Abstract
As one of the gastroesophageal reflux disease (GERD), reflux esophagitis (RE) severely affects patients’ daily lives. Costunolide (Cos), pertains to a sesquiterpene lactone, performs multiple pharmacological activities including inhibited acute liver injury, anti-inflammation and anti-oxidant. We carried out our study to investigate the anti-inflammatory effect and protective effects of Cos against esophageal tissue damage caused by gastric acid refluxing. The determination of anti-inflammatory effects of Cos were conducted using lipopolysaccharide (LPS)-induced RAW 264.7 cell inflammatory model. The ameliorative effects of Cos on RE were confirmed on RE controlled rats model. The results indicated that Cos reduced nitrite production and inhibited cellular inflammation via regulating the activation of NF-κB. In addition, gastric acid reflux increased expression levels of inflammatory proteins (COX-2, TNF-α and IL-1β) in esophageal tissues, while Cos treatment significantly downregulated the expression of these proteins by inhibiting activation of NF-κB. Furthermore, through observing histological stain, Cos significantly improved esophageal damage caused by gastric acid reflux. Therefore, we suggested that Cos has the potential to be a material of natural drug for the treatment of reflux esophagitis caused by acid reflux.
Similar content being viewed by others
Introduction
According to the American Society of Gastroenterology, the definition of gastroesophageal reflux disease (GERD) refers to some symptoms and complications that resulted in the contents of stomach refluxing into the esophagus or other parts (oral or lung) [1]. Population-based studies from 2005 to 2014 have shown that the increase of the incidence of symptomatic GERD in the world including the area of Australia, North America, East Asia, Europe, Middle East, and South America. Especially, the incidence rate in East Asia has increased by about 3 times in 10 years (2.5–7.8%) [2, 3]. Heartburn and regurgitation are the most typical symptoms of GERD, simultaneously, chest pain and sleep disorders are also symptoms that may be associated with GERD [4]. As one of the GERD, reflux esophagitis (RE) severely affects patients’ daily lives. Proton pump inhibitors (e.g., omeprazole) and histamine type 2 receptor antagonists (e.g., ranitidine) are the main drugs for the treatment of this disease while one-third of the patients have not shown a reduction in symptoms when taking drugs [5, 6].
Costunolide (Cos; Fig. 1a), pertains to a sesquiterpene lactone, is mainly extracted from plants of the families Saussurea and Magnoliaceae [7]. In previous studies, Cos has been shown to exhibit excellent physiological activities on a variety of disease of cell models and animal models, such as inducing apoptosis of HL-60 and U20S cancer cell lines by regulating reactive oxygen species [8, 9], and inhibiting the differentiation of inflammatory CD4+ cells and the apoptosis of prostate cancer cells [10, 11]. In animal models, it inhibited mice acute liver injury caused by LPS/d-galactosamine through inhibiting the NF-κB signaling pathway [12] and ameliorated lipoteichoic acid-induced acute lung injury by regulating the MAPK signaling pathway [13].
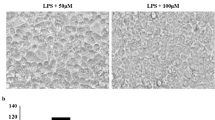
The chemical structure of Cos (a), and effect of Cos on NO production (b), cell viability (c), observation and quantitative result of cell morphology (d) in RAW 264.7 cells induced by LPS. Concentration of nitrite oxide and cytotoxicity were measured by Griess Reagent and Cell Viability, Proliferation & Cytotoxicity Assay Kit, respectively. Morphology of cells was captured by optical microscopy and quantitative result of cell morphology was analysis using Image J program. Scale bar was 200 μm. Data are expressed as mean ± standard deviation. All experiments were performed in three independent experiments. ###p < 0.001 vs. normal cells; ***p < 0.001 vs. LPS-stimulated cells
Many studies on the protective effects of natural plant extracts and single plant extract constituents on experimental RE. For example, the phenolic component, chlorogenic acid, promoted regression of RE by inhibiting the inflammatory response [14], and the extract of Artemisia campestris protected against the injury of esophageal mucosal caused by acid reflux via regulating the oxidation and antioxidant balance [15]. The fruit extract and its biomarker (scopoletin) of Morinda citrifolia aqueous, in addition to the extract of myrtle berry seed (Myrtus communis) were also reported to inhibit RE and gastric ulcer [16, 17]. However, there are no studies about the effect of Cos on experimental animal model of RE.
