Abstract
The aim of this study was to investigate the effect of Lactobacillus plantarum HFY09 on gastric injury induced by HCl/ethanol in Kunming mice. The results showed that HFY09-H inhibited any increases in gastric juice volume, maintained the normal pH value of gastric acid, and reduced the damage caused to the gastric mucosa and gastric wall, the inhibition rate on the injury area reaches 63.70%. Compared with the negative control group, HFY09 increased the levels of serum somatostatin (SS) and vasoactive intestinal peptide (VIP), and also decreased the levels of substance P (SP), endothelin-1 (ET-1), interleukin-6 (IL-6), interleukin-12 (IL-12), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). In addition, real time fluorescent quantitative PCR (Q-PCR) also confirmed that high-dose HFY09 (109 CFU/kg/day) upregulated the mRNA expression of copper/zinc superoxide dismutase (Cu/Zn-SOD), manganese superoxide dismutase (Mn-SOD), catalase (CAT), endothelial nitric oxide synthase (eNOS), and neuronal nitric oxide synthase (nNOS), and downregulated the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). At the same time, the results of the HFY09 treatment group were similar to those of the ranitidine treatment group. These results indicate that HFY09 can prevent gastric injury induced by HCl/ethanol in vivo. Therefore, HFY09 may play a potential role in the treatment of gastric diseases.
Similar content being viewed by others
Introduction
Gastric injury is an infectious or chemical attack factor that breaks down the mucosal defense system, which will cause epithelial damage or ulcers in the stomach [1, 2]. Current research shows that bacteria, drugs, smoking, and alcohol consumption can cause gastric mucosal damage. Alcohol is the main component of alcohol. It is fat soluble and can produce acetaldehyde, which has a strong carcinogenic effect that begins from the time it enters the mouth and continues when it enters the blood circulation. Although the gastric mucosa can metabolize ethanol and acetaldehyde through alcohol dehydrogenase and acetaldehyde dehydrogenase, the carcinogenic effect of acetaldehyde cannot be underestimated [3]. Therefore, alcoholism can cause gastric bleeding, gastric erosion, and long-term drinking can cause gastric diseases, cancer, alcoholic hepatitis, fatty liver and other alcoholic diseases [3,4,5]. HCl may also cause gastric mucosal damage, and ethanol may reach the mucosa and induce rupture of blood vessel wall cells [6].
Probiotics and some new biological drugs have been widely studied as new therapies to treat stomach-related diseases. There is growing evidence that probiotics are beneficial to host health [7]. Numerous studies have confirmed that yeast and lactobacillus are the main resident microorganisms in the stomach that are beneficial to the human body. Among them, yeast is colonized in the gastric secretory area, while lactobacillus is colonized in the non-secretory area, and this is regarded as the main force that maintains the micro-ecological balance in the stomach [8]. Kwon et al. [9] studied ethanol-induced gastritis and liver injury in mouse models and found that Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 played a significant role in repairing gastritis by stimulating the immune system and fibroblasts. Senol et al. [10, 11] found that rats pretreated with a probiotic mixture exhibited significantly reduced acute gastric mucosal damage caused by aspirin or ethanol. Oliveira et al. [12] showed that animal experiments have also confirmed that Lactobacillus reuteri DSM 17938 can prevent the gastric damage caused by ethanol.
Lactobacillus plantarum HFY09 is a Lactobacillus that was isolated from natural fermented yak yoghurt and identified by our research team. In this study, we used a solution of hydrochloric acid and ethanol to establish a mouse model of chemical gastric injury to study the preventive effects of Lactobacillus plantarum H0FY9 on gastric injury in mice. The results can provide insight for the development of probiotics. However, due to the use of a variety of probiotics, the mechanisms governing treatment and gastric-related diseases require further study.
Materials and methods
Laboratory strain
Lactobacillus plantarum HFY09 was stored in the China General Microbiological Culture Collection Center (CGMCC No. 16633, Beijing, China). HFY09 was isolated from Sichuan yak yoghurt. Lactobacillus delbrueckii subspecies Bulgaria (LB; CGMCC No. 1.16075) was used as the comparative strain of HFY09.
