Abstract
Evodiamine is an active alkaloid member found in Traditional Chinese Herb (TCH) Evodia rutaecarpa. It has been reported to exhibit remarkable biological and medicinal activities including anticancer and anti-inflammatory. This study was designed to investigate the anticancer effects of evodiamine against human liver cancer and evaluate its effects on cell migration, cell invasion, cellular apoptosis and PI3K/AKT pathway. The results showed that evodiamine exhibits potent antiproliferative effects against two human liver cancer cell lines (HepG2 and PLHC-1) with an IC50 of 20 µM. Nonetheless, the cytotoxic effects of evodiamine were comparatively low against the normal cells as evident from the IC50 of 100 μM. The growth inhibitory effects of evodiamine were found to be due to the induction of apoptosis as revealed by the DAPI, AO/EB and annexin V/PI staining assays. The induction of apoptosis was also associated with upregulation of Bax and downregulation of Bcl-2 expression in a concentration dependent manner. The wound healing and transwell assay revealed that evodiamine caused a significant decline in the migration and invasion of the HepG2 and PLHC-1 cells. Investigation of the effects of evodiamine on the PI3K/AKT signalling revealed that evodiamine inhibited the phosphorylation of PI3K and AKT proteins. Taken together, the results showed that evodiamine inhibits the growth of human liver cancer via induction of apoptosis and deactivation of PI3K/AKT pathway. The results point towards the therapeutic potential of evodiamine in the treatment of liver cancer.
Similar content being viewed by others
Introduction
Traditional Chinese herbs (TCH) are considered as promising and novel sources of chemotherapy adjuvants and antitumor remedies [1]. Additionally, TCH assists in improving the efficiency of chemotherapy and eliminating/lowering its hazardous side-effects. Over the years, researchers have extensively undertaken clinical and experimental investigations to augment the effectiveness of chemotherapy. Interestingly, several bioactive molecules have been extracted from TCH which exhibit potent antitumor activity in epidemiological as well as experimental models. Evodia rutaecarpa is a popular TCH, locally known as “Wu-Chu-Yu”. It has been an important constituent of Traditional Chinese Medicine from a long period of time prescribed for treatments of several ailments including postpartum hemorrhage, headache and gastrointestinal disorders [2, 3]. Evodia rutaecarpa is rich source of alkaloid which is believed to be responsible for its bioactivities [3]. The total content of evodiamine in Evodia species ranges from 0.072 to 2.52% [4]. Alkaloids have been reported to exhibit a spectrum of biological activities including antiproliferative and antitumor activities against an array of human cancer cell lines [5].
Evodiamine is one of the key alkaloids isolated from Evodia rutaecarpa, which has been shown to exhibit remarkable bioactivities [6]. Evodiamine has been reported to show uterotonic, thermoregulatory, vasodilatory, anti-obesity, anti-inflammatory, antinociceptive, catecholamine secretion and testosterone secretion effects [7, 8]. Studies have also reported cytotoxic effects of evodiamine against colon, prostate and liver cancer [9]. The antitumor activity of evodiamine have been attributed to its potential of proliferation inhibition, apoptosis initiation, invasion inhibition and metastasis suppression against several human cancer cells including lung cancer, colon cancer, cervical cancer, melanoma, leukemic T-lymphocyte, prostate cancer and breast cancer cells [10].
Liver cancer is one of the dangerous and prevalent types of primary liver malignancies across the globe. Currently, it is ranked as second among high mortality cancers and with each passing year there is an alarming increase in liver cancer incidences across the globe [11]. The key treatment options for liver cancer include surgical resection, organ transplant and radiofrequency ablation. The only proven potential anti-liver cancer agent for chemotherapy is sorafenib, which amplifies the patient’s survival [12]. However, high cost and low availability of these treatments generate an emergency for novel and efficient agents that can assist us with better outcome against liver cancer.
A previous study has reported the anticancer effects of evodiamine against human hepatocellular carcinoma cells [13]. However, the anticancer effects of evodiamine against human liver cancer cells via modulation of PI3K/AKT pathway and its effects on liver cancer cell migration and invasion have not been studied. Against this backdrop, the present study was designed to investigate the anticancer effects of evodiamine against human liver cancer cells (PLHC-1 and HepG2) and to evaluate its effects on PI3K/AKT signalling, cell migration and invasion.