In the present study, we firstly examined the anti-inflammatory effect of Cos on cell model and proved that Cos has good anti-inflammatory activity. In order to investigate whether it has a protective effect on RE, we determined the protective effect of Cos on experimental RE. We found that oral administration of Cos significantly ameliorated the esophageal mucosal damage caused by gastric acid reflux, increased the pH of gastric content and inhibited the expression of COX-2, TNF-α and IL-1β in RE rat esophageal tissue, by regulating the NF-κB signaling pathway. Our results further verified the anti-inflammatory effects of Cos and provided a potential alternative drug for treating RE.
Materials and methods
Materials
Cos (purity > 99%, Fig. 1a) was purchased from Chem Faces (Wuhan, Hubei, China). Ranitidine and LPS were purchased from Sigma-Aldrich (St Louis, MO, USA). Luminol reagent, primary antibodies of β-actin, iNOS, COX-2, TNF-α, IL-1β, p-IκBα, and p-NF-κB, and secondary antibodies content with horseradish peroxidase (HRP) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The antibody of lamin B1 was purchased from the company of Cell Signaling Technology (Danvers, MA, USA). The protein assay reagent and PVDF membranes were obtained from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Penicillin, streptomycin, Dulbecco’s modified Eagle’s medium (DMEM), and fetal bovine serum (FBS) were purchased from Welgene (Namcheon-ro, South Korea).
Cell culture
RAW 264.7 mouse macrophage cell line used in this study was obtained from the American Type Culture Collection (ATCC). Recovered cells growth in medium containing DMEM, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FBS in an incubator with 5% CO2, at 37 °C.
Cell viability and NO production determination
Cells were cultured in 96-well plates at a density of 1 × 106 cells/ml by incubation for 24 h, at 37 °C. The incubated cells were pretreated with Cos (3.75, 7.5, and 15 μM) for 1 h. Then 1 μg/ml of LPS was added and the cells incubated for another 18 h. The supernatant of the cells medium and cells was used for measuring the concentration of NO by the Griess reagent (Promega, USA), and the cytotoxicity was evaluated by using a cell viability, proliferation, and cytotoxicity assay kit (DoGenBio Co., Ltd., Korea), according to the manufacturer’s protocol, respectively.
Cell morphology observation and quantitative analysis
Cell morphology was observed using inverted microscope eclipse TS 100 (Nikon, Instruments INC., 1300 Walt Whitman Road, Melville, N.Y., USA). Quantitative result for cell morphology was analysis using Image J program. The calculation was based on roundness formula.
A: the cell area, dmax: the maximum diameter [18].
Cellular protein extraction and western blot sample preparation
Cells were cultured in 6-well plates at a density of 6 × 105 cells/ml by incubating for 24 h. Afterward, the cells were pretreated with Cos (7.5 and 15 μM) for 1 h. Then 1 μg/ml of LPS was added to medium for incubating another 1 or 18 h. After washing (three times) with iced phosphate-buffered saline, radio immunoprecipitation assay lysis buffer was used for cells lysing, containing protease inhibitor cocktail (USB Corporation Cleveland, OH, USA) and incubated on ice for 10–15 min. The resulting lysate was centrifuged at 4 °C, 13,000 rpm for 15 min to collect the supernatant, which was stored at − 80 °C in an ultra-low-temperature freezer, for next step.
