Abstract
The mass usage of veterinary pharmaceuticals in farms has contributed to environmental pollution in vicinity waters, soils, and sediments from farms and composting facilities. In the present study, we investigated the usage of four antibiotics (viz., lincomycin, sulfamethazine, sulfamethoxazole, and trimethoprim) to understand their contamination routes from livestock manure piles. Residual levels of these antibiotics in a nearby reservoir were set as a positive control (Site 1), and a swine manure pile in a farm (Site 2) and a soil sample around the manure pile (Site 3) were selected for this study. Artificial rainwater was flowed into the manure sample (Site 2), the soil sample around the manure pile (Site 3), and a soil sample around the vicinity river (Site 4). A stream sample (Site 5) around the manure pile and river water near the manure pile (Site 6) were also collected. For qualitative and quantitative analyses, analytical validation was performed, and all the four antibiotics were detected at Site 1 in the concentration range of 0.03–1.6 µg/L. Lincomycin was the antibiotic with the highest detection level. At Site 2, the detection level of all antibiotics remained at 0.3–17.3 µg/L, and their residual amounts were continuously detected in subsequent samples with approximately 30-fold decrease. The migration of antibiotics was confirmed to be independent of pH value. Therefore, this study indicates that farm manure pile should be thoroughly managed for antibiotic contamination in vicinity areas with periodical monitoring, especially waterways.
Similar content being viewed by others
Introduction
Pharmaceuticals and personal care products (PPCPs) are increasingly being used at homes, hospitals, and livestock farms, as a result of which they are frequently detected in soil environments, effluents of sewage treatment plants, rivers, reservoirs, and sediments [1,2,3]. Especially, there has been an increasing interest in the environmental contamination caused by perfumes, shower supplies, insect repellents, and pharmaceutical substances, including antibiotics [4, 5]. In the case of more than 60% of antibiotics, unchanged antibiotics could be excreted to the outside of livestock bodies via feces and urine and hence could be easily introduced into the aquatic environment [6].
PPCPs that are introduced into the aquatic ecosystem can cause acute and chronic toxicity to aquatic organisms [7, 8]. It has been reported that the metabolites of these PPCPs can also enter the aquatic environment and cause adverse effects on the aquatic ecosystem [9]. Household sewage and factory wastewater are collected and treated through sewage pipes at a sewage treatment plant [5]. Although the effluent emission limit in the sewage treatment plant is set to pass biological standards and physicochemical standards, it cannot be considered that the treatment of trace organic substances such as PPCPs is performed in a perfect manner [9, 10].
In several countries, livestock manures containing large amounts of medicinal substances are discharged into the environment with appropriate treatment at wastewater treatment facilities, composting facilities, and liquefaction facilities for self-treatment or consignment treatment [11]; however, the majority of livestock manure does not go through an appropriate treatment process. There are several cases of inflow into the surrounding soil and rivers due to the rapid expansion of intensive livestock facilities as a nonpoint source [12].
In this manner, untreated PPCPs from sewage treatment plant effluents and unexpected and livestock manure runoffs flow into various water systems and disturb the aquatic ecosystem [2, 10, 11]. When sludge, a byproduct of sewage treatment, is used for improving farmland, PPCPs can be easily contaminated in agricultural environments and probably remain in the agricultural environment and get absorbed by growing crops [13].
This study was conducted to trace the contamination routes of livestock antibiotics that contaminate waterways from livestock manure piles, which were not transported to treatment facilities but stored in livestock farms for composting process and then introduced into vicinity soils and rivers via factitious water. The level of residual antibiotics in reservoirs as control and that in composting manure pile, vicinity soils and waterways, and rivers were measured.
Materials and methods
Sampling
Samples were collected in May 2014 at the upstream point in Ulsan, South Korea. The sampling point was selected as the point where manure flowed into the river due to surface runoff, and for the control group, a reservoir was selected in the same tributary. Samples were transported to the laboratory immediately after collection and stored in a refrigerator at ≤ 4 °C. Pretreatment and instrument analysis were completed within 2 weeks of sample collection.