Materials and methods
Cell culture, chemicals and cultural conditions
Human normal liver THLE-2 and liver cancer HepG2 and PLHC-1 cells were collected from the Cell Bank of Type Culture Collection of Chinese Academy of Science, Shanghai, China. All three cell lines were seeded in RMPI-1640 cultural medium containing 10% of fetal bovine serum (GIBCO BRL) and potential antibiotics streptomycin (100 μg/ml) and penicillin (100 U/ml). Seeding of the cell lines was performed in an incubator under an atmosphere of 5% CO2, 95% air and 37° of temperature. Equivalent amounts phosphate buffered saline (PBS) was used as vehicle control. All the chemicals and reagents involved in this study were bought from Sigma-Aldrich including evodiamine (> 98% purity).
The viability assay
The effects of evodiamine on cell viability of liver cancer HepG2 and PLHC-1, and normal liver THLE-2 cells were determined via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. In brief, 1.4 × 105 cells/well of each cell line was cultured separately overnight using 96-well plates. Following overnight incubation, each well plate was supplied with different doses of evodiamine (0 to 640 μM) for a time interval of 48 h. After drug treatment, all cell lines were stained with 50 μl of MTT solution of concentration 5 mg/ml and resultant formazan crystals were dissolved using DMSO (dimethyl sulphoxide). Finally, microplate spectrophotometer (BioTek Instruments, Inc., Winooski, United States) at 570 nm was used to record absorbance for optical density calculations.
Apoptotic assays
The apoptosis was detected by 4ʹ,6-diamidino-2-phenylindole (DAPI), acridine orange/ethidium bromide (AO/EB) and annexin V/PI staining assays. The cancerous HepG2 and PLHC-1 cells were harvested at exponential phase of growth followed by loading onto 24-well plates. After the incubation period, HepG2 and PLHC-1 cells were subjected to evodiamine treatment at different doses (0 to 640 μM) for 48 h. In case of DAPI staining assay, cells were harvested, washed with PBS and fixed at room temperature with 80% ethanol for 30 min. Thereafter, fixative was discarded and cells were rewashed with PBS thrice prior to 50 min of incubation in dark at 25 °C with 1 μg/ml of DAPI solution. In case of AO/EB staining assay, cells were deprived of fixation and were loaded with 100 µl of freshly prepared AO/EB solution (100 µg/ml). Both DAPI and AO/EB stained HepG2 and PLHC-1 cells were immediately loaded onto Nikon fluorescence microscope (Nikon Inc., Japan) for apoptosis measurements. Annexin V/PI staining assay was used to determine the percentage of the apoptotic liver cancer cells as described previously [14].
Transwell assay
Cell invasion assay was executed using transwell chambers coated with Matrigel (BD Biosciences) bearing membranes of 8 µm of pore size (Corning Co., NY, United States). Fresh cell culture of HepG2 and PLHC-1 cells was placed onto upper transwell chambers maintain serum free cultural medium with a concentration of 4.2 × 104 cells. These cells were supplemented with different evodiamine doses (0 to 640 μM). Lower transwell were only filled with RMPI medium (600 µl) and FBS (20%). Afterwards, transwell chambers were placed under incubation for 24 h followed by removal of non-invasive cells by scrubbing. Invaded cells were fixed with paraformaldehyde fixative and then stained with crystal violet (10%). Finally, invaded cells were numbered under a microscope (Olympus, japan).
Wound healing assay
HepG2 and PLHC-1 cells were seeded in fresh culture media till 85% confluence. Thereafter, a plastic scraper was used create a wound followed by PBS washing. The cultural medium was completely removed and replaced with a fresh one to maintain different concentrations of evodiamine (0 to 640 μM). Then cells were incubated for 48 h at 37 °C followed by two times of washing using PBS. Finally, the wound was investigated under a light microscope (Nikon, Tokyo, Japan).
Western blotting
The effects of evodiamine on the apoptosis and PI3K/AKT pathway allied proteins were examined by western blotting. After treatment of HepG2 and PLHC-1 cells with variant evodiamine doses (0 to 640 μM), cells were subjected for lysing using RIPA buffer (Beyotime, Beijing, China). Bicinchonic Acid assay was performed to monitor the protein content among each lysate. About 35 µg of proteins was subjected to separation via SDS-PAGE followed by electrophoretic transferal to PVDF membranes (Millipore Corp, Atlanta, GA, United States). These membranes were blocked using non-fat dry milk (5%) as blocking agent for 1 h at room temperature. Afterwards, membranes were treated overnight at 4 °C with indicated primary antibodies. Followed by horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. In the end, the protein signals were recorded using ECL (enhanced chemiluminescence) reagent (Pierce, Rockford, United States.
