Abstract
Aralia continentalis (A. continentalis) is a medicinal plant belonging to Araliaceae, it has been reported to exert anti-cancer, anti-bacterial, anti-inflammatory, anti-platelet and anti-oxidative activities. But the potential mechanism for the anti-inflammatory effect of compounds isolated from the roots of A. continentalis is still insufficient. So, we evaluated whether compounds isolated from the roots of A. continentalis exert anti-inflammatory effects and elucidated its potential mechanism in RAW264.7 cells. The concentrated residue was subsequently suspended in H2O and partitioned with n-hexane, methylene chloride (CH2Cl2), ethyl acetate (EtOAc) and n-butanol (n-BuOH). The fractions were subjected to sequential column chromatography over silica-gel, RP-18, MPLC, recycling and preparative HPLC to isolated the novel compound. The novel compound was identified as 18-nor-ent-pimara-9(11),15-diene-4β-ol and confirmed anti-inflammatory activity. The 18-nor-ent-pimara-9(11),15-diene-4β-ol dose-dependently blocked NO production and inhibited iNOS, COX-2, TNF-α and IL-1β expression in LPS-stimulated RAW264.7 cells. The 18-nor-ent-pimara-9(11),15-diene-4β-ol inhibited LPS-stimulated degradation of IκB-α and nuclear accumulation of p65, which resulted in the suppression of NF-κB activation in RAW264.7 cells. Also, the 18-nor-ent-pimara-9(11),15-diene-4β-ol attenuated the phosphorylation of p38 and ERK1/2 in LPS-induced RAW264.7 cells. These results suggest that the nor-ent-pimara-9(11),15-diene-4β-ol isolated from the roots of A. continentalis may have grate potential for the development of anti-inflammatory drugs.
Similar content being viewed by others
Introduction
In recent years, interest and expectations for medicinal resource plants have been increased, and many studies have been conducted to extract effective ingredients from natural resources and find functional materials because compounds present in medicinal resource plants have a variety of physiological vitality and are useful for living things [1,2,3].
Inflammatory reactions are reported to be involved in various pathological mechanisms, such as the introduction of bacteria and viruses into the body, in which the immune cells recognize and protect the body by secreting various inflammatory intermediaries, thereby promoting the growth of cancer cells, increasing insulin resistance, and worsening arteriosclerosis. The macrophages are known to be one of the important immune cells controlling the inflammatory response, responding to the early stages of the tumor necrosis factor-α (TNF-α), cytokines and lipopolysaccharide (LPS) infection and playing a pivotal role in the defense of the host and in maintaining the star. In particular, they are known to be involved in inflammatory reactions such as prostaglandin E2 (PGE2), nitric oxide (NO), cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS). These substances are reported to be involved in the development of inflammatory diseases. Inhibition of inflammatory factors is very effective in preventing the onset and progression of inflammatory diseases [4,5,6]. The nuclear factor kappa-B (NF-κB) activation is significantly associated with inflammatory diseases and much attention is focused on developing anti-inflammatory drugs targeting NF-κB. The mitogen-activated protein kinases (MAPK) cascade is one of the important signaling pathways in immune responses, and several recent studies reported that inhibition of MAPKs in mast cells can be an suitable target for pharmacological treatment of inflammatory diseases [7, 8].
Aralia continentalis (A. continentalis) is a medicinal plant belonging to Araliaceae, it has been reported to exert anti-cancer, anti-bacterial, anti-inflammatory, anti-platelet and anti-oxidative activities. The root of A. continentalis is known to contains essential oil (α-pinene, β-pinene and sabinene) 1–2%, stearic acid 0.07%, resin, salicylic acid and diterpenic acid I, II, copper, manganese and nickel [9, 10]. We isolated 18-nor-ent-pimara-9(11),15-diene-4β-ol from the roots of A. continentalis in previous study [11]. In this study, we investigated anti-inflammatory activity of 18-nor-ent-pimara-9(11),15-diene-4β-ol isolated from the ethanol extract of the roots A. continentalis.