Selection of analytical items in PPCPs
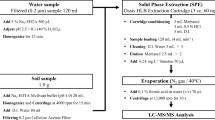
Although there are several different substances corresponding to PPCPs, four antibiotics, viz., lincomycin, sulfamethazine, sulfamethoxazole, and trimethoprim, were analyzed in this study to determine their levels in vicinity soils and waterways after runoff of livestock manure. The structure of the four livestock antibiotics is shown in Additional file 1: Fig. S1.
Sample preparation and instrumental analysis
In the case of soil and manure samples (Sites 2 to 4), 200 mL of a pH solution prepared using 3.5 M sulfuric acid was poured into 20 g of the sample to confirm the effect of nonpoint contamination. The experiment was conducted under three pH conditions (4, 5, and 6). A 0.2-μm PTFE syringe filter (Whatman, Sigma-Aldrich, St. Louis, MI, USA) was used to remove solid substances as impurities from the leachate. The filtered sample was pretreated in the same manner as done for the water sample. An automatic extraction device (SPE-DEX, Horizon Technology 4790, Salem, NH, USA) equipped with a hydrophilic–lipophilic balance (HLB) cartridge (Waters, Milford, MA, USA) was used for the extraction of PPCPs from the sample. Before extraction, 3.5 M sulfuric acid was added to adjust the sample pH to 5, and 400 μL of ethylenediaminetetraacetic acid [EDTA], 0.1 μg/mL) and 20 μL of internal standard (Sulfathiazole-d4, Ibuprofen-13C3, 17β-estradiol-13C2) were added to the samples.
Methyl tertiary butyl ether (MTBE), methanol, and distilled water were pre-flowed into the cartridge (Oasis HLB 200 mg), and then the sample was passed, washed with distilled water, and dried. The analyte was eluted with a 10% methanol/MTBE mixed solution and methanol and then concentrated, after which qualitative and quantitative analyses were conducted by liquid chromatography/mass spectrometry (LC/MS/MS, Waters Acquity Xevo TQ-S MS/MS, UK). The analyte was subjected to electrospray ionization using the multiple reaction monitoring method. Specific analytical conditions (column properties, column oven temperature, mobile phase, MS conditions, etc.) are represented in Additional file 1: Fig. S2.
Quality assurance and quality control
To prepare the calibration curve, a standard mixture solution was prepared at a concentration range of 0.01–2 μg/L (5 points) and pretreated in the same manner as done for the sample. The correlation coefficient (R2) of the calibration curve was ≥ 0.99, and the analytes were quantified using the internal standard method [4]. For checking the matrix effect of the interfering substances contained in the actual sample, a standard substance was injected into the sample (adjusted to 0.5 μg/L concentration) and measured three times. According to the result, the accuracy in the actual sample was 77–129%, and the precision (relative standard deviation) was 0–19%, indicating that the effect of the interfering substances was acceptable. Furthermore, for the strict quantification of PPCPs to be analyzed, the limit of quantitation was defined as a concentration with a signal/noise (S/N) ratio of ≥ 10, in the range of 0.02–0.04 μg/L, for each substance.
Results
Four types of veterinary antibiotics, including lincomycin, sulfamethoxazole, sulfamethazine, and trimethoprim, were analyzed in this study. We attempted to investigate the level of contamination of these veterinary antibiotics from the manure pile in the livestock farm that flowed to the nearby soils and into the nearby river via unexpected runoff. Six samples were collected, and a water sample of a nearby reservoir (Site 1) was used as a control. In addition to Site 1, manure (Site 2), soil around the manure pile (Site 3), soil around the vicinity river (Site 4), water of the vicinity stream around the manure pile (Site 5), and river water (Site 6) were collected and used for analysis (Fig. 1).
As mentioned earlier, leachates of samples from Sites 2, 3, and 4 were obtained by flowing artificial rainwater (pH 5). The experiment was conducted three times per site, and the result was expressed as the average and deviation of the level of each antibiotic compound (Fig. 2). At Site 1, all the four types of antibiotics were identified, among which the residual concentration of lincomycin was the highest, with the detection level reaching 1.6 µg/L. Other antibiotics were detected less than 12 times the concentration of lincomycin. Therefore, we believe that the vicinity reservoir was already contaminated with these antibiotics.