Statistical analysis
The data from independent triplicate experiments were indicated as mean ± SD. For statistical comparison, student’s t-test was used for each assay. A probability value of P < 0.05 was taken as significant.
Results
Evodiamine exerted antiproliferative effects on HepG2 and PLHC-1 cells
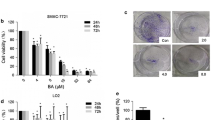
The antiproliferative effects of evodiamine (Fig. 1a) were evaluated by using MTT assay. The results showed that evodiamine caused a significant (P < 0.05) decrease in the viability of the human HepG2 and PLHC-1 liver cancer cells (Fig. 1b and IC). The antiproliferative effects of evodiamine against the liver cancers cells exhibited a dose dependent pattern. The IC50 of evodiamine against the human liver cancer cells (HepG2 and PLHC-1) was found to be 20 μM. Nonetheless, the antiproliferative effects of evodiamine were found to be less profound against the normal THLE-2 normal liver cells as evident from the IC50 of 100 μM (Fig. 1d). Taken together, these results indicate selective anticancer effects of evodiamine against the liver cancer cells.
Evodiamine inhibits the proliferation of liver cancer cells a Chemical structure of evodiamine. b Viability of evodiamine treated PLHC-1 cells. c Viability of evodiamine treated HepG2 cells. d Viability of evodiamine treated THLE-2 cells. The results depicted that evodiamine inhibited the proliferation of the two liver cancer cell lines and with exceptionally low toxicity against the normal liver cell lines. The experiments were performed in triplicate and expressed as mean ± SD (*P < 0.05)
Evodiamine induced apoptotic cell death in HepG2 and PLHC-1 cells
Several assays were used to determine if evodiamine exerts antiproliferative effects in liver cancer cells via induction of apoptosis. The results of the DAPI staining revealed that evodiamine caused alterations in the nuclear morphology of the HepG2 and PLHC-1 cells such as nuclear condensation and fragmentation suggestive of apoptosis (Fig. 2). AO/EB staining assay results revealed that evodiamine treatment increased the number of early and late stage apoptotic cells along with necrotic cells (Fig. 3). Further, annexin V/PI showed that apoptotic cell percentage increased from 0.92% in control group to 35.99% in evodiamine treated PLHC-1 cells and from 2.46% to 30.4% against HepG2 cells (Fig. 4a). Evodiamine induced apoptotic cell death in both HepG2 and PLHC-1 cells were further supported by western blotting data. The results of western blotting showed considerable increase in Bax protein levels and downregulation of Bcl-2 levels (Fig. 4b).
Evodiamine induced apoptosis in liver cancer cells. AO/EB staining was used to study apoptosis in HepG2 and PLHC-1 cells after evodiamine treatment at indicated concentrations. Results showed amplified yellow-green, orange-red and red fluorescence indicating early apoptosis, late apoptosis and necrotic cells. The experiments were performed in triplicate
Induction of apoptosis in liver cancer cells by evodiamine a Quantification of apoptotic cell percentage of evodiamine treated HepG2 and PLHC-1 cells by Annexin V/PI staining assay. Results showed that apoptotic cell percentage of the liver cancer cells increased remarkably with increasing evodiamine doses. b Western blotting assay showing increase in Bax and decrease in Bcl-2 expression in HepG2 and PLHC-1 cells. The experiments were performed in triplicate
Evodiamine retarded migration and invasion of HepG2 and PLHC-1 cells
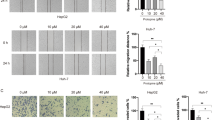
To analyze the effects of evodiamine on migration and invasion of HepG2 and PLHC-1 cells, wound healing and transwell chambers assays were performed. Herein, it was observed that evodiamine inhibited the migration of the HepG2 and PLHC-1 cell lines. The wound width of treated groups showed decreased considerably as compared to that of control group (Fig. 5). In case of PLHC-1 cells, the invasion percentage of the HepG2 and PLHC-1 cells was significantly (P < 0.05) decreased upon evodiamine treatment and exhibited in a dose dependent pattern (Fig. 6).