Materials and methods
Chemical reagents
Lipopolysaccharide (LPS) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against phospho-IκB-α, IκB-α, phospho-extracellular signal-regulated kinase1/2 (p-ERK1/2), ERK1/2, phospho-p38 (p-p38), p38, p65 and β-actin were purchased from Cell Signaling technology (Bervely, MA, USA).
Isolation and confirmation of 18-nor-ent-pimara-9(11),15-diene-4β-ol from roots of A. continentalis
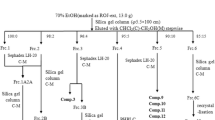
The roots of A. continentalis (5 kg) were extracted with 70% ethanol for 2 days. The ethanol-soluble fraction was filtered and concentrated using a vacuum evaporator at 40 °C. This extracts were suspended in distilled water and successively partitioned with n-hexane, dichloromethane (CH2Cl2), ethyl acetate (EtOAc) and n-butanol (n-BuOH). Repeated chromatography of the n-hexane fraction over a SiO2 column with an n-hexane:EtOAc solvent system (50:1 to 1:1 gradient), resulted in 14 sub-fractions. The novel compound [18-nor-ent-pimara-9(11),15-diene-4β-ol] was isolated and identified by spectroscopic methods, including 1H (700 MHz) and 13C NMR (175 MHz). Preparative HPLC was performed on a Shimadzu system (LC-8A pump and SPD-20A UV/VIS detector, Kyoto, Japan) using a YMC-Pack ODS A column (I.D. 250 × 20 mm), and a mixed solvent system of acetonitrile:H2O (50:50 to 90:10 gradient, 50 min.) at a flow rate of 12 ml/min.
Cell culture
Mouse macrophage cell line, RAW264.7 cells were purchased American Type Culture Collection (ATCC, Virginia, USA) and grown in Dulbecco's Modified Eagle (Lonza, Walkerscille, MD, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 ug/ml streptomycin. The cells were cultured in an incubator containing 5% CO2 at 37 °C. Trypsin–EDTA (Sigma, St. Louis, MO, USA) was used to detach the cells from a T75 flask. The novel compound was dissolved in dimethyl sulfoxide (DMSO) and treated to cells. DMSO was used as a vehicle and the final DMSO concentration did not exceed 0.1% (v/v).
Cell viability
Cell viability was performed by MTT assay. Briefly, cells were plated at a density of 1 × 106 cells/well in 12-well plate and incubated for 24 h. The cells were treated with 18-nor-ent-pimara-9(11),15-diene-4β-ol at the indicated concentrations for 24 h. Then, the cells were incubated with 200 μl of MTT solution (1 mg/ml) for an additional 2 h. The resulting crystals were dissolved in DMSO. The formation of formazan was measured by reading absorbance at a wavelength of 570 nm using UV/Visible (Perkin Elmer, Nowolk, CT, USA).
Nitric oxide generation assay
RAW264.7 cells were incubated 12-well plate for overnight. The cells were pretreated with 18-nor-ent-pimara-9(11),15-diene-4β-ol at the indicated concentrations for 6 h and then co-treated with LPS (1 μg/ml) for 18 h. NO levels were evaluated by Griess assay. Briefly, 50 μl of the cell culture supernatants were mixed with 50 μl of Griess reagent (Sigma Aldrich, St. Louis, MO, USA) and followed by reaction for 10 min at the room temperature. After 10 min, absorbance values were determined using a UV spectrophotometer (Perkin Elmer, Nowolk, CT, USA) at 540 nm.
Isolation of cytosol and nucleus fraction
Nucleus fractions from RAW264.7 cells after treatment of 18-nor-ent-pimara-9(11),15-diene-4β-ol and LPS were prepared using a nuclear extract kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer's protocols. Briefly, we harvested RAW264.7 cells with cold 1Xhypotonic buffer and reacted at 4 ℃ for 15 min. After adding detergent and vortexing for 10 s, the cells were centrifuged at 15,000 rpm for 10 min at 4 °C and the supernatants (cytoplasmic fraction) were collected and stored at − 80 °C for further analysis. The cell pellets were used for nuclear fraction collection. Cell pellets were re-suspended with lysis buffer by pipetting up and down, and incubated at 4 °C for 30 min under shaking. After 30 min, nuclear suspensions were centrifuged at 15,000 rpm for 10 min at 4 °C, and the supernatants (nuclear fraction) were stored at − 80 °C for further analysis.