Maintenance of rats and experimental RE surgery
Forty, male, 7-week-old Sprague-Dawley rats were housed in standard cages and maintained in a breeding room under controlled temperature (21–25 °C) and 12 h light/dark cycle for 1 week, with ad libitum access to food and water. All steps of the experiment were carried out according the animal welfare recommendations of the Institutional Animal Care and Use Committee of Chonbuk National University Laboratory Animal Center in South Korea (IACUC; CBNU 2017-00079, date of approval, August 5, 2018). Before surgery, rats were fasted for 18 h but supplied with water. Rats were divided into five groups with 8 in each group, randomly. Group 1 was the normal group (no oral administration was given). Group 2 was the vehicle group (RE-control), orally administered with physiological saline. Groups 3 and 4 were treated by oral administration of Cos at 15 and 30 mg/kg, respectively. Group 5 was the positive control, orally administered with ranitidine at 30 mg/kg. Oral administration was performed 2 h before surgery to induce RE. Following respiratory anesthesia, rats’ laparotomies were carried out (except for the normal group), 2 cm incision was made to expose the stomach, and then the forestomach and pylorus were ligated. After 4 h, all rats were sacrificed. The esophagus and stomach were removed, the tissue was longitudinally dissected to expose the internal lesions, and images were taken to calculate the damage ratio of the esophageal mucosa. The esophageal tissue was then aliquoted and placed in an − 80 °C ultra-low-temperature freezer for later use.
Esophageal lesion index and histological study
The gross damage ratio of esophageal tissue was analyzed by Image J software and calculated, as described in our previous study [19]. For histological analysis, the esophageal tissue was cut into about 2 mm size, fixed in 10% neutral-buffered formalin (NBF), paraffin-embedded, sectioned (5 μm) and hematoxylin–eosin stained. Digital images of the sections were collected by using a Lecia microscope (Leica Microsystems, Germany) at 10× magnification.
Determination of gastric contents pH
4 h after the operation, all rats were sacrificed. The gastric contents were collected in a 15 ml tube, centrifuged at 2500 rpm for 10 min, and the supernatant was transferred to a new tube to measure the pH value with a pH meter EL20 (Columbus, Ohio, USA).
Extraction of esophageal tissue proteins
For extracting tissue protein, esophageal tissue was homogenized and lysed with iced cytoplasmic lysis buffer containing protease inhibitor cocktail then incubated on ice for another 30 min. Following centrifugation of the lysate at 13,000 rpm, 4 °C for 3 min, and the cytoplasmic protein was collected. Subsequently, the pellet was re-suspended with nuclear lysis buffer. Following incubation on ice for 30 min, the lysate was centrifuged at 13,000 rpm, 4 °C for 15 min, the nuclear protein was collected. All the protein samples were stored at − 80 °C in an ultra-low-temperature freezer until used.
Western blot analysis
Protein samples were loaded and separated on 8% and 10% polyacrylamide gel by SDS-polyacrylamide gel electrophoresis and then transferred to PVDF membranes and blocked with 5% skim milk for 1 h 30 min at room temperature. Then the 1:1000 primary antibodies were added to membranes for incubating at 4 °C overnight, followed by incubation with 1:10,000 secondary antibodies for 2 h at room temperature. Protein bands were colored by luminol reagent and captured by Bio-Rad imaging software (NY, USA). Densiometric analysis was performed using the Image Lab 5.1 program (NY, USA).
Statistical analysis
All experiments were carried out in three independent replicates, and all data were expressed as average value ± standard deviation. One-way ANOVA and Fisher’s LSD multiple comparison test were conducted using SPSS 12.0 K for Windows. p < 0.05 was the significance level.