Evodiamine inhibits the migration of the liver cancer cells. Wound healing assay was executed to monitor HepG2 and PLHC-1 cell migration ability after evodiamine treatment. Results showed that migratory capability of HepG2 and PLHC-1 cells was arrested by test drug. The experiments were performed in triplicate
Evodiamine inhibits the invasion of the liver cancer cells. Transwell invasion assay was executed to monitor HepG2 and PLHC-1 cells invasion ability following evodiamine treatment. Results showed that invasiveness of HepG2 and PLHC-1 cells was suppressed upon evodiamine treatment. The experiments were performed in triplicate and expressed as mean ± SD (*P < 0.05)
Evodiamine blocked PI3K/AKT pathway in HepG2 and PLHC-1 cells
Effects on PI3K/AKT pathway by evodiamine were studied by using western blotting assay. The results indicated that the expression levels of p-PI3K, p-AKT and PI3K decreased and those of AKT remained almost constant (Fig. 7).
Evodiamine deactivates the PI3K/AKT signalling pathway. Western blotting assay was performed to determine the expressions of PI3K/AKT pathway linked proteins. Results indicated the expression of p-PI3K. p-AKT and PI3K decreased whereas the expression of AKT remained intact. The experiments were performed in triplicate
Discussion
Natural products provide a vast variety of chemical entities many of which prove to novel drugs and lead molecules [15]. Since time immemorial, natural products have served humanity in one or the other way to improve life as well as health issues. The exploration of plant-based drugs has led to the development of several antitumor drugs from alkaloids [16]. Alkaloids are mostly plant secondary metabolites and predominantly found in certain species of blooming plants [5]. Evodia rutaecarpa is rich in alkaloid content with evodiamine alkaloid as a key constituent responsible for its high medicinal value [3]. Although, evodiamine has been reported to induce apoptosis in human hepatocellular carcinoma cells [13], the anticancer effects of evodiamine on liver cancer cells via deactivation of PI3K/AKT signalling cascade has not been studied. Additionally, the effect of evodiamine on the migration and invasion of liver cancer cells is still unclear. The results of the present study evodiamine induced dose-dependent toxicity against liver cancer HepG2 and PLHC-1 cells with an IC50 of 20 µM. Nonetheless, the cytotoxicity of evodiamine against the normal liver cells were significantly lower than the normal cells. There results indicate selective anticancer effects of evodiamine and are consistent with previous studies wherein evodiamine has been reported of exert anticancer effects against lung cancer, colon cancer, cervical cancer and melanoma cells [17, 18]. Previous studies have shown that evodiamine exerts anticancer effects by inducing apoptotic cell death in a number of human cancer cell lines such as murine L929 fibroblastoma, breast NCI/ADR-RES cells, prostate LNCap, DU145 and PC-3 cancer cells, leukemic U937 cells and A375-S2 melanoma cells via alterations in balance of proapoptotic Bax and antiapoptotic Bcl-2 protein levels [19]. Consistently, in the present study, it was found that evodiamine induced apoptosis in the HepG2 and PLHC-1 liver cancer cells via induction of apoptosis [13]. Western blotting indicated that evodiamine downregulated Bcl-2 and upregulated the Bax protein levels in both HepG2 and PLHC-1 cells in a concentration-dependent manner. Next the effects of evodiamine were also investigated on the migration and invasion of the liver cancer cells. It was found that evodiamine inhibited the liver cancer cell migration and invasion. These results suggest that evodiamine may prove to beneficial for the treatment of metastatic cancers. Additionally, these results are in agreement with a previous study wherein evodiamine has been found to suppress the migration and invasion of the liver cancer cells [20]. The PI3K/AKT pathways has been shown to be aberrantly activated in several cancer types and is responsible for the growth, development, and tumorigenesis of different human cancers [21]. As such it is an important drug target for the management of human cancer including liver cancer. Interestingly, in the present study it was found that evodiamine could deactivate the PI3K/AKT pathway in liver cancer cells indicative of its potential as lead molecule for the treatment of liver cancer.
Conclusion
The results of the present investigation revealed potent anticancer activity of evodiamine alkaloid against human liver cancer cells. Evodiamine induced anticancer effects via stimulation of apoptosis and blocking of PI3K/AKT signalling pathway. Evodiamine also suppressed the migration and invasion of liver cancer cells. Taken together, evodiamine may prove a lead molecule in the development of liver cancer chemotherapy. However further in vivo research endeavors are recommended.
Availability of data and materials
Not applicable.