SDS-PAGE and western blot analysis
After treatment with 18-nor-ent-pimara-9(11),15-diene-4β-ol and LPS, cells were washed twice with cold 1 × phosphate-buffered saline (1XPBS), and lysed in radio immune precipitation assay (RIPA) buffer (Boston Bio Products, Ashland, MA, USA) supplemented with protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO, USA) and phosphatase inhibitor cocktail (Sigma Aldrich, St. Louis, MO, USA), and centrifuged at 15,000 rpm for 10 min at 4 °C. Protein concentration was determined by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL, USA) using bovine serum albumin (BSA) as the standard.
We separated the equal proteins on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred them to nitrocellulose membrane. The nitrocellulose membranes were blocked with 5% non-fat dry milk in tris-buffered saline containing 0.05% tween 20 (TBS-T) by stirring at room temperature for 1 h and then incubated with specific primary antibodies in 5% skim dry milk in 0.05% TBS-T at 4 °C for overnight. After three washes with TBS-T, the blots were incubated with horse radish peroxidase (HRP)-conjugated immunoglobulin G (IgG) for 1 h at room temperature and chemiluminescence was detected with ECL western blotting substrate and visualized in Chemi Doc MP Imaging system (Bio-rad, CA, USA).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
After treatment of 18-nor-ent-pimara-9(11),15-diene-4β-ol and LPS, total RNA was extracted from RAW264.7 cells using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and total RNA (1 μg) was synthesized using a Verso cDNA Kit (Thermo Scientific, Pittsburgh, PA, USA) according to the manufacturer’s protocol. PCR was performed using PCR Master Mix Kit (Promega, Madison, WI, USA) and mouse primers for iNOS, COX-2, IL-1β and GAPDH (Table 1).
Statistical analysis
All the data are shown as mean ± SD (standard deviation). Statistical analysis was determined by Student’s t-test. Differences with *P or #P < 0.05 were considered statistically significant.
Results and discussion
Confirmation of 18-nor-ent-pimara-9(11),15-diene-4β-ol from A. continentalis
The chemical structure of 18-nor-ent-pimara-9(11),15-diene-4β-ol was confirmed by comparing the UV, 1H-NMR, 13C-NMR and 2D-NMR spectral data to published spectra. 18-nor-ent-pimara-9(11),15-diene-4β-ol appreared as white amorphous powder [11]; 1H-NMR (700 MHz, CDCl3) δ 5.82 (1H, dd, J = 17.5, 10.5 Hz, H-15), 5.38 (1H, qui, J = 2.1 Hz, H-11), 4.93 (1H, dd, J = 17.5, 2.1 Hz, Ha-16), 4.87 (1H, dd, J = 10.5, 2.1 Hz, Hb-16), 2.36 (1H, m, H-8), 2.03 (1H, m, Ha-12), 1.99 (1H, m, Ha-6), 1.82 (1H, m, Ha-1), 1.79 (1H, m, Ha-3), 1.77 (1H, m, Hb-12), 1.74 (1H, m, H-5), 1.73 (1H, m, Ha-7), 1.60 (1H, dt, J = 7.0, 3.5 Hz, Ha-2), 1.56 (1H, m, Hb-6), 1.47 (1H, dt, J = 14.0, 3.5 Hz, Hb-2), 1.41 (1H, dq, J = 12.6, 2.1 Hz, Ha-14), 1.36 (1H, m, Ha-3), 1.26 (1H, m, Hb-7), 1.24 (1H, m, Hb-1), 1.21 (3H, s, H-19), 1.04 (1H, m, Hb-14), 1.03 (3H, s, H-20), 0.97 (3H, s, H-17); 13C-NMR (175 MHz, CDCl3) δ 150.5 (C-9), 150.3 (C-15), 116.3 (C-11), 109.1 (C-16), 73.2 (C-4), 47.3 (C-5), 43.1 (C-3), 41.7 (C-14), 40.3 (C-1), 38.7 (C-10), 37.6 (C-12), 34.9 (C-13), 29.4 (C-8), 26.5 (C-7), 24.3 (C-20), 23.4 (C-19), 22.4 (C-17), 20.9 (C-2), 17.5 (C-6); ESI–MS m/z 297 [M + Na]+. The HPLC chromatogram and UV spectrum of the 18-nor-ent-pimara-9(11),15-diene-4β-ol was shown in Fig. 1.