Mouse model of gastric damage
Sixty Kunming (KM) mice (6 weeks old, male) were randomly divided into 6 groups (n = 10) after adaptive feeding for 1 week. Namely, Normal group, negative control group, ranitidine group, Lactobacillus delbrueckii subspecies Bulgaria group (LB group), low concentration HFY09 group (HFY09-L group) and high concentration HFY09 group (HFY09-H group). The normal and negative control groups were intragastrically administered saline solution, the ranitidine group was intragastrically administered 50 mg/kg of ranitidine. The HFY09-L and HFY09-H mice were intragastrically administered 1.0 × 108 CFU/kg and 1.0 × 109 CFU/kg HFY09 suspension, respectively, and LB mice were intragastrically administered 1.0 × 109 CFU/kg LB suspension. On the first 14 days, the normal group and the negative control group were provided with free diet and drinking water, the ranitidine group was given ranitidine solution, the Lactobacillus plantarum HFY09 group was given low-and high-dose bacterial suspension, and LB suspension was administered to the LB group. All groups were processed for 14 days.
After the 15th day, the mice were fasted for 24 h and were then treated with induction solution HCl/ethanol solution (60% ethanol and 40% 150 mmol/L HCl mixture) by gavage. The induced dose was 10 mL/kg [5, 13]. Except for the normal group, the other groups were given the same dose of HCl/ethanol solution, and the normal group was given the same volume of normal saline. Half an hour later, mouse blood was obtained and centrifuged at 4 °C, 4000 rpm, 10 min. The amount of gastric secretion and pH value of the gastric juice were measured. The area of gastric injury was analyzed by ImageJ software, and the inhibition rate of gastric injury was calculated: Inhibitory rate of gastric injury (%) = area of gastric injury/area of gastric tissue × 100 [14]. The specific administration method and dosage are shown in Table 1. This study was approved by the Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (KJQN201901619), Chongqing, China.
Mouse serum ET-1, SP, SS, and VIP level measurement
Serum was collected from the mice and stored at − 80 °C. The serum endothelin-1 (ET-1) (ml001965), substance P (SP) (ml001885), somatostatin (SS) (ml101826), and vasoact iveintestinal peptide (VIP) (ml001911) levels were determined according to kit instructions (Shanghai Enzyme Linked Biotechnology Co., Ltd, Shanghai, China).
Mouse cytokine IL-6, IL-12, TNF-α, and IFN-γ level measurement
The levels of cytokines interleukin-6 (IL-6) (ml002293), interleukin-12 (IL-12) (ml037868), tumor necrosis factor-α (TNF-α) (ml209800), and interferon-γ (IFN-γ) (ml22709) in serum were measured according to the corresponding kit instructions (Shanghai Enzyme Linked Biotechnology Co., Ltd, Shanghai, China).
Pathological observation of gastric tissue in mouse
One third of the gastric tissues of mice were removed and placed in 10% formalin neutral fixative solution for 48 h for histopathological analysis. After hematoxylin–eosin (HE) staining [15], the histopathological morphology was observed.
mRNA expression determination (Q-PCR assay)
Real time fluorescent quantitative PCR (Q-PCR) is a method for measuring the total amount of products after each polymerase chain reaction (PCR) cycle using fluorescent chemicals in a DNA amplification reaction.[13, 16]. The objective genes and housekeeper gene were all reacted by fluorescence quantitative PCR to obtain cycle threshold (CT) values, and calculate the relative expression of mRNA in samples by relative comparison and quantitative method (2−ΔΔCt method) [17, 18]. Q-PCR was used to detect the relative expression of antioxidant enzymes (copper/zinc superoxide dismutase (Cu/Zn-SOD), manganese superoxide dismutase (Mn-SOD), catalase (CAT)), inflammatory regulatory factors (endothelial nitric oxide synthase (eNOS), neuronal nitric oxide synthase (nNOS), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2)), and epidermal growth factor (EGF) and receptor (EGFR) mRNA in the gastric tissues of different groups of mice. Table 2 shows the corresponding gene primer sequence.
Statistical analysis
The data are expressed as the mean ± SD. The difference between the mean values of each group was evaluated by single factor analysis. The significant difference was indicated by the analysis of variance (ANOVA) of the Duncan multi-range test (P < 0.05). All figures were drawn with Origin 8.0 software [19].
Results
Determination of gastric mucosal injury
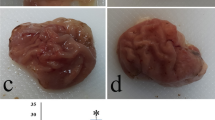
As shown in Fig. 1 and Table 3, compared with the normal group, the gastric mucosa in the negative control group exhibited obvious bleeding and erosion, with the largest area of gastric mucosa damage. After treatment with HFY09, ranitidine, and LB, the area of injury was significantly reduced (P < 0.05), and ranitidine has the most obvious inhibitory effect on gastric injury. The rate of inhibition by HFY09-H was between the rates of ranitidine and HFY09-L. Therefore, HFY09 can reduce the effect of gastric injury on gastric mucosa.
