Abstract
Background
Vaginal discharge syndrome (VDS) is a common condition. Clinical management targets sexually transmitted infections (STIs) and bacterial vaginosis (BV); there is limited focus on Candida infection as cause of VDS. Lack of Candida treatment coverage and, if present, antifungal resistance may result in VDS treatment failure. This study aimed to determine the prevalence of Candida infection, antifungal resistance, and coinfections in Namibian women with VDS.
Methods
A cross-sectional study was performed using 253 vaginal swabs from women with VDS in Namibia. Demographic data was collected, and phenotypic and molecular detection of Candida species was performed followed by fluconazole susceptibility testing of Candida isolates. BV was diagnosed using Nugent score microscopy; molecular detection of Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis was performed.
Results
Candida species was detected in 110/253 women (43%). Ninety women (36%) had Candida albicans and 24 (9.5%) had non-albicans Candida species. The non-albicans species detected were 19 (17%) Candida glabrata, 4.0 (3.5%) Candida krusei, and 1.0 (0.9%) Candida parapsilosis. Candida albicans were more frequently isolated in younger (p = 0.004) and pregnant women (p = 0.04) compared to non-albicans Candida species. Almost all (98%) Candida albicans isolates were susceptible to fluconazole while all non-albicans Candida species were fluconazole resistant. STIs were diagnosed in 92 women (36%): 30 (12%) with C. trachomatis, 11 (4.3%) N. gonorrhoeae, and 70 (28%) T. vaginalis; 98 (39%) women had BV. Candida infection alone was diagnosed in 30 women (12%), combined with STIs in 42 women (17%) and was concurrent with BV in 38 women (15%). Candida infection was more often detected in swabs from women without C. trachomatis detected (6.4% vs. 16%; OR 0.30; 95% CI 0.10–0.77, p = 0.006).
Conclusions
The high prevalence of Candida infection, especially those due to non-albicans Candida species that are resistant to fluconazole, is a great concern in our setting and may lead to poor treatment outcomes. Access to microbiological testing for Candida species in the context of syndromic management is warranted.
Similar content being viewed by others
Background
Vaginal discharge syndrome (VDS) is the most common gynaecological condition among women of reproductive age [1]. Vulvovaginal candidiasis (VVC) is the most common aetiology of VDS, accounting for about 90% of symptomatic vaginal infections [2, 3]. Up to 75% of healthy women face symptomatic VVC at least once in their childbearing years, with some experiencing intermittent and often obdurate forms of the disease [4, 5]. A previous study from Namibia reported that symptomatic VVC was present in 61/335 (18.2%) of women newly diagnosed with human immunodeficiency virus (HIV) that were enrolled for antiretroviral therapy [6]. Additionally, recurrent VVC (RVVC) may occur in 10%-20% of women with symptomatic VVC [7]. In two recent studies, RVVC, defined as four or more symptomatic episodes per year, was estimated to occur in 37,390 females per year in Namibia and in over a million females per year in South Africa [8, 9]. There are several factors contributing to RVVC, which include treatment failure, co-infections and antifungal resistance [10].
The aetiology in more than 90% of the VVC cases is Candida albicans [11]. Non-albicans Candida species have emerged as an important aetiology of VVC as its prevalence and antifungal resistance is a mounting problem globally [10, 11]. The most significant of these non-albicans Candida species is Candida glabrata owing to its intrinsic resistance or low susceptibility to azoles [12]. Other than C. glabrata, C. krusei, C. tropicalis, C. parapsilosis and, very rarely, Saccharomyces cerevisiae, are other potential pathogens that may lead to VVC [13].
In addition to VVC, VDS may also be caused by sexually transmitted infections (STIs), such as Chlamydia trachomatis, Trichomonas vaginalis, Neisseria gonorrhoeae, and bacterial vaginosis (BV) [2, 3]. In Namibia, like other low- to middle-income countries (LMICs), syndromic management of VDS is the standard of care, where clinicians treat patients empirically, without aetiological diagnosis, based on a set of symptoms [14]. This approach is associated with under-treatment as STIs frequently remain asymptomatic, but over-treatment with unnecessary use of antibiotics also occurs [15, 16]. Determination of the microbiological aetiology of VDS is essential to guide empirical treatment algorithms and to guide effective prescription of antifungal drugs for presumed candidiasis. Most of such studies focus on STIs and do not include Candida species, or antifungal resistance [15, 17, 18]. Comprehensive evaluation of microbial aetiology of VDS is essential to better understand the occurrence of Candida species, as well as the intersection with STIs and BV. The aim of this study was to determine the prevalence of Candida species, fluconazole resistance and co-infections in swabs collected from women with VDS in Namibia.
Methods
Study design, setting and population
This cross-sectional study was conducted using 253 vaginal swabs collected from women with VDS between February and July 2021 at primary healthcare facilities across Namibia. The vaginal swabs were collected by healthcare workers, which were sent to the diagnostic laboratory for routine diagnostic testing.