At Site 2, all the four antibiotics were detected in the manure pile in the concentration range of 0.3–17.3 µg/L, and the concentration of trimethoprim was found to be the lowest in this study. At Site 3, trimethoprim was no longer detected, and lincomycin was detected at 2.5 µg/L. The other two antibiotics were detected at < 0.15 µg/L concentration. Site 3 was not far from the place where the manure was stored, and hence the leachate was contaminated with large amounts of antibiotics. At Site 4, trimethoprim and lincomycin were not detected, whereas the other two antibiotics were detected at < 0.5 µg/L concentration. However, at Sites 5 and 6, lincomycin was recovered, and sulfamethoxazole and sulfamethazine were determined in the concentration range of approximately 0.1–0.9 µg/L (Fig. 2).
Figure 3 illustrates a comparison of accumulated concentrations of each of the four antibiotics. The total amount of antibiotics detected in this study was significantly higher at Site 2 than in other sampling sites because of the greater contribution of sulfamethazine and sulfamethoxazole among the antibiotics. However, the detection levels of these sulfa-type antibiotics at Site 2 were found to be 90 times higher than those in other sites, as the level of remaining antibiotics in the soil was high. Moreover, lincomycin and trimethoprim might be less tightly adsorbed onto soil matrices compared with other sulfa-type antibiotics (Fig. 3), although some adsorption of lincomycin might be assumed.
Figure 4 shows the relationship between pH level and translocation of residual antibiotics in the soil samples adjacent to the manure pile (Site 3) according to the pH condition of artificial rainwater. The experiments were conducted in triplicate, and the average and deviation of each antibiotic were calculated (Fig. 4). The influence of different pH values of artificial rainwater was not observed. Therefore, even during an intense acid rainfall, the degree of leaching of antibiotics may not differ significantly. The reason for using the Site 3 sample was that it had a roof on Site 2, assuming that it was not affected by rainfall.
Discussion
The total amount of antibiotics and anticoccidials used in Korean livestock farms was reported to be almost 984 tons in 2018, and the total amount of antibiotics used in pig farms was 492 tons, which was 50% [14]. Because these antibiotics remain in the meat of each animal and are presumably delivered to consumers, it is important to monitor the residuals of these antibiotics with regular periodic schedules. A recent study reported that an antibiotic was detected in 26 of 58 chicken meat samples, with the detection rate reaching 45%. Amoxicillin was detected in 9 cases in the concentration range of 1.43–3.41 µg/kg, enrofloxacin was detected in 7 cases in the concentration range of 0.35–0.73 µg/kg, and sulfamethoxazole was detected in 6 cases in the concentration range of 0.03–0.37 µg/kg [15]. More recently, it has been reported that residual antibiotics cause continuous genetic selection to induce antibiotic resistance to nontarget microorganisms, which may influence veterinarians in prescribing antibiotics [16]. The majority of pharmaceuticals are not biodegradable and enter into the environment, including the aquatic ecosystem, causing bioaccumulation and toxicity [3]. In a previous study in which the residuals of 22 antibiotics in the Haihe River in China were identified, the detection level of sulfamethoxazole was 201 ng/L, and for ciprofloxacin and erythromycin, the bioaccumulation factor was averaged at 3262 and 4492 L/kg, respectively [3].
Moreover, unlike conventional pesticides, these residual pharmaceutical substances exist in a very low concentration range of a few ng/L to several µg/L in the aquatic environment [2, 4, 8]. Furthermore, considering that the combined toxicity of these antibiotics has been reported, there is probably an urgent need for studies investigating not only the single toxicity but also the combined toxicity exhibited by antibiotics [7].