Determination of volume and pH of mouse gastric juice
The volume of gastric juice and pH value of gastric juice in each group are shown in Table 4. The normal mice had the least amount of gastric juice and the highest pH value of gastric juice. The negative control group was the opposite. The HFY09-H and ranitidine group can effectively ameliorate the adverse changes in gastric juice volume and pH caused by HCl/ethanol in mice, which are manifested the pH value of gastric juice and the amount of gastric juice were closer to that of healthy mice.
Pathological observation of mouse gastric tissue
A pathological section of gastric tissue in mice with acute gastric injury induced by HCl/ethanol is shown in Fig. 2. The gastric mucosa of normal mice is intact, and there is no inflammatory cell infiltration. However, the gastric mucosal epithelial cells are significantly reduced, the intercellular space is significantly increased in negative control group of mice, and gastric damage is the most obvious. After treatment with HFY09 and ranitidine, the gastric mucosal structure of mice was improved, the structure of each layer was clear, and the damage was reduced. Compared with the normal group, the gastric mucosal cells in the HFY09 low-dose group were still partially exfoliated, but also improved. Therefore, HFY09 has a protective effect on gastric injury to a certain extent, with a stronger effect at high concentrations.
Determination of ET-1, SP, SS, and VIP levels in mouse serum
The levels of SS and VIP were the highest, and ET-1 and SP were the lowest in the normal group (Table 5). However, four indexes of gastric ulcer in the negative control group were opposite to those in the normal group. After treatment, the levels of serum SS and VIP were significantly increased (P < 0.05), and the levels of SP and ET-1 were significantly decreased (P < 0.05). The ability of HFY09 to regulate serum SS, SP, VIP and ET-1 was between the raninidine and LB groups, and the effect was higher than that of LB group.
Determination of IL-6, IL-12, TNF-α, and IFN-γ levels in mouse serum
The levels of IL-6, IL-12, TNF-α, and IFN-γ were the lowest in healthy mice. However, the level of inflammatory factors was increased in mice with gastric injury induced by HCl/ethanol, and decreased after treatment (Table 6). The decrease was the greatest in the ranitidine group, followed by the HFY09-H treatment group. The effect of reducing inflammatory factors in the HFY09-H group was stronger than that in the HFY09-L group.
Gene expression of Cu/Zn-SOD, Mn-SOD, and CAT in the mouse gastric tissue
The expression of Mn-SOD, Cu/Zn-SOD, and CAT in the mouse stomach was detected by Q-PCR. The results in Fig. 3 show that the expression levels of Mn-SOD, Cu/Zn-SOD, and CAT mRNA were the highest in the gastric tissues of healthy mice, and were lowest in the negative control group. Mn-SOD, Cu/Zn-SOD, and CAT in the ranitidine group and the HFY09-H group were higher than those in the negative control group, and the expression level of ranitidine group was higher than that of the HFY09-H treatment group. The expression level of LB was similar to that of HFY09-L.
Gene expression of eNOS, iNOS, nNOS, and COX-2 in the mouse gastric tissue
The expression of COX-2, iNOS, eNOS, and nNOS in the mouse gastric tissue is shown in Fig. 4. The expression of eNOS and nNOS was the highest in healthy mice, while COX-2 and iNOS were the lowest. The negative control group was the opposite. HFY09 and ranitidine have obvious therapeutic effect, and ranitidine has the best improvement effect. The high-dose group (HFY09-H) expression was significantly lower than that in the low-dose group (HFY09-L) and the LB treatment group, and the difference between HFY09-H group and the ranitidine group was small. These data indicated that HFY-09 could inhibit inflammation, protect the gastric tissue of gastric injury mice induced by HCl/ethanol, and the effect was stronger at high concentrations.
Gene expression of EGF and EGFR in the mouse gastric tissue
Figure 5 shows the gene expression of EGF and EGFR in the mouse stomach. The expression level of EGF and EGFR in the negative control group induced by inducer was significantly lower than that in the healthy mice (P < 0.05). The effects of ranitidine, LB, and HFY09 on the gene expression of EGF and EGFR were significantly different from those of the control group (P < 0.05). The gene expression of EGF and EGFR in the HFY09 high-dose group was lower than that in the ranitidine treatment group, but was significantly higher than that in the LB group and the HFY09 low-dose group. At the same dose, the effect of HFY09-H was more pronounced than that of HFY09-L.