The laboratory requisition forms were assessed for inclusion and exclusion in the study. Vaginal swabs from women aged 18 to 49 years of age with ‘vaginal discharge’ as diagnostic indication recorded by the clinician were included, while swabs were excluded if more than two weeks old.
Detection and identification of Candida isolates
Vaginal swabs were inoculated on the chromogenic Candida agar plates (CHROMagar™, France), which were incubated (Thermo Scientific, USA) at 37 °C for 24–36 h. Colonies isolated on chromogenic Candida agar were used to identify Candida spp. according to colony colour, as per manufacturer’s instructions. In each test, the reference strain C. albicans American Type Culture Collection (ATCC) 14053 was used for quality control. If there were no visible colonies within 3 days, the sample was considered negative for Candida. Colonies were inoculated on the Sabouraud Dextrose Agar (SDA) plates (Oxoid, United Kingdom) in order to be stored with 50% glycerol (Merck, Germany) at − 20 °C prior to DNA extraction.
Molecular confirmation of Candida species
Deoxyribonucleic acid (DNA) extraction and purification from vaginal swabs were performed using the Quick-DNA™ Fungal/ Bacterial Miniprep Kit (Zymo Research, USA) according to the manufacturer’s guidelines. Multiplex PCR was performed using the One Taq® Quick-Load 2 × Master Mix (New England BioLabs, USA), a universal Candida primer pair targeting ITS1 and ITS2 and C. albicans specific primers according to Rad et al. [19]. The PCR products were then analysed with the 50 bp DNA ladder (New England BioLabs, USA) by gel electrophoresis through a 2% agarose gel and ultraviolet visualisation [19]. Candida albicans ATCC 14053, C. glabrata CBS2175, C. parapsilosis CBS2195, C. tropicalis CBS94, and C. krusei CBS473 were included in each PCR reaction as positive controls; nuclease-free water (BioConcept, Switzerland) was used as negative control.
Fluconazole antifungal susceptibility patterns of Candida isolates
The fluconazole susceptibility of Candida isolates was determined using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) microbroth dilution (MBD) method to determine the minimum inhibitory concentration (MIC) [20]. The EUCAST breakpoints were used to assign the Candida species into the clinical categories “susceptible”, “intermediate” and “resistant” [20]. Quality control isolates included C. parapsilosis ATCC 22019 (susceptible) and C. krusei ATCC 6258 (resistant) [21].
Detection of bacterial vaginosis
A Gram-stained vaginal smear prepared from vaginal swab was examined under a microscope and evaluated for BV by Nugent scoring [22, 23]. Nugent scores from 0 to 3 are considered as “Normal”; 4 to 6 as “Intermediate”; and 7 to 10 as “BV” [24].
Detection of sexually transmitted infections
Molecular detection of Chlamydia trachomatis and N. gonorrhoeae was performed using the LightMix 480 HT CT/NG assay (TIB MOLBIOL, Berlin, Germany), while a validated in-house real time-PCR assay, as described elsewhere, was performed for detection of T. vaginalis [25].
Statistical analyses
Data were captured into a study-specific Epi Info™ database version 7.2.4.0 (Centres for Disease Control and Prevention (CDC), USA) and exported into RStudio version 2021.09.1 (RStudio, USA) for analysis. Data are presented as absolute value with proportion, and median with range. The chi-squared test, with Fisher’s Exact if appropriate, was used to compare dichotomous variables between groups, while the Mann–Whitney test was used for continuous variables between groups. Logistic regression was used to calculate associations of age and pregnancy between Candida albicans and non-albicans Candida species isolated from women with yeast infections. A p value < 0.05 was considered statistically significant.
Results
Study population
A total of 253 vaginal swabs from women with VDS were included. The median age of these women was 29 years (interquartile range (IQR) 24–34), 58 (23%) were HIV-infected and 60 (24%) were pregnant.
Candida isolates were detected in vaginal swabs from 110 (43%; 110/253) women; there was no association with any of the demographic variables (Table 1). In addition, Chlamydia trachomatis was detected from 30 women (12%), N. gonorrhoeae from 11 (4.3%) and T. vaginalis from 70 (28%). Bacterial vaginosis was present in 98 women (39%) while 69 (27%) and 98 (39%) belonged to an intermediate and normal Nugent score category.
Any Candida infection was less likely detected in swabs from women with Chlamydia trachomatis (6.4% vs. 16%; OR 0.30; 95% CI 0.10–0.77, p = 0.006). There was no relationship between Candida infection and age, HIV, N. gonorrhoeae, T. vaginalis, and BV.
Distribution of Candida species recovered from vaginal swabs
Among the 110 women who tested positive for Candida, 114 Candida isolates were detected using culture methods, i.e. both C. albicans and C. glabrata were detected in four vaginal swabs. C. albicans was the most common isolate (n = 90, 79%), followed by the following non-albicans Candida species: C. glabrata (n = 19, 17%), C. krusei (n = 4, 3.5%) and C. parapsilosis (n = 1, 0.9%) (Table 2). Molecular methods confirmed phenotypic identification in all isolates (100% concordance).
There were significant differences between women with C. albicans and non-albicans Candida spp. with