In the present study, for investigating the four types of livestock antibiotics, a reservoir adjacent to a livestock farm was selected as a control, and samples collected from a compost site (swine manure pile), three vicinity soils, and connected stream and river were selected as the sampling sites to determine the degree of leaching of antibiotics from the antibiotic-contaminated manure. All the four types of antibiotics were detected in the reservoir, designated as the control group, with the detection level being 0.03–1.6 µg/L. In particular, the detection concentration of lincomycin was the highest among the examined antibiotics. In our previous study, lincomycin was also detected in all the water samples in Ulsan, with the detection level ranging from 13 to 2620 ng/L and the average value being 317 ng/L [4]. Therefore, in the case of lincomycin, continuous and regular monitoring is essential considering the detection frequency and level in the city.
Furthermore, lincomycin was detected at a significantly higher level than the level detected in the vicinity of effluents (165 ng/L) from the sewage treatment plant in Busan, South Korea [10], which was judged to elevate the probability of occurrence of antibiotic resistance. Therefore, considering the issue of public health, it is important to suggest and reorganize the reason for the high detection level of lincomycin in the national or local management strategy on antibiotics.
The levels of three antibiotics, lincomycin, sulfamethazine, and sulfamethoxazole, remained at high concentrations (~ 10 µg/L) at the compost site (Site 2), and the detection level of trimethoprim was 0.3 µg/L (Fig. 2). The application of livestock manures as fertilizing composts could be suggested as a reason for the presence of antibiotic residues in the agricultural environment. Samples were collected from vicinity soils, sediments, and rivers from the composting facility to examine the antibiotic residues, and the detection level was ND–222.84 µg/L [17]. In a previous study that investigated seven antibiotics, sulfamethazine was detected at an average level of 20.30–28.38 µg/L in nearby soils with composting facilities, and sulfamethoxazole was also detected at a level of 0.77–5.43 µg/L [17]. The total amount of sulfa-type antibiotics detected in that study was 24.39–38.82 µg/L, which was similar to the antibiotic level detected in the manure pile sample (25.3 µg/L) in the present study (Fig. 3). Therefore, the composting facility, which collects a large amount of swine manure, might be the strong nonpoint source of pollution that contaminated the nearby soils and streams with an elevated concentration of antibiotics.
On the other hand, in the previous study in which the soil adjacent to the compost facility in Gangwon province was selected as the sampling area, tylosin was detected at a significantly higher concentration of 84.47–222.84 µg/L [17], indicating the need to conduct periodic monitoring for tylosin in Ulsan. Moreover, in the nearby river samples, sulfa-type antibiotics were detected at a concentration range of 0.97–14.85 µg/L, and a dilution effect of approximately 10 times was observed [17]. These results are similar to those obtained in the present study, in which a dilution effect of approximately 15 times was found (Fig. 3). However, the previous study reported a higher concentration of antibiotics in sediment samples than in soil and water samples, with the detection level of sulfa-type antibiotics being 71.96–120.91 µg/L [17]. Therefore, it is reasonable to monitor sediment samples in future studies to understand the characteristics of antibiotic residues in comparison with this study.
To confirm that rainwater falls to the manure pile area and becomes runoff, thereby contaminating the vicinity soil and streams, artificial rainwaters were flowed into the samples from Sites 2, 3, and 4 in this study. After flowing the water into the manure and soil samples, the degree of antibiotic contamination in the neighboring soils, streams, and rivers was confirmed. When the soils that were presumably contaminated by the antibiotics were analyzed, it was observed that sulfa-type antibiotics were adsorbed onto the soil matrix and contaminated the nearby river at very low concentrations (Fig. 3). Moreover, this phenomenon was not dependent on the pH value (Fig. 4). Similar to these results, higher concentrations of antibiotics were detected in samples as closer to the composting facility in the previous study [17]. However, in the case of sediments, it was also confirmed that the distance from the composting facility did not influence the degree of contamination of antibiotics [1].
Availability of data and materials
Not applicable.