mRNA expression level of EGF and EGFR in mouse gastric. a–e In the same column, values with different letters are significantly different (P < 0.05) according to Duncan’s multiple-range test. Normal = normal mice; Negative control = mice treated with HCl/ethanol (10 mL/kg); Ranitidine = 50 mg/kg per day by gavage; HFY09 = mice treated with HCl/ethanol (15th day) and doses (L, H) of Lactobacillus plantarum HFY09 (108, 109 CFU/kg per day); LB = mice treated with HCl/ethanol (15th day) and Lactobacillus delbrueckii subsp. bulgaricus (109 CFU/kg per day)
Discussion
Acute gastric injury is usually caused by acute alcohol abuse. The main symptoms are damage to the cytoplasmic membrane at the top of the epithelium, causing cell shedding, multiple gastric erosions, and ulcers [20]. If the blood vessels are involved, the injury can cause major bleeding and inflammation of the gastric mucosa. Animal experiments show that by directly observing gastric mucosa tissue with ulceration, the extent of gastric damage can be determined by measuring the ulcerated area [18, 21].
Lactic acid bacteria (LAB) has special physiological function, can produce organic acids, enzymes, etc., can regulate the normal flora of gastrointestinal tract, maintain the micro ecological balance, improve the gastrointestinal function, inhibit the growth of putrefactive bacteria in intestinal tract. Many studies have found that probiotics exert a certain therapeutic effect on various intestinal diseases, but there are fewer studies on the effect of probiotics such as Lactobacillus, Bifidobacterium, and Saccharomyces on gastric-related diseases [22].
In the current study, by consulting relevant studies [23,24,25] and international standards, the concentration of bacterial in the beverage must be higher than 107 CFU/mL, which is why 109 CFU/mL lactobacillus is often used in animal experiments [26]. Therefore, we chose 109 CFU/kg and 108 CFU/kg as research concentrations to investigate the preventive effect of Lactobacillus plantarum HFY09 on acute gastric injury.
The gastric juice of healthy mice did not affect the gastric mucosa. However, when gastric mucosa is stimulated, the secretion of gastric juice will increase and the pH value will decrease [18]. These changes aggravate the damage of the gastric mucosa, which may cause gastritis or gastric ulcers, and long-term serious injuries may cause gastric cancer.[13, 18, 27]. On the one hand, in the current study, the pH value of gastric juice in normal group was higher than that in negative control group. HFY09 treatment effectively reduced the amount and increased the pH value of gastric juice, so as to certain preventive effect in alcohol induced gastric injury. On the other hand, by analyzing the area of gastric injury and calculating the inhibition rate of gastric injury with ImageJ software, the degree of gastric injury in each group can be directly compared. There was no erosion and bleeding in the gastric mucosa of healthy mice (Table 3). However, the gastric mucosa of the negative control group was damaged obviously. Compared with the degree of gastric injury in the negative control group, the area of gastric injury in the low and high dose HFY09 group was significantly reduced (P < 0.05), and the inhibition rates for gastric injury were 46.95% and 63.70%, respectively, but the inhibition rate in HFY09-H was lower than that in ranitidine group (84.46%). These results suggest that HFY09 has protective effect on gastric injury to some extent.
In addition, histopathological observation is a kind of pathological examination, which is used to examine the pathological changes in organs, tissues or cells [13, 14]. In order to study and evaluate the protective effect of HFY09 on the gastric injury induced by HCl/ethanol, we analyzed the gastric tissue sections of mice. The preventive effect of HFY09 on gastric injury was manifested in the decrease of epithelial cell abscission and the decrease of cell gap.
Gastrointestinal hormones such as SP, ET, SS and VIP can not only regulate gastric acid secretion, but also regulate gastrointestinal movement [28]. Endothelin (ET) is an endothelium-derived vasoconstrictor composed of 21 amino acids. It is the most potent vasoconstrictor peptide found thus far and plays an important role in gastric mucosal injury [13]. Some studies showed that ET mainly causes damage by reducing the internal blood flow and weakening the protective mechanism of the gastric mucosa, which will cause severe ulceration and play a key role in the pathogenesis of ulcerative colitis [29]. Gastrointestinal hormones that regulate gastric juice secretion include SS and VIP [30]. SS is a strong inhibitor of gastric acid secretion. It has an inhibitory effect on cAMP production in parietal cells, which not only inhibits the release of GAS in G cell secreted granules, but also directly inhibits GAS gene expression. GAS is related to gastric acid secretion [31]. When the stomach is irritated, the decrease in SS will aggravate the secretion of gastrointestinal fluid and the inflammation of the gastrointestinal tract [31, 32].