The effect of 18-nor-ent-pimara-9(11),15-diene-4β-ol on NO production and iNOS, COX-2, TNF-α and IL-β expression in LPS-stimulated RAW264.7 cells
The nitric oxide (NO) has been known as one of the most multifaceted players in the immune system. It is included in the pathogenesis and control of tumors, infectious diseases, chronic degenerative diseases and autoimmune processes [12, 13]. NO is synthesized in mammalian cells by a family of three NO synthesis (NOS). The neuronal NOS (nNOS, Type I NOS) and endothelial NOS (eNOS, type III NOS) have been classified as constitutive NOS because these are continuously present in cells, while an inducible NOS (iNOS, type II NOS), is revealed only after exposure to specific stimulants such as cytokines and bacterial endotoxic lipopolysaccharide (LPS) in macrophages and hepatocytes [14,15,16,17,18,19].
So, in the study, to determine if the 18-nor-ent-pimara-9(11),15-diene-4β-ol could reduce NO generation by LPS, RAW264.7 cells were pretreated with the 18-nor-ent-pimara-9(11),15-diene-4β-ol (0, 5, 10, 20 μM) for 6 h and then co-treated with LPS (1 μg/ml) for the additional 18 h. As shown in Fig. 2a, 18-nor-ent-pimara-9(11),15-diene-4β-ol significantly decreased LPS-mediated overproduction of NO in RAW264.7 cells. To exclude the cytotoxic effect of 18-nor-ent-pimara-9(11),15-diene-4β-ol from the inhibitory effect of 18-nor-ent-pimara-9(11),15-diene-4β-ol against NO production, we tested cell viability after the treatment of 18-nor-ent-pimara-9(11),15-diene-4β-ol using MTT assay. As shown in Fig. 2b, 18-nor-ent-pimara-9(11),15-diene-4β-ol showed no cytotoxicity. Since NO production is regulated by iNOS, COX-2, IL-1β and TNF-α expression, the effect of 18-nor-ent-pimara-9(11),15-diene-4β-ol on iNOS, COX-2, IL-1β and TNF-α expression was evaluated by RT-PCR. As shown in Fig. 2c, the treatment of 18-nor-ent-pimara-9(11),15-diene-4β-ol attenuated the mRNA expression of iNOS, COX-2, TNF-α and IL-1β. Thus, 18-nor-ent-pimara-9(11),15-diene-4β-ol attenuated the overproduction of NO by inhibiting LPS-induced iNOS, COX-2, TNF-α and IL-1β overexpression in RAW264.7 cells.
The effect of 18-nor-ent-pimara-9(11),15-diene-4β-ol on NO production and iNOS, COX-2, TNF-α and IL-1β in LPS-stimulated RAW264.7 cells. a RAW264.7 cells were pretreated with 18-nor-ent-pimara-9(11),15-diene-4β-ol for 6 h and then co-treated with LPS (1 μg/ml) for 18 h. The determination of NO from the cell culture media was measured by Griess assay. b RAW264.7 cells were treated with 18-nor-ent-pimara-9(11),15-diene-4β-ol at the indicated concentrations for 24 h. Cell viability was measured using MTT assay system and expressed as % cell viability. c For RT-PCR, RAW264.7 cells were pre-treated with novel compound at the indicated concentrations for 6 h and then co-treated with LPS (1 μg/ml) for the additional 18 h. Total RNA was isolated and RT-PCR was performed for iNOS, COX-2, TNF-α and IL-1β. Values given are the mean ± SD (n = 3). *P < 0.05 compared to the cells without the treatment, and #P < 0.05 compared to the cells treated with LPS alone. GAPDH was used as an internal control for RT-PCR
The effect of 18-nor-ent-pimara-9(11),15-diene-4β-ol on NF-κB signaling activation in LPS-induced RAW264.7 cells
The nuclear factor kappa-B (NF-κB) serves to control the activation of inflammation. NF-κB is not a single gene but a family of closely related transcription factors that contain five genes: NF-κB1(p50/p105) NF-κB2(p52/p100), RelA(p65), c-Rel, and RelB. The activities of NF-κB are strictly regulated by interaction with inhibitory IκB protein. As with the NF-κB transcription factors, there are several IκB proteins (IκBα, IκBβ, IκBγ and IκBε) that have different affinities for individual NF-κB dimers. The activation of NF-κB dimers is the result of IKK-mediated, phosphorylation-induced degradation of the IκB inhibitor, which enables the NF-κB dimers to enter the nucleus and activate specific target gene expression. The degradation and phosphorylation of IκB in response to LPS lead to NF-κB translocation to the nucleus. This event is related to the activation of a wide range of NF-κB-responsive pro-inflammatory genes [20,21,22,23,24,25].