Results
Cos inhibited the NO production and no effect on cell viability in LPS-induced RAW 264.7 cells
To evaluate the cytotoxicity of Cos and its inhibitory effect on NO production, cells were plated in 96-well plates at a density of 1 × 106 cells/ml. Following pretreatment with Cos (3.75, 7.5, 15 μM) for 1 h, then 1 μg/ml of LPS was added for incubating another 18 h. The results indicated that LPS induced increased production of NO, whereas pretreatment with Cos significantly reduced NO production in a dose-dependent manner (Fig. 1b). As shown in Fig. 1c, no cytotoxicity was observed until the concentration up to 15 μM. Compared with normal cells, LPS-induced cells exhibited cell spreading, an irregular shape, and pseudopod formation, which was improved by treatment with Cos. The result of roundness factor which is a quantitative parameter of cell morphology (the closer to 1, the more regular cell morphology) was showed in Fig. 4d.
Cos suppressed the protein expression levels of iNOS, COX-2, and TNF-α in LPS-induced RAW 264.7 cells
To evaluate the inhibitory effect of Cos on inflammatory mediators and pro-inflammatory cytokines in LPS-induced RAW 264.7 cells, cells were plated in 6-well plates at a density of 6 × 105 cells/ml. Following pretreatment with Cos (7.5 and 15.0 μM) for 1 h, LPS (1 μg/ml) was then added and the cells incubated for another 18 h. The results showed that LPS induced a marked increase in the expression levels of iNOS (Fig. 2a), COX-2 (Fig. 2b), and TNF-α (Fig. 2c) while co-treatment with Cos down-regulated the expression levels of these proteins at concentration 15 μM.
Effects of Cos on the expression levels of iNOS (a), COX-2 (b), and TNF-α (c) in RAW264.7 cells induced by LPS were analyzed by western blotting. Data are expressed as mean ± standard deviation. All experiments were performed in three independent experiments. ###p < 0.001, ##p < 0.01, #p < 0.05 vs. normal cells; ***p < 0.001, **p < 0.01, *p < 0.05 vs. LPS-stimulated cells
Cos suppressed the activation of NF-κB in LPS-induced RAW 264.7 cells
To observe the effect of Cos on NF-κB activation, cells were plated in 6-well plates at a density of 6 × 105 cells/ml. Following pretreatment with Cos (7.5 and 15.0 μM) for 1 h, LPS (1 μg/ml) was then added for incubating another 1 h. LPS induced an increase in the phosphorylation of IκBα and NF-κB (Fig. 3). Pretreatment with Cos dramatically inhibited the phosphorylation of IκBα and NF-κB induced by LPS at concentration 15 μM.
Effects of Cos on the phosphorylation of NF-κB (a), IκBα (b) in RAW264.7 cells induced by LPS were analyzed by western blotting. Data are expressed as mean ± standard deviation. All experiments were performed in three independent experiments. ##p < 0.01, #p < 0.05 vs. normal cells; *p < 0.05 vs. LPS-stimulated cells
Cos alleviated the damage of esophageal tissue induced by gastric acid reflux
To demonstrate the protective effect of Cos on RE, we induced acute esophageal reflux by surgery. As shown in Fig. 4a-i, reflux of gastric acid caused black-red hemorrhagic lesions in the entire rat esophagus, and hypertrophy of the esophageal tissue. However, this condition gradually decreased with the treatment of Cos, which was manifested as a reduction in the extent of hemorrhagic lesions and tightening of the esophagus structure. Especially at the Cos concentration of 30 mg/kg, the rate of esophageal lesions was reduced by about 25% compared with the RE-control group (Fig. 4a-ii). The ranitidine positive control group decreased the rate of esophageal lesions by about 45%.
