed using ChemiDoc XRS imager (Bio-Rad) with the Image Lab Software (version 4.1, Bio-Rad).
Data analysis
Data analysis was performed using SPSS software (IBM, NY, USA) with Student’s t-test. Data are presented as mean ± standard deviation (SD). Values of p < 0.05, < 0.01, or < 0.001 were considered statistically significant.
Results and discussion
Effect of isoacteoside on cell viability
The wide diversity of natural products found in plants enables the discovery of novel compounds to combat chronic diseases such as those caused by obesity. Isoacteoside, a phenylethanoid glycoside and an isomer of acteoside (Fig. 1), has been isolated from a number of plant species, including Magnolia denudata, where it is found in the flower [3]. A few studies have demonstrated the physiological activity of isoacteoside. Isoacteoside inhibits a-amylase and lipase [7] and inhibits the production of pro-inflammatory cytokines in the human mast cell line HMC-1, by blocking the caspase‑1, MAPK, and NF-kB signaling pathways [8]. Isoacteoside attenuates acute kidney injury induced by severe acute pancreatitis and septic acute lung injury. In addition, it exerts antioxidant, anti-inflammatory, and anticancer effects [9,10,11]. However, the in vitro bioactivity of isoacteoside with regard to its anti-obesity effects has not been studied. In this study, the anti-obesity effects of isoacteoside were investigated in 3T3-L1 pre-adipocytes.
The effects of isoacteoside on the viability of 3T3-L1 cells are shown in Fig. 2. Despite increasing the concentration of isoacteoside, cell viability remained unchanged until a certain concentration was reached. After 24 h of isoacteoside treatment, cell viability was approximately 100.67 ± 0.15, 102.64 ± 0.31, 103.42 ± 0.58, 100.88 ± 0.17, 98.57 ± 0.93, 28.78 ± 2.77 and 7.82 ± 0.19% in comparison to the control group for 100, 300, 500, 1000, 1500, 2000, and 2500 μM isoacteoside-treatment groups, respectively. After 48 h of isoacteoside treatment, cell viability was approximately 104.51 ± 4.25, 100.80 ± 9.11, 104.80 ± 5.48, 106.10 ± 7.33, 48.41 ± 3.67, 3.10 ± 0.07, 3.09 ± 0.02% in comparison to the control group for 100, 300, 500, 1000, 1500, 2000, and 2500 μM isoacteoside-treatment groups, respectively. After 72 h of isoacteoside treatment, cell viability was similar to 48 h (data was not shown). These data suggest that isoacteoside does not significantly affect cell viability below a concentration of 1000 μM. Therefore, we inferred that while isoacteoside has almost no effect on cell viability, it might have other effects, such as on the adipogenic differentiation of 3T3-L1 cells.
Effect of isoacteoside on differentiation of 3T3-L1 cells
The pre-adipocytes were treated with differentiation medium, containing DMEM with IBMX, DEX, and INS in order to generate lipid droplets. These lipid droplets developed a red color upon staining with oil red O, as observed from the cell culture plates (Fig. 3A) and by optical microscopy (Fig. 3B). However, although the cells were treated with differentiation medium, lipid concentration decreased from 100% (control without treatment) to 68.69 ± 1.86% and 33.85 ± 0.22%, following treatment 75 and 150 mM isoacteoside, respectively. This result indicates that with an increase in the concentration of isoacteoside, the lipid content decreases (Fig. 3C). These results suggest that isoacteoside inhibits the differentiation of 3T3-L1 cells and thus the formation of lipid droplets.