To elucidate the effect of 18-nor-ent-pimara-9(11),15-diene-4β-ol on NF-κB signaling activation, we investigated a western blot for IκB-α degradation and phosphorylation in LPS-stimulated RAW264.7 cells. As shown in Fig. 3a, LPS induced successive IκB-α degradation and phosphorylation at 30 min after the stimulation. However, pretreatment of 18-nor-ent-pimara-9(11),15-diene-4β-ol blocked LPS-stimulated IκB-α degradation and phosphorylation in a dose-dependent manner. Cytosol to nucleus p65 translocation due to IκB-α degradation by various stimuli are essential for NF-κB activation. Thus, we performed whether 18-nor-ent-pimara-9(11),15-diene-4β-ol inhibit the nuclear translocation of p65. As shown in Fig. 3b, LPS raised an amount of p65 in the nucleus of RAW264.7 cells. However, pretreatment of 18-nor-ent-pimara-9(11),15-diene-4β-ol dose-dependently blocked LPS-induced p65 translocation. Translocate p65 into the nucleus binds to the NF-κB binding site and extends NF-κB transcriptional activity. These data show that 18-nor-ent-pimara-9(11),15-diene-4β-ol may suppress NF-κB activation by inhibition of p65 translocation into the nucleus via blocking the IκB-α degradation.
The effect of 18-nor-ent-pimara-9(11),15-diene-4β-ol on NF-κB signaling activation in LPS-stimulated RAW264.7 cells. a, b RAW264.7 cells were pretreated with 18-nor-ent-pimara-9(11),15-diene-4β-ol for 6 h and then co-treated with LPS (1 μg/ml) for 20 min. c RAW264.7 cells were pretreated with 18-nor-ent-pimara-9(11),15-diene-4β-ol for 6 h and then co-treated with LPS (1 μg/ml) for 20 min. After the treatment, the cytosol and nucleus were prepared. For Western blot analysis, the cell lysates were subjected to SDS-PAGE and the Western blot was performed using antibodies against p-IκB-α, IκB-α and p65. β-actin was used as internal control
The effect of 18-nor-ent-pimara-9(11),15-diene-4β-ol on MAPKs signaling activation in LPS-stimulated RAW264.7 cells
The mitogen-activated protein kinases (MAPKs) pathway plays important role in transmitting signals from cell surface to the nucleus and manages cellular functions such as growth, migration, differentiation, proliferation and death. In addition, MAPKs pathway is the important regulator in the activation pro-inflammatory cytokines in different cell types, including T cells, epithelial cells, dendritic cells and macrophages. Some defects in MAPKs signaling are related to carcinogenesis by increased production of pro-inflammatory cytokines, cell proliferation, growth factors and anti-cancer factors. Generally growth factors are involved in activation of extracellular signal regulated kinases 1 and 2 (ERK1/2), whereas stress stimuli activates the c-Jun N-terminal kinases (JNK) and p38 MAPKs [24, 25]. Excessive activation of ERK1/2 has been associated with tumors. Also, deregulations of JNK and p38 MAPKs pathways are associated with cancer developments [26, 27]. As MAPKs have serious roles in cancer and inflammation, it has been focused and shown by different scientists that targeting MAPKs suppression is beneficial for cancer remedy [28,29,30,31,32,33].