Change history
18 June 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1186/s13765-022-00706-w
References
McCulloch M, See C, Shu XJ, Broffman M, Kramer A, Fan WY, Gao J, Lieb W, Shieh K, Colford JM Jr (2006) Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non–small-cell lung cancer: meta-analysis of randomized trials. J Clin Oncol 24:419–430
Jiang J, Hu C (2009) Evodiamine: a novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules 14:1852–1859
Sheu JR (1999) Pharmacological effects of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa. Cardiovasc Drug Rev 17:237–245
Tang X, Huang Z, Chen Y, Liu Y, Liu Y, Zhao J, Yi J (2014) Simultaneous determination of six bioactive compounds in Evodiae fructus by high-performance liquid chromatography with diode array detection. J Chromatogr Sci 52:149–156
Mondal A, Gandhi A, Fimognari C, Atanasov AG, Bishayee A (2019) Alkaloids for cancer prevention and therapy: current progress and future perspectives. Eur J Pharmacol 858:172472
Yu H, Jin H, Gong W, Wang Z, Liang H (2013) Pharmacological actions of multi-target-directed evodiamine. Molecules 18:1826–1843
Choi YH, Shin EM, Kim YS, Cai XF, Lee JJ, Kim HP (2006) Anti-inflammatory principles from the fruits of Evodia rutaecarpa and their cellular action mechanisms. Arch Pharm Res 29:293–297
Sachita K, Kim Y, Yu HJ, Cho SD, Lee JS (2015) In vitro assessment of the anticancer potential of evodiamine in human oral cancer cell lines. Phytother Res 29:1145–1151
Qiu C, Gao LN, Yan K, Cui YL, Zhang Y (2016) A promising antitumor activity of evodiamine incorporated in hydroxypropyl-β-cyclodextrin: pro-apoptotic activity in human hepatoma HepG2 cells. Chem Cent J 10:1–1
Zhang Y, Wu LJ, Tashiro S, Onodera S, Ikejima T (2004) Evodiamine induces tumor cell death through two different pathways: Apoptosis and necrosis. Acta Pharmacol Sin 25:83–89
Mittal S, El-Serag HB (2013) Epidemiology of hepatocellular carcinoma: Consider the population. J Clin Gastroenterol 47:S2–S6
Zhu YJ, Zheng B, Wang HY, Chen L (2017) New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin 38:614–622
Li YL, Zhang NY, Hu X, Chen JL, Rao MJ, Wu LW, Li QY, Zhang B, Yan W, Zhang C (2018) Evodiamine induces apoptosis and promotes hepatocellular carcinoma cell death induced by vorinostat via downregulating HIF-1α under hypoxia. Biochem Biophys Res Commun 498:481–486
Lukanova A, Kaaks R (2005) Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiol Prev Biomarkers 14:98–107
Khursheed A, Rather MA, Rashid R (2016) Plant-based natural compounds and herbal extracts as promising apoptotic agents: their implications for cancer prevention and treatment. Adv Biomed Pharma 3:245–269
Tao H, Zuo L, Xu H, Li C, Qiao G, Guo M, Lin X (2020) Alkaloids as anticancer agents: a review of chinese patents in recent 5 years. Recent Pat Anticancer Drug Discov 15:2–13
Yang J, Wu LJ, Tashiro S, Onodera S, Ikejima T (2008) Nitric oxide activated by p38 and NFkappaB facilitates apoptosis and cell cycle arrest under oxidative stress in evodiamine-treated human melanoma A375–S2 cells. Free Radic Res 42:1–11
Fei XF, Wang BX, Li TJ, Tashiro S, Minami M, Xing DJ, Ikeijma T (2003) Evodiamine, a constituent of Evodiae Fructus, induces anti-proliferating effects in tumor cells. Cancer Sci 94:92–98
Zhang Y, Wu LJ, Tashiro S, Onodera S, Ikejima T (2003) Intracellular regulation of evodiamine induced A375–S2 cell death. Biol Pharm Bull 26:1543–1547
Ogasawara M, Suzuki H (2004) Inhibition by evodiamine of hepatocyte growth factor-induced invasion and migration of tumor cells. Biol Pharm Bull 27:578–582
Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB (2005) Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4(12):988–1004
Acknowledgements
The authors acknowledge The Seventh Medical Centre of PLA General Hospital, Beijing, 100700, China to conduct the presented protocol.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JJ and JZ designed the study. JJ, KK, and YL carried out bulk of the experiments. and collect the data. JJ and JZ performed the statistical analysis. JZ supervised the work and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s13765-022-00706-w"
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, J., Kang, X., Liu, Y. et al. RETRACTED ARTICLE: Inhibition of human liver cancer cell growth by evodiamine involves apoptosis and deactivation of PI3K/AKT pathway. Appl Biol Chem 63, 67 (2020). https://doi.org/10.1186/s13765-020-00551-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-020-00551-9