Effects of Cos on esophageal reflux induced esophageal mucosal damage in rats. Gross (a-i), the ratio of esophageal mucosal damage (a-ii) was calculated by Image J program, microscopic (scale bar 200 μm) (b), and pH of the gastric contents (c). N, normal rats; Veh, RE controlled rats; Cos15, RE controlled rats treated with Cos 15 mg/kg; Cos30, RE controlled rats treated with Cos 30 mg/kg; R, RE controlled rats treated with ranitidine 30 mg/kg. **p < 0.01, ***p < 0.001 vs. RE controlled rats. Data are expressed as mean ± standard deviation
Cos alleviated histological changes induced by gastric acid reflux and increased the pH of the gastric contents
We performed histologically examined the esophageal tissue. In the normal group, we observed normal esophageal tissues, including the mucosa, submucosa, muscularis externa, and adventitia. However, the esophageal mucosa and submucosa caused by gastric acid reflux almost fell off, and the muscle layer tissue became loose, whereas, the esophageal changes were gradually reduced by the treatment of Cos (Fig. 4b). Furthermore, the gastric acid pH of the RE-control group was 2.21–2.65, while the gastric acid pH of the Cos treatment group (30 mg/kg) was 2.72–3.38, which was significantly different from RE-control group). As well as the gastric acid pH of the ranitidine treatment group was 2.90–3.94, similar to the Cos treatment group (30 mg/kg) (Fig. 4c).
Cos suppressed the protein expression levels of inflammatory proteins in esophageal tissue
To investigate whether Cos affects the expression of inflammatory factors and pro-inflammatory mediators in esophageal tissues, we performed western blotting analysis on rat esophageal tissues. Gastric acid reflux led to a significant increase in the protein expression levels of COX-2, TNF-α, and IL-1β while treatment with Cos significantly inhibited the expression levels of these three proteins, particularly, at a Cos concentration of 30 mg/kg (Fig. 5). These findings indicated that Cos was effective in suppressing esophageal inflammation caused by acid reflux.
Effects of Cos on the expression levels of COX-2 (a), TNF-α (b), and IL-1β (c) in esophageal tissue were measured by western blotting. N, normal rats; Veh, RE controlled rats; Cos15, RE controlled rats treated with Cos 15 mg/kg; Cos30, RE controlled rats treated with Cos 30 mg/kg; R, RE controlled rats treated with ranitidine 30 mg/kg. ###p < 0.001, ##p < 0.01 vs. normal rats; **p < 0.01, *p < 0.05 vs. RE controlled rats. Data are expressed as mean ± standard deviation
Cos suppressed the phosphorylation of NF-κB and IκBα in esophageal tissue
To investigate whether Cos treatment affects the phosphorylation of NF-κB and IκBα. As shown in Fig. 6, gastric acid reflux activated NF-κB, which increased its phosphorylation level, and the phosphorylation of the NF-κB inhibitory protein-IκBα, is also increased. In contrast, Cos significantly inhibited the phosphorylation levels of both NF-κB and IκBα, especially at 30 mg/kg, and suggest that the NF-κB signaling pathway may be associated with RE.
Effects of Cos on the phosphorylation of NF-κB (a), IκBα (b) in esophageal tissue were measured by western blotting. N, normal rats; Veh, RE controlled rats; Cos15, RE controlled rats treated with Cos 15 mg/kg; Cos30, RE controlled rats treated with Cos 30 mg/kg; R, RE controlled rats treated with ranitidine 30 mg/kg. ###p < 0.001, ##p < 0.01 vs. normal rats; **p < 0.01, *p < 0.05 vs. RE controlled rats. Data are expressed as mean ± standard deviation
Discussion
Inflammation is an innate immune response that occurs when the body is stimulated by irritants, such as pathogens, bacterial or tissue damage, to protect the host from damage [20]. However, persistent inflammation leads to excessive inflammatory mediators and cellular effectors that can lead to a variety of inflammation-related diseases and even cancer [21]. RE is also an inflammatory disease due to long-term reflux may lead to esophageal damage and even esophageal carcinogenesis. The incidence rate of RE is gradually increasing worldwide and imposes a great burden on patients in daily life and economic status [22]. Finding drugs with good therapeutic effects and small side effects to treat this disease is an urgent problem.