Effects of isoacteoside on lipid accumulation in differentiating 3T3-L1 cells. A Images of cell culture plate of 3T3-L1 cells after staining lipid droplets with oil red O. B Images of 3T3-L1 cells obtained using a light microscope after staining lipid droplets with oil red O. C Lipid content of 3T3-L1 cells following isoacteoside treatment. The percentage was normalized based on the differentiated control group (+ / −) as 100. ***p < compared to the differentiated cell group without isoacteoside treatment
Effect of isoacteoside on the expression of mRNAs related to adipogenesis and lipogenesis
To observe the extent of inhibition of adipogenesis and lipogenesis by isoacteoside, qRT-PCR was performed to quantify the expression levels of the related genes (Fig. 4). The primer sequences used for this experiment are listed in Table 1. The mRNA expression of peroxisome proliferator-activated acceptor gamma (PPARγ) was 10.78 ± 2.62% in the undifferentiated control group (− / −), compared to that in the differentiated control group (+ / −). The mRNA expression of PPARγ decreased to 42.34 ± 1.08% and 13.13 ± 0.17%, following treatment with 75 μM and 150 μM isoacteoside (Fig. 4A), respectively. The mRNA expression of CCAAT/enhancer-binding protein alpha (C/EBPα) was 2.65 ± 0.17% in the undifferentiated control group (− / −), compared to that in the differentiated control group (+ / −). The mRNA expression of C/EBPα decreased to 46.15 ± 0.70% and 8.73 ± 1.09% upon treatment with 75 μM and 150 μM isoacteoside, respectively (Fig. 4B). The mRNA expression of perilipin (PLIN1) was 0.63 ± 0.06% in the undifferentiated control group (− / −), compared to that in the differentiated control group (+ / −). The mRNA expression of PLIN1 decreased to 45.25 ± 2.53% and 6.13 ± 1.58%, upon treatment with 75 μM and 150 μM isoacteoside, respectively (Fig. 4C). These results suggest that isoacteoside suppresses the expression of genes related to adipogenesis and lipogenesis.
Quantitative real-time PCR (qRT-PCR) analysis of adipogenesis- and lipogenesis-related genes after isoacteoside treatment of 3T3-L1 cells. A Relative mRNA level of PPARγ was normalized with that of β-actin gene of each group. B Relative mRNA level of C/EBPα was normalized with that of β-actin gene of each group. C Relative mRNA level of PLIN1 (perilipin) was normalized with that of β-actin gene of each group. The levels of mRNA for each group were normalized based on the differentiated control group (+ / −). Primer sequences used for qRT-PCR analysis are shown in Table 1. *p < 0.05, **p < 0.01, ***p < 0.001 compared to the differentiated cell group without treatment of isoacteoside
Effect of isoacteoside on the expression of adipogenesis and lipogenesis-related proteins
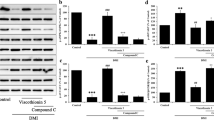
The expression of three proteins involved in the adipogenesis of 3T3-L1 cells and lipid droplet accumulation is shown in Fig. 5A. The expression of PPARγ in the undifferentiated control group (− / −) was 17.16 ± 3.85% compared to that in the differentiated control group (+ / −). PPARγ expression levels were 38.50 ± 3.14% and 30.02 ± 8.42% in the groups treated with 75 μM and 150 μM isoacteoside, respectively, compared to that in the differentiated control group (+ / −) (Fig. 5B). The expression of C/EBPα in the undifferentiated control group (− / −) was 12.53 ± 1.72% compared to that in the differentiated control group (+ / −). C/EBPα expression levels were 33.16 ± 3.42% and 18.81 ± 0.22% in the groups treated with 75 μM and 150 μM isoacteoside, respectively, compared to that in the differentiated control group (+ / −) (Fig. 5C). The expression of PLIN1 in the undifferentiated control group (− / −) was 8.22 ± 1.55% compared to that in the differentiated control group (+ / −). PLIN1 expression levels were 31.70 ± 1.79% and 6.35 ± 2.78% in the groups treated with 75 μM and 150 μM isoacteoside, respectively, compared to that in the differentiated control group (+ / −) (Fig. 5D).