To further examine whether decrease of MAPKs activation by 18-nor-ent-pimara-9 (11), 15-diene-4β-ol treatment is associated with the regulation of p38, ERK1/2 and JNK activation, we investigated the effects of 18-nor-ent-pimara-9 (11), 15-diene-4β-ol on phosphorylation of of p38, ERK1/2 and JNK in LPS-stimulated RAW264.7 cells. As shown in Fig. 4, increase of of p38, ERK1/2 and JNK phosphorylation was discovered in RAW264.7 cells by LPS. However, 18-nor-ent-pimara-9(11), 15-diene-4β-ol suppressed phosphorylation of p38, ERK1/2 and JNK, indicating that 18-nor-ent-pimara-9(11), 15-diene-4β-ol blocks the inflammatory response by inhibiting of p38, ERK1/2 and JNK activation in LPS-induced RAW264.7 cells.
The effect of 18-nor-ent-pimara-9(11),15-diene-4β-ol on MAPK signaling activation in LPS-stimulated RAW264.7 cells. RAW264.7 cells were pretreated with 18-nor-ent-pimara-9(11),15-diene-4β-ol for 6 h and then co-treated with LPS (1 μg/ml) for 20 min. For Western blot analysis, the cell lysates were subjected to SDS-PAGE and the Western blot was performed using antibodies against p-ERK1/2, p-p38 and Total p-38 and Total ERK1/2 were used as internal control
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- LPS:
-
Lipopolysaccharide
- MTT:
-
3-(4,5-Dimethylthizaol-2-yl)-2,5-diphenyl tetrazolium bromide
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- NO:
-
Nitric oxide
- ERK1/2:
-
Extracellular signal-regulated kinase ½
- NF-κB:
-
Nuclear factor kappa-B
- MAPK:
-
Mitogen-activated protein kinases
- JNK:
-
C-Jun N-terminal kinases
References
Kim KH, Ko KI, Kang EJ, Yang EK, Park SN (2004) A research trend of natural product on well-being industry. J Soc Cosmet Sci Korea 30:239–343
Ahn SK, Goo YM, Ko KH, Lee SJ, Moon YH, Lee SS, Kim JW, Lee SS (2014) Study on the evaluation of nutritional values and antioxidant activities for herbal medicine by-products. J Agric Life Sci 48:101–110
Kim EY, Baik IH, Kim JH, Kim SR, Rhyu MR (2004) Screening of the Antioxidant Activity of Some Medicinal Plants. Korean J food sci technol 36:333–338
Lee SE, Lee JH, Kim JK, Kim GS, Kim YO, Soe JS, Choi JH, Lee ES, Noh HJ, Kim SY (2011) Anti-inflammatory activity of medicinal plant extracts. Kor J Med Crop Sci 19:217–226
McDaniel ML, Kwon G, Hill JR, Marshall CA, Corbett JA (1996) Cytokines and nitric oxide in islet inflammation and diabetes. Proc Soc Exp Biol Med 211:24–32
Kim DH, Park SJ, Jung JY, Kim SC, Byun SH (2009) Antiinflammatory effects of the aqueous extract of Hwangnyenhaedok-tang in LPS-activated macrophage cells. Kor J Herbol 24:39–47
Lawrence T, Fong C (2010) The resolution of inflammation: Anti-inflammatory roles for NF-κB. Int J Biochem Cell Biol 42:519–523
Kim HK (2014) Role of ERK/MAPK signalling pathway in anti-inflammatory effects of Ecklonia cava in activated human mast cell line-1 cells. Asian Pac J Tro Med 7:703–708
Han WS (2005) Isolation of the Antimicrobial Compounds from Aralia cordata Thunb. Extract Kor J Med Crop Sci 13:182–185
Park SY, Kim JW (1995) Cytotoxic polyacetylenes from Aralia cordata. Yakhak Hoeji 39:681–688
Lee JE, Sim SJ, Jeong W, Choi CW, Kim N, Park Y, Kim MJ, Lee DH, Hong SS (2020) Diterpenoids and phenolic analogues from the roots of Aralia continentalis. J Asian Nat Prod Res 13:1–8
Yang SA, Im NK, Jhee KH, Lee IS (2008) Effects of Aralia continentalis Kitagawa on antiplatelet and antioxidative activities. J Life Sci 18:357–362