Cos, as a sesquiterpene lactone present in M. sieboldii or plants of the same genus, has been shown to display various physiological activities, including antioxidant, anti-inflammatory, and anticancer. To explore the anti-inflammatory effects and protective effects of Cos on RE, we carried out experiments in vitro (LPS-induced RAW 264.7 cells inflammation) and in vivo (experimental RE).
Nitric oxide (NO) production in human body is induced nitric oxide synthase (NOS) including neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS). NO synthesized by nNOS and eNOS is beneficial for nerve transmission and vasodilation, whereas that synthesized by iNOS is harmful and can promote the development of inflammation and aggravate the inflammatory response [23]. Cyclooxygenase-2 (COX-2) plays a more important role in inflammation and cancer development because it is highly induced under various stimulating conditions [24]. TNF-α is also involved in the development of various inflammations [25]. In our study, Cos co-treatment significantly inhibited the NO production as well as the expression levels of iNOS, COX-2, and TNF-α induced by LPS in a dose-dependent manner. Furthermore, Cos co-treatment also down-regulated the phosphorylation of NF-κB and IκBα in cells. It means that Cos have a good anti-inflammatory effect on cell model.
The experimental RE model in rat has already been used to evaluate the protective effects of drug or plant extracts [7,8,9]. In this study, we also created the experimental RE through surgery that introduced before [19]. The chronic and repeated exposure to gastric contents is well-recognized as causing esophageal mucosal hemorrhage and hyperplasia, and sparse tissue structure [26]. Histologically, the esophageal tissue of RE usually exhibits mucosal shedding, submucosal hemorrhage, and cell infiltration, and the muscle layer tissue appears sparse and irregular [27]. The results of this study showed that severe lesions and mucosal shedding occurred in the esophageal tissue, due to gastric acid reflux, whereas oral administration of Cos significantly reduced these symptoms. Furthermore, ranitidine is a commonly used drug for the treatment of gastroesophageal reflux disease such as hyperacidity. As a H2 receptor antagonist, ranitidine usefully inhibits gastric acid secretion and reduce stomach acid by competitively blocking the binding of histamine to the H2 receptor on the parietal cells [5, 6]. In our study, the gastric acid pH of the Cos treatment group (30 mg/kg) was significantly different from RE-control group. As well as similar to the ranitidine treatment group.
NF-κB is a transcription factor which plays a crucial role in the activation and development of cells of the innate immune system [28, 29]. In a stable cellular environment, NF-κB is inactive and anchored to the cytoplasm by its inhibitory protein, IκBα [30]. Once the cell is stimulated, it immediately initiates a rapid response mode. The inhibitory protein, IκBα, undergoes phosphorylation and degradation, leading to release of the NF-κB dimer, which then transfers to the nucleus and binds to a specific site for transcription of the target gene, such as COX-2, TNF-α, and IL-1β [31, 32]. Once the stimulus is resolved, the process returns to the latent state. Many studies had proved that the NF-κB signaling pathway is involved in the occurrence and development of inflammation-related diseases and cancer [33, 34]. To investigate the interrelation between NF-κB signaling pathway and RE, we furthermore performed western blotting assay on the esophageal tissue proteins. Results showed that Cos treatment significantly inhibited the increase of expression levels of COX-2, TNF-α, and IL-1β caused by gastric acid refluxing in the esophageal tissue. In addition, we also evaluated the phosphorylation of NF-κB and IκBα in the esophageal tissue. It was evident that Cos significantly inhibited the activation of NF-κB via down-regulating the phosphorylation levels of NF-κB and IκBα in esophageal tissue of RE rats (Fig. 6).
Availability of data and materials
Not applicable.