Western blot analysis of adipogenesis- and lipogenesis-related proteins after isoacteoside treatment of 3T3-L1 cells. A Proteins related to adipogenesis and lipogenesis were quantified by western blotting. B Levels of PPARγ were normalized to that of β-actin of each group. C Levels of CEBPα were normalized to that of β-actin of each group. D Levels of PLIN1 (perilipin) were normalized to that of β-actin of each group. The percentage was normalized using the differentiated control group (+ / −) as 100. ***p < 0.001 compared to the differentiated cell group without isoacteoside treatment
PPARγ and C/EBPα are two important proteins involved in adipogenesis and lipogenesis, respectively [12]. PPARγ is a nuclear receptor protein within the PPAR group and acts as a transcription factor involved in the differentiation of pre-adipocytes and lipid metabolism [13]. PPARγ targets fat-specific marker genes such as genes encoding adipocyte fat acid-binding protein (aP2) and sterol regulatory element-binding protein 1 (SREBP-1). PPARγ promotes adipose tissue production and reduces the expression of leptin, thereby inhibiting lipolysis and promoting lipid accumulation [14,15,16]. C/EBPα is also a transcription factor that binds aP2 with PPARγ and regulates adipogenesis [17, 18]. C/EBPα is PPARγ-dependent, indicating that C/EBPα alone cannot induce adipogenesis in the absence of PPARγ [19]. Perilipin is a protein found in lipid droplets present on the surface of differentiated adipocytes, where it blocks access to lipases to regulate lipogenesis and promotes PPARγ expression [20]. Therefore, our results indicate that the expression of adipogenesis and lipogenesis-related proteins was suppressed by isoacteoside treatment (Fig. 5). In general, natural products have few side effects on the human body, except in a few cases [21].
The present study aimed to investigate whether isoacteoside inhibits adipogenesis and lipogenesis in 3T3-L1 pre-adipocytes. Although the viability of 3T3-L1 cells was not affected by isoacteoside (< 1000 µM), anti-obesity effects were observed at concentrations lower than 1000 µM. This was supported by the fact that lipid content decreased in the cell culture after treatment with 75 μM and 150 μM isoacteoside, as shown by oil red O staining and the expression levels of adipogenesis- and lipogenesis-related proteins and mRNAs. These results showed that isoacteoside exerted anti-obesity effects on 3T3-L1 cells.
Although, more studies are needed to support this promising mechanism, this study might provide implication in animal and human anti-obesity effect. However, isoacteoside will need to be compared for their efficacy with lipid-lowering medications, such as simvastatin, mevinolin, or orlistat. In the future, in vivo studies analyzing other tissues involved in obesity are required to validate the anti-obesity effects of isoacteoside.
Availability of data and materials
Not applicable.
Abbreviations
- DEX:
-
Dexamethasone
- ECL:
-
Enhanced chemiluminescence
- HRP:
-
Horseradish peroxidase
- IBMX:
-
3-Isobutyl-1-methylxanthine
- INS:
-
Insulin
- PVDF:
-
Polyvinylidene fluoride
- SD:
-
Standard deviation
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TBS:
-
Tris-buffered saline
- PPARγ:
-
Peroxisome proliferator-activated acceptor gamma
- CEBPα:
-
CCAAT/enhancer-binding protein alpha
- WST-1:
-
Water-soluble tetrazolium-1
References
NCD-RisC (2017) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390:2627–2642
Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B (2006) Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17:4–12
Joo YH, Nam MH, Chung N, Lee YK (2020) UPLC-QTOF-MS/MS screening and identification of bioactive compounds in fresh, aged, and browned Magnolia denudata flower extracts. Food Res Int 133:109192