The VIP level is closely related to immune regulation and the inflammation resulting from gastric injury [32]. At the same time, VIP can inhibit the transcription of iNOS in vivo and prevent damage to the gastric mucosa caused by the conversion of iNOS into excessive NO [33]. SP is an excitatory gastrointestinal hormone, and its production will increase under stress and will cause secretion of a large amount of gastric juice and strong acidity in the stomach, resulting in damage to the gastric mucosa [13, 34]. Table 5 shows that HCl/ethanol induction has led to an imbalance of gastrointestinal hormone secretion in mice, with significant increases (P < 0.05) in serum ET-1 and SP, and significant reductions (P < 0.05) in SS and VIP. HFY09 can alleviate the change of gastrointestinal hormones, which is manifested by increasing the level of serum SS and VIP, reducing the level of ET-1 and SP. Its effect is similar to that of ranitidine.
Another effective method involves controlling the amount of pro-inflammatory factors. Animal experiments show that the release of a large amount of oxygen free radicals caused by ethanol destroys the redox balance in the body, causes a variety of immune responses, causes the immune system in the body to release excessive pro-inflammatory factors, and triggers inflammatory reactions [35, 36]. The levels of IL-6, IFN-γ and other inflammatory cytokines in healthy people were lower than those in patients with inflammatory diseases [37]. Some researchers have found that probiotics can decrease the gastric damage caused by ethanol through their own anti-inflammatory properties. Oliveira et al. found that Lactobacillus reuteri DSM 17938 supplement can protect the mucosal adhesions caused by ethanol-induced gastric injury in mice [12]. Studies by Suo et al. found that Lactobacillus fermentum Suo can reduce the levels of the serum inflammatory factors IL-6, IL-12 and IFN-γ in mice with gastric injury induced by HCl/ethanol [5]. In the current study, ranitidine, LB, and HFY09 reduced the levels of IL-6, et al. inflammation-related factors in serum. Among them, the effect of ranitidine is the most obvious, followed by high-dose HFY09, indicating that high-dose HFY09 is effective at preventing gastric damage.
Many studies have shown that the expression of antioxidant related genes is related to inflammation, tumor and other chronic diseases (e.g., Cu/Zn SOD, Mn-SOD, and CAT) [38, 39]. Studies show that there are much higher quantities of xanthine oxidase in the gastrointestinal tract as compared to other tissues. Ethanol will be converted into acetaldehyde as a result of gastrointestinal metabolism. Acetaldehyde will further produce oxygen free radicals under the action of xanthine oxidase, which will lead to oxidative damage of the gastric mucosa [40]. Therefore, oxygen free radicals are one of the important mechanisms involved in ethanol-induced gastric injury. Cu/Zn SOD and Mn-SOD can scavenge superoxide, hydrogen peroxide, and hydroxyl and lipid peroxides, regulate gastric injury, and even enable recovery to normal levels [37].
According to the results of this study, Compared with the injury group, HFY09-H upregulated the expression of Cu/Zn SOD, Mn-SOD, and CAT mRNA, indicating that HCl/ethanol-induced gastric oxidative metabolism free radicals can be eliminated. HFY09 has a certain antioxidant effect in gastric injury, and can inhibit gastric injury. NO is liposoluble and can freely cross the cell membrane and be highly oxidized. Its production depends on nitric oxide synthetase (NOS), including nNOS, eNOS, and iNOS [41]. nNOS and eNOS produce low levels of NO and protect the gastric mucosa to a certain extent [14, 42, 43]. It was found that the occurrence of gastric ulcers was related to the over-inhibition of eNOS and nNOS levels [42]. In addition, we also measured the expression mediators of related inflammatory factors in gastric tissue, such as iNOS and COX-2, which inhibit the production of inflammatory cytokines (such as IL-6).
COX-2 is an isoenzyme of COX. Cytokines and endotoxin can induce COX-2 production, participate in inflammatory reactions, and promote the development of inflammation [13, 44]. Therefore, COX-2 is highly expressed when cells are stimulated by inflammation. Qian et al. [13] found that raw liubao tea polyphenols can downregulate the expression of COX-2, thus decreasing inflammation and subsequently reducing gastric injury. In addition, there is a synergistic effect resulting from the interaction of iNOS with COX-2, which can further decrease the inflammatory response [45]. In the current study, Lactobacillus plantarum HFY09 also effectively controlled the expression of iNOS and COX-2 in mouse gastric tissue, and increased the expression of nNOS and eNOS, relieving gastric injury.