Lee SG, Jo DJ, Chang HJ, Kang H (2015) Antioxidant and anti-inflammatory activities of ethanol extracts from Aralia continentalis Kitagawa. Journal of Naturopathy 4:10–14
Eo HJ, Kim DS, Sim SJ, Park GH, Song JH, Jeong JB, Kim N (2018) Anti-inflammatory effect of leaves extracts from Aralia cordata through inhibition of NF-κB and MAPKs Signaling in LPS-stimulated RAW264.7 cells. Korean J Plant Res 31:634–640
Cuzik TJ, Korbut R, Adamek-cuzik T (2003) Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol 54:469–487
Muchel T, Feron O (1997) Nitric oxide synthases: which, where, how, and why? J Clin Invest 100:2146–2152
Goldstein IM, Ostwald P, Roth S (1996) nitric oxide: a review of its role in retinal function and disease. Vision Res 36:2979–2994
Kroncke KD, Fehsel K (1997) Kolb-Bachofen V (1997) Nitric oxide: cytotoxicity versus cytoprotection—how, why, when and where? Nitric Oxide 1:107–120
Lala PK, Chakraborty C (2001) Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol 2:149–156
Sharma JN, Al-Omran A, Parvathy SS (2007) Role of nitric oxide in inflammatory diseases. Inflammopharmacology 15:252–259
Liu T, Zhang Joo LD, Sun SC (2017) NF-κB signaling in inflammation. Signal Trans Targeted Ther 12:e17023
Dolcet X, Llobet D, Pallares J, Matias-Guiu X (2005) NF-κB in development and progression of human cancer. Virchows Arch 446:475–482
Gilmore TD (2006) Introduction to NF-κB: player, pathways, persperctive. Oncogene 25:6680–6684
Gadjeva M, Tomczak MF, Zhang M, Wang YY, Dull K, Rogers AB, Erdman SE, Fox JG, Carroll M, Horwitz BH (2004) A role for NF-κB subunits p50 and p65 in the inhibition of lipopolysaccharide-induced shock. J Immunol 173:5786–5793
Abraham E (2003) Nuclear Factor–κB and its role in sepsis-associated organ failure. J Inferct Dis 187:364–369
Chea SW (2005) Function and activation of NF-κB in immune system. Korean J Otolaryngol 48:284–288
Manzoor Z, Koo JE, Koh YS (2014) Mitogen-activated protein kinase signaling in inflammation-related carcinogenesis. J Bacteriol Virol 44:297–304
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183
Huang P, Han J, Hui L (2010) MAPK signaling in inflammation-associated cancer development. Protein Cell 1:218–226
Wagner EF, Nebreda AR (2009) Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 9:537–549
Sebolt-Leopold JS, Herrera R (2004) Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer 4:937–947
Arthur JS, Ley SC (2013) Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13:679–692
Chen H, Sohn J, Zhang L, Tian J, Chen S, Bjeldanes LF (2014) Anti-inflammatory effects of chicanine on murine macrophage by down-regulating LPS-induced inflammatory cytokines in IκBα/MAPK/ERK signaling pathways. Eur J Pharmacol 724:168–174
Acknowledgements
The authors would like to thank all of the researcher who contributed to this study.
Funding
This work was supported by a grant from National Institute of Forest Science (FP0400-2017–01).
Author information
Authors and Affiliations
Contributions
GHP directed, and HJE, YP and SSH designed the study. HJE, YP and SSH performed the experiments. GHP and HJE drafted manuscript. YP and SSH corrected the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eo, H.J., Park, Y., Hong, S.S. et al. Anti-inflammatory effects of 18-nor-ent-pimara-9(11),15-diene-4β-ol isolated from the roots of Aralia continentalis on LPS-induced in RAW264.7 cells. Appl Biol Chem 63, 74 (2020). https://doi.org/10.1186/s13765-020-00553-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-020-00553-7