Abbreviations
- GERD:
-
Gastroesophageal reflux disease
- RE:
-
Reflux esophagitis
- LPS:
-
Lipopolysaccharide
- Cos:
-
Costunolide
- NO:
-
Nitrite oxide
- iNOS:
-
Inducible nitrite oxide synthase
- COX-2:
-
Cyclooxygenase-2
- IL-1β:
-
Interleukin-1β
- TNF-α:
-
Tumor necrosis factor
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- IκBα:
-
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- NBF:
-
Neutral-buffered formalin
References
Katz PO, Gerson LB, Vela MF (2013) Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 108:308–328
El-Serag HB, Sweet S, Winchester CC, Dent J (2014) Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 63:871–880
Jung HK (2011) Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J Neurogastroenterol Motil 17:14–27
Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, Liberati A, O’Connell D, Oxman AD, Phillips B, Schünemann H, Edejer TT, Vist GE, Williams JW Jr (2004) Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 4:38
Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME (2013) Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002095.pub5
Colin-Jones DG (1995) The role and limitations of H2-receptor antagonists in the treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 9:9–14
Park SH, Choi SU, Lee CO, Yoo SE, Yoon SK, Kim YK, Ryu SY (2001) Costunolide, a sesquiterpene from the stem Bark of Magnolia sieboldii, inhibits the RAS-Farnesyl-Proteintransferase. Planta Med 67:358–359
Park HJ, Kwon SH, Han YN, Choi JW, Miyamoto K, Lee SH, Lee KT (2001) Apoptosis-inducing costunolide and a novel acyclic monoterpene from the stem bark of Magnolia sieboldii. Arch Pharm Res. 24:342–348
Zhang C, Lu T, Wang GD, Ma C, Zhou YF (2016) Costunolide, an active sesquiterpene lactone, induced apoptosis via ROS-mediated ER stress and JNK pathway in human U2OS cells. Biomed Pharmacother 80:253–259
Park E, Song JH, Kim MS, Park SH, Kim TS (2016) Costunolide, a sesquiterpene lactone, inhibits the differentiation of pro-inflammatory CD4+ T cells through the modulation of mitogen-activated protein kinases. Int Immunopharmacol 40:508–516
Chen J, Chen B, Zou Z, Li W, Zhang Y, Xie J, Liu C (2017) Costunolide enhances doxorubicin-induced apoptosis in prostate cancer cells via activated mitogen-activated protein kinases and generation of reactive oxygen species. Oncotarget 8:107701–107715
Wang Y, Zhang X, Zhao L, Shi M, Wei Z, Yang Z, Guo C, Fu Y (2017) Costunolide protects lipopolysaccharide/D-galactosamine-induced acute liver injury in mice by inhibiting NF-κB signaling pathway. J Surg Res 220:40–45
Chen Z, Zhang D, Li M, Wang B (2018) Costunolide ameliorates lipoteichoic acid-induced acute lung injury via attenuating MAPK signaling pathway. Int Immunopharmacol 61:283–289
Kang JW, Lee SM (2014) Protective effects of chlorogenic acid against experimental reflux esophagitis in rats. Biomol Ther 22:420–425
Jabri MA, Tounsi H, Abdellaoui A, Marzouki L, Sebai H (2018) Protective effects of Artemisia campestris extract against gastric acid reflux-induced esophageal mucosa injuries. Pathophysiology 25:63–69
Kwon OJ, Choo BK, Lee JY, Kim MY, Shin SH, Seo BI, Seo YB, Rhee MH, Shin MR, Kim GN, Park CH, Roh SS (2016) Protective effect of Rhei Rhizoma on reflux esophagitis in rats via Nrf2-mediated inhibition of NF-κB signaling pathway. BMC Complement Altern Med 16:7
Mahattanadul S, Ridtitid W, Nima S, Phdoongsombut N, Ratanasuwon P, Kasiwong S (2011) Effects of Morinda citrifolia aqueous fruit extract and its biomarker scopoletin on reflux esophagitis and gastric ulcer in rats. J Ethnopharmacol 134:243–250