Joo YH, Chung N, Lee YK (2020) Anti-obesity effect of fresh and browned Magnolia denudata flowers in a high fat diet murine model. J Funct Foods 75:104227
Ji L, -Ping, Ren-Chao T, Xiao-Meng S, Hao-Yue Z, Shuai S, Ai-Zhen X, Zheng-Tao W, Li Y (2021) Comparison of main chemical composition of Plantago asiatica L. and P. depressa Willd. seed extracts and their anti-obesity effects in high-fat diet-induced obese mice. Phytomedicine 81:153362
Yan Y, Du C, Li Z, Zhang M, Li J, Jia J, Li A, Qin X, Song Q (2018) Comparing the antidiabetic effects and chemical profiles of raw and fermented Chinese Ge-Gen-Qin-Lian decoction by integrating untargeted metabolomics and targeted analysis. Chin Med 13:54
Yang Q, Qi M, Tong R, Wang D, Ding L, Li Z, Huang C, Wang Z, Yang L (2017) Plantago asiatica L. seed extract improves lipid accumulation and hyperglycemia in high-fat diet-induced obese mice. Int J Mol Sci 18:1393
Nam SY, Kim HY, Yoou MS, Kim AH, Park BJ, Jeong HJ, Kim HM (2015) Anti-inflammatory effects of isoacteoside from Abeliophyllum distichum. Immunopharmacol Immunotoxicol 37:258–264
Yang X, Guo F, Peng Q, Liu Y, Yang B (2019) Suppression of in vitro and in vivo human ovarian cancer growth by isoacteoside is mediated via sub-G1 cell cycle arrest, ROS generation, and modulation of AKT/PI3K/m-TOR signaling pathway. J BUON 24:285–290
Luo YN, He NN, Xu J, Wang R, Cao W, Chen D (2021) Isoacteoside attenuates septic acute lung injury by inhibiting inflammation, oxidative stress and endothelial hyperpermeability in mice. https://doi.org/10.21203/rs.3.rs-501345/v1
Wang B, Li XH, Song Z, Li ML, Wu XW, Guo MX, Zhang XH, Zou XP (2021) Isoacteoside attenuates acute kidney injury induced by severe acute pancreatitis. Mol Med Rep 23:287
Hu ED, Tontonoz P, Spiegelman BM (1995) Transdifferentiation of myoblasts by the adipogenic transcription factors PPARγ and C/EBPα. Proc Natl Acad Sci USA 92:9856–9860
Rosen ED, Spiegelman BM (2000) Molecular regulation of adipogenesis. Annu Rev Cell Dev Bi 16:145–171
Kallen CB, Lazar MA (1996) Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3L3-L1 adipocytes. Proc Natl Acad Sci USA 93:5793–5796
Picard F, Kurtev M, Chung NJ, Topark-Ngarm A, Senawong T, de Oliveira RM, Leid M, McBurney MW, Guarente L (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429:771–776
Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N (1998) Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem 273:16710–16714
Lee HW, Hur J, Park HY, Kim Y, Ha SK (2021) Glycolaldehyde disrupts insulin signaling and glucose uptake through adipogenesis. Appl Biol Chem 64:1–14
Wu Y, Tan F, Zhang T, Xie B, Ran L, Zhao X (2020) The anti-obesity effect of lotus leaves on high-fat-diet-induced obesity by modulating lipid metabolism in C57BL/6J mice. Appl Biol Chem 63:1–11
Rosen ED, Hsu CH, Wang XZ, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM (2002) C/EBPα induces adipogenesis through PPARγ: a unified pathway. Gene Dev 16:22–26
Arimura N, Horiba T, Imagawa M, Shimizu M, Sato R (2004) The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J Biol Chem 279:10070–10076
Zeng ZP, Jiang JG (2010) Analysis of the adverse reactions induced by natural product-derived drugs. Brit J Pharmacol 159:1374–1391
Acknowledgements
This research was supported by a grant of Korea University.
Funding
Korea University funded this study.
Author information
Authors and Affiliations
Contributions
CGC wrote and revised the manuscript and performed the experiments. DJL performed the experiments. NC and YHJ supervised the study, interpreted the data, and edited the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, C.G., Lee, D.J., Chung, N. et al. Anti-obesity effects of isoacteoside on 3T3-L1 adipocytes. Appl Biol Chem 65, 33 (2022). https://doi.org/10.1186/s13765-022-00701-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-022-00701-1