References
Awad YM, Kim KR, Kim SC, Kim K, Lee SR, Lee SS, Ok YS (2015) Monitoring antibiotic residues and corresponding antibiotic resistance genes in an agroecosystem. J Chem 2015:974843
Bojarski B, Kot B, Witeska M (2020) Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals 13:189
Gao L, Shi Y, Li W, Liu J, Cai Y (2012) Occurrence, distribution and bioaccumulation of antibiotics in the Haihe River in China. J Environ Monit 14:1247–1254
Kwon H-O, Sim W-J, Kim H-Y, Oh J-E, Choi S-D (2011) Distribution of pharmaceuticals and personal care products (PPCPs) in main rivers of Ulsan, Korea. J Korean Soc Environ Anal 14:158–164
Ryu J, Oh J, Snyder SA, Yoon Y (2014) Determination of micropollutants in combined sewer overflows and their removal in a wastewater treatment plant (Seoul, South Korea). Environ Monit Assess 186:3239–3251
Chee-Sanford JC, Mackie RI, Koike S, Krapac IG, Lin YF, Yannarell AC, Maxwell S, Aminov RI (2009) Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J Environ Qual 38:1086–1108
Ding L, Zang L, Zhang Y, Zhang Y, Wang X, Ai W, Ding N, Wang H (2017) Joint toxicity of fluoroquinolone and tetracycline antibiotics to zebrafish (Danio rerio) based on biochemical biomarkers and histopathological observation. J Toxicol Sci 42:267–280
Ebert I, Bachmann J, Kühnen U, Küster A, Kussatz C, Maletzki D, Schluter C (2011) Toxicity of the fluoroquinolone antibiotics enrofloxacin and ciprofloxacin to photoautotrophic aquatic organisms. Environ Toxicol Chem 30:2786–2792
Ebele AJ, Abdallah MAE, Harrad S (2017) Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg Contam 3:1–16
Lee EY, Sim WJ, Oh JE (2008) Analysis of pharmaceutical compounds in water using solid phase extraction (SPE) and Liquid Chromatography/Mass Spectrometry (LC/MS). J Korean Soc Environ Anal 11:130–143
Kim JP, Jin DR, Lee W, Chae M, Park J (2020) Occurrence and removal of veterinary antibiotics in livestock wastewater treatment plants. South Korea. Processes 8:720
Sun B, Zhang L, Yang L, Zhang F, Norse D, Zhu Z (2012) Agricultural non-point source pollution in China: causes and mitigation measures. Ambio 41:370–379
Al-Farsi RS, Ahmed M, Al-Busaidi A, Choudri BS (2017) Translocation of pharmaceuticals and personal care products (PPCPs) into plant tissues: a review. Emerg Contam 3:132–137
NIFDSE (2019) Nationwide antibiotic consumption and resistance monitoring. Natl Inst Food Drug Saf Eval Chengju Korea 2018:9–17
Lee HJ, Cho SH, Shin D, Kang HS (2018) Prevalence of antibiotic residues and antibiotic resistance in isolates of chicken meat in Korea. Korean J Food Sci Anim Resour 38:1055–1063
Jung WK, Shin S, Park YK, Lim SK, Moon DC, Park KT, Park YH (2020) Distribution and antimicrobial resistance profiles of bacterial species in stray cats, hospital-admitted cats, and veterinary staff in South Korea. BMC Vet Res 16:109
Ok YS, Kim SC, Kim KR, Lee SS, Moon DH, Lim KJ, Sung JK, Hur SO, Yang JE (2011) Monitoring of selected veterinary antibiotics in environmental compartments near a composting facility in Gangwon Province, Korea. Environ Monit Assess 174:693–701
Acknowledgements
Not applicable.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03040570).
Author information
Authors and Affiliations
Contributions
MKP and JYO conducted experiments. MKP, SEL, and SDC analyzed data. SEL and SDC wrote the manuscript. SDC revised the manuscript. SDC conceived and designed this research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Fig. S1.
Structure of the four livestock antibiotics. Fig. S2. Flow chart for specific analytical conditions (column properties, column oven temperature, and SPE elution).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, MK., Oh, JY., Lee, SE. et al. Determination of veterinary pharmaceutical runoffs from a swine manure pile using LC–MS/MS. Appl Biol Chem 63, 69 (2020). https://doi.org/10.1186/s13765-020-00559-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-020-00559-1