EGF can promote epithelial proliferation, tissue repair, and cellular protection. It plays an important role in protecting gastric mucosa from damage factors and maintaining the integrity of the gastrointestinal mucosa. EGF exerts its biological effects through its receptor, EGFR [39]. Our results showed that the expression of EGF and EGFR in the gastric tissue of HCl/ethanol-induced gastric injury mice was significantly lower than that of normal mice. EGF, as a protective factor, combines with its receptor EGFR to accelerate ulcer healing, increase tissue repair ability, and maintain ulcer healing quality [46, 47]. In the current study, the treatment of HFY09 significantly increased the expression level of EGF and EGFR genes in the gastric tissue of mice with gastric injury, indicating that HFY09 has a protective effect on the gastric tissue of mice with gastric injury, and the effect is stronger at a higher dose.
A model of acute gastric injury induced by HCl/ethanol was used to evaluate the preventive effect of Lactobacillus plantarum HFY09 on gastric injury. The results showed that HFY09 inhibited the secretion of gastric juice, maintained the normal pH value of gastric acid. Compared with the negative control group, the levels of SP, ET-1, IL-6, IL-12, TNF-α, and IFN-γ in the HFY09-H group decreased, while the levels of SS and VIP increased. Further experiments found that HFY09 increased the expression of eNOS, nNOS, Cu/Zn SOD, Mn-SOD, EGF, and EGFR, and decreased the expression of iNOS and COX-2 in gastric tissues, indicating that HFY09 could inhibit the apoptosis of gastric mucosal epithelial cells and play a biological role, which was positively correlated with the concentration. The results show that HFY09 can be used in the production of probiotic preparations, providing a theoretical basis for the application of probiotics.
Availability of data and materials
All data and materials used in this study are included in this published article.
References
Zhao TY, Zhang Y, Mu SS, Park JP, Bu HS, Leng XY, Wang SM (2020) Protective effects of genipin on ethanol-induced acute gastric injury in mice by inhibiting NLRP3 inflammasome activation. Eur J Pharmacol 867:172800
Lim JM, Song CH, Park SJ, Park DC, Jung GW, Cho HR, Bashir KMI, Ku SK, Choi JS (2019) Protective effects of triple fermented barley extract (FBe) on indomethacin-induced gastric mucosal damage in rats. BMC Complement Altern Med 19:1–11
Salaspuro M (2017) Key role of local acetaldehyde in upper GI tract carcinogenesis. Best Pract Res Clin Gastroenterol 31:491–499
Zhao X, Sun P, Li GJ, Yi RK, Qian Y, Park KY (2018) Polyphenols in Kuding tea help prevent HCl/ethanol-induced gastric injury in mice. Food Funct 9:1713–1725
Suo HY, Zhao X, Qian Y, Sun P, Zhu K, Li J, Sun BZ (2016) Lactobacillus fermentum Suo attenuates HCl/ethanol induced gastric injury in mice through its antioxidant effects. Nutrients 8:1–17
Alrashdi AS, Salama SM, Alkiyumi SS, Abdulla MA, Hadi AHA, Abdelwahabet SI, Taha MM, Hussiani J, Asykin N (2012) Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum sambac against HCl/Ethanol-induced gastric mucosal injury in rats. Evidence-based Complement Altern Med 2012:15
Chen HF, Nie QX, Xie M, Yao HYY, Zhang K, Yin JY, Nie SP (2019) Protective effects of β-glucan isolated from highland barley on ethanol-induced gastric damage in rats and its benefits to mice gut conditions. Food Res Int 122:157–166
Coconnier MH, Liévin V, Bernet-Camard MF, Hudault S, Servin AL (1997) Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob Agents Chemother 41:1046–1052
Kwon EK, Kang GD, Kim WK, Han MJ, Kim DH (2017) Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate ethanol-induced gastritis and hepatic injury in mice. J Funct Foods 38:389–398
Senol A, Isler M, Karahan AG, Kiliç GB, Kuleaşan H, Goren I, Saritas U, Kaya S, Ciris M, Akturk O, Aridogan BC, Demirin H, Cakmakci LM (2011) Effect of probiotics on aspirin-induced gastric mucosal lesions. Turk J Gastroenterol 22:18–26