Yu HY, Lim KP, Xiong SJ, Tan LP, Shim W (2013) Functional morphometric analysis in cellular behaviors: shape and size matter. Adv Healthc Mater 2:1188–1197
Nan L, Nam HH, Choo BK, Park JC, Kim DG, Lee JH, Moon KH (2018) An ethanolic extract of Allium hookeri root alleviates reflux esophagitis and modulates NF-κB signaling. Evid Based Complement Alternat Med. https://doi.org/10.1155/2018/1834681
Cameron B, Landreth GE (2010) Inflammation, microglia, and Alzheimer’s disease. Neurobiol Dis 37:503–509
Liu J, Tang J, Zuo Y, Yu Y, Luo P, Yao X, Dong Y, Wang P, Liu L, Zhou H (2016) Stauntoside B inhibits macrophage activation by inhibiting NF-κB and ERK MAPK signaling. Pharmacol Res 111:303–315
Chen MJ, Wu MS, Lin JT, Chang KY, Chiu HM, Liao WC, Chen CC, Lai YP, Wang HP, Lee YC (2009) Gastroesophageal reflux disease and sleep quality in a Chinese population. J Formos Med Assoc 108:53–60
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615
Tiano HF, Loftin CD, Akunda J et al (2002) Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res 62:3395–3401
Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF (2007) The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 48:751–762
Sandhu DS, Fass R (2018) Current trends in the management of gastroesophageal reflux disease. Gut Liver 12:7–16
Nakamura K, Ozawa Y, Furuta Y, Miyazaki H (1982) Effects of sodium polyacrylated (PANa) on acute esophagitis by gastric juice in rats. Jpn J Pharmacol 32:445–456
Zhang Q, Lenardo MJ, Baltimore D (2017) 30 Years of NF-κB: a blossoming of relevance to human pathobiology. Cell 168:37–57
Zhang ZQ, Rigas B (2006) NF-κB, inflammation and pancreatic carcinogenesis: NF-κB as a chemoprevention target (Review). Int J Oncol 29:185–192
Hayden MS, Ghosh S (2008) Shared principles in NF-κB signaling. Cell 132:334–362
Hayden MS, West AP, Ghosh S (2006) NF-κB and the immune response. Oncogene 25:6758–6780
Charrad R, Berraïes A, Hamdi B, Ammar J, Hamzaoui K, Hamzaoui A (2016) Anti-inflammatory activity of IL-37 in asthmatic children: correlationwith inflammatory cytokines TNF-α, IL-β, IL-6 and IL-17A. Immunobiology 221:182–187
Guo H, Zhang Y, Cheng BC, Fu X, Zhu P, Chen J, Chan Y, Yin C, Wang Y, Hossen M, Amin A, Tse AK, Yu ZL (2018) An ethanolic extract of the aerial part of Siegesbeckia orientalis L. inhibits the production of inflammatory mediators regulated by AP-1, NF-κB and IRF3 in LPS-stimulated RAW 264.7 cells. Biosci Trends 12:330–337
Miller SA, White JA, Chowdhury R, Gales DN, Tameru B, Tiwari AK, Samuel T (2018) Effects of consumption of whole grape powder on basal NF-κB signaling and inflammatory cytokine secretion in a mouse model of inflammation. J Nutr Intermed Metab 11:1–8
Acknowledgements
Not applicable.
Funding
This research was carried out with the support of the National Research Foundation of Korea (NRF) (NRF-2017R1D1A3B03036020).
Author information
Authors and Affiliations
Contributions
HHN and BKC conceived and designed the experiments, LN did most of the experiments, performed the data analyses and wrote the manuscript, HHN helped perform the analysis with constructive discussion. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
There are on competing interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nan, L., Nam, HH. & Choo, BK. Costunolide inhibits inflammation in LPS-induced RAW264.7 cells and ameliorates gastric acid reflux-induced esophageal injury in rat model. Appl Biol Chem 63, 33 (2020). https://doi.org/10.1186/s13765-020-00514-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-020-00514-0