Senol A, Isler M, Karahan AG, Kiliç GB, Kuleaşan H, Kaya S, Keskin M, Goren I, Saritas U, Aridogan BC, Delibas N (2011) Preventive effect of probiotics and α-tocopherol on ethanol-induced gastric mucosal injury in rats. J Med Food 14:173–179
Oliveira AP, Souza LKM, Araújo TSL, Araújo SD, Nogueira KM, Sousa FBM, Silva RO, Pacífico DM, Martins CS, Brito GAC, Souza MHLP, Medeiros JVR (2019) Lactobacillus reuteri DSM 17938 protects against gastric damage induced by ethanol administration in mice: role of TRPV1/substance P axis. Nutrients 11:208
Qian Y, Zhang J, Fu XW, Yi RK, Sun P, Zou M, Long XY, Zhao X (2018) Preventive effect of raw Liubao tea polyphenols on mouse gastric injuries induced by HCl/ethanol via anti-oxidative stress. Molecules 23:2848
Liu BH, Zhang CF, Zhang J, Zhao X (2019) Wu shan shen cha (Malus asiatica nakai. leaves)-derived flavonoids alleviate alcohol-induced gastric injury in mice via an anti-oxidative mechanism. Biomolecules 9:1–13
Liu B, Fang Y, Yi RK, Zhao X (2019) Preventive effect of blueberry extract on liver injury induced by carbon tetrachloride in mice. Foods 8:1–13
Zhao X, Song JL, Yi RK, Li GJ, Sun P, Park KY, Suo HY (2018) Comparison of antioxidative effects of insect tea and its raw tea (Kuding Tea) polyphenols in kunming mice. Molecules 23:204
Zhao X, Qian Y, Li GJ, Tan J (2015) Preventive effects of the polysaccharide of Larimichthys crocea swim bladder on carbon tetrachloride (CCl4)-induced hepatic damage. Chin J Nat Med 13:521–528
Liu BH, Feng XX, Zhang J, Wei Y, Zhao X (2019) Preventive effect of anji white tea flavonoids on alcohol-induced gastric injury through their antioxidant effects in kunming mice. Biomolecules 9:1–13
Li GJ, Long XY, Pan YN, Meng X, Zhao X (2018) Colitis reducing effects of Lactobacillus plantarum YS-4 in dextran sulfate sodium-induced C57bl/6J mice. Biomed Res 29:768–774
Kang GD, Kim DH (2017) Ponciretin attenuates ethanol-induced gastric damage in mice by inhibiting inflammatory responses. Int Immunopharmacol 43:179–186
Liu WX, Shang PJ, Liu TL, Xu H, Ren DJ, Zhou W, Wen A, Ding Y (2017) Gastroprotective effects of chebulagic acid against ethanol-induced gastric injury in rats. Chem Biol Interact 278:1–8
Shang M, Sun J (2016) Vitamin D/VDR, probiotics, and gastrointestinal diseases. Curr Med Chem 24:876–887
Zhang J, Zhou XR, Chen BS, Chen BS, Long XY, Mu JF, Pan YN, Song JJ, Zhao X, Yang ZN (2018) Preventive effect of lactobacillus plantarum CQPC10 on activated carbon induced constipation in Institute of Cancer Research (ICR) mice. Appl Sci 8:1–15
Suo HY, Zhao X, Qian Y, Li GJ, Liu ZH, Xie J, Li J (2014) Therapeutic effect of activated carbon-induced constipation mice with Lactobacillus fermentum suo on treatment. Int J Mol Sci 15:21875–21895
Qian Y, Suo HY, Du M, Zhao X, Li J, Li GJ, Song JL, Liu ZH (2015) Preventive effect of lactobacillus fermentum lee on activated carbon-induced constipation in mice. Exp Ther Med 9:272–278
Lam EKY, Tai EKK, Koo MWL, Wong HPS, Wu WKK, Yu L, So WHL, Woo PCY, Cho CH (2007) Enhancement of gastric mucosal integrity by Lactobacillus rhamnosus GG. Life Sci 80:2128–2136
Xie M, Chen HH, Nie SP, Tong W, Yin JY (2017) Gastroprotective effect of gamma-aminobutyric acid against ethanol-induced gastric mucosal injury. Chem Biol Interact 272:125–134
Zhao X, Yi RK, Qian Y, Park KY (2018) Lactobacillus plantarum YS-3 prevents activated carbon-induced constipation in mice. J Med Food 21:575–584
Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ (2009) Glucagon-like peptide-1 receptor activation modulates pancreatitis- associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 58:2148–2161
Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, Poitras AP (2002) Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol 282:948–952
Qian Y, Zhao X, Kan J (2013) Preventive effect of resistant starch on activated carbon-induced constipation in mice. Exp Ther Med 6:228–232
Matveyenko AV, Dry S, Cox HI, Moshtaghian A, Gurlo T, Galasso R, Butler AE, Butler PC (2009) Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 58:1604–1615
Tatarkiewicz K, Gu BG, Roy PD (2012) No evidence of drug-induced pancreatitis in rats treated with exenatide for 13 weeks. Diabetes Obes Metab 15:417–426
Kawabata A, Kinoshita M, Nishikawa H, Kuroda R, Nishida M, Araki H, Arizono N, Oda Y, Kakehi K (2001) The protease-activated receptor-2 agonist induces gastric mucus secretion and mucosal cytoprotection. J Clin Invest 107:1443–1450
Mei XT, Xu DH, Xu S, Zheng YP, Xu SB (2012) Novel role of Zn(II)-curcumin in enhancing cell proliferation and adjusting proinflammatory cytokine-mediated oxidative damage of ethanol-induced acute gastric ulcers. Chem Biol Interact 197:31–39
Li GJ, Sun P, Wang R, Zhou YL, Qian Y, Zhao X (2014) Preventive effect of polysaccharide of Larimichthys crocea Swim bladder on reserpine induced gastric ulcer in ICR Mice. Korean J Physiol Pharmacol 18:183–190
Li F, Lu DY, Zhong Q, Tan F, Li WF, Zhao LW (2019) Lactobacillus fermentum HFY06 reduced CCl4-induced hepatic damage in Kunming mice. RSC Adv 10:1–9
Long XY, Zhao X, Wang W, Zhang Y, Wang HW, Liu XQ, Suo HY (2019) Protective effect of silkworm pupa oil on hydrochloric acid/ethanol-induced gastric ulcers. J Sci Food Agric 99:2974–2986
Chen SC, Zhao X, Sun P, Qian J, Shi YH, Wang R (2017) Preventive effect of Gardenia jasminoides on HCl/ethanol induced gastric injury in mice. J Pharmacol Sci 133:1–8
Sugata H, Ueno T, Shimosegawa T, Yoshimura T (2003) Direct detection of nitric oxide and its roles in maintaning gastric mucosal integrity following ethanol-induced injury in rats. Free Radic Res 37:159–169
Li F, Huang GB, Tan F, Yi RK, Zhou XR, Mu JF, Zhao X (2020) Lactobacillus plantarum KSFY06 on d-galactose-induced oxidation and aging in Kunming mice. Food Sci Nutr 8:379–389
Ignarro LJ, Byrns RE, Sumi D, Nigris FD, Napoli C (2006) Pomegranate juice protects nitric oxide against oxidative destruction and enhances the biological actions of nitric oxide. Nitric Oxide Biol Chem 15:93–102
Kawaratani H, Tsujimoto T, Douhara A, Takaya H, Moriya K, Namisaki T, Noguchi R, Yoshiji H, Fujimoto M, Fukui H (2013) The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm 2013:10
Attiq A, Jalil J, Husain K, Ahmad W (2018) Raging the war against inflammation with natural products. Front Pharmacol 9:1–27
Sakthivel KM, Guruvayoorappan C (2016) Acacia ferruginea inhibits inflammation by regulating inflammatory iNOS and COX-2. J Immunotoxicol 13:127–135
Zhao X, Cheng Q, Qian Y, Yi RK, Gu LJ, Wang SS, Song JL (2017) Insect tea attenuates hydrochloric acid and ethanol-induced mice acute gastric injury. Exp Ther Med 14:5135–5142
Yang Y, Yin B, Lv L, Wang ZY, Chen ZY, Wen X, Zhang YG, Sun WJ, Li Y, Zhao X (2017) Gastroprotective effect of aucubin against ethanol-induced gastric mucosal injury in mice. Life Sci 189:44–51
Acknowledgements
Not applicable.
Funding
This research was funded by Children's Research Institute of National Center for Schooling Development Programme and Chongqing University of Education (CSDP19FS01103), the Science and Technology Research Program of Chongqing Municipal Education Commission (KJZD-K201901601) and Research Project of Chongqing University of Education (KY2015TBZC), China.
Author information
Authors and Affiliations
Contributions
FL performed the majority of the experiments and wrote the manuscript; HL, GR, XL and RY contributed to the data analysis; FT and XZ designed and supervised the study, XZ checked the final manuscript, HS revised the manuscript and provided funding for the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (201904037B), Chongqing, China.
Competing interests
The authors of this manuscript state that they do not have conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, F., Sun, H., Ran, G. et al. Preventive effect of Lactobacillus plantarum HFY09 on HCl/ethanol-induced gastric injury in mice. Appl Biol Chem 63, 49 (2020). https://doi.org/10.1186/s13765-020-00536-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-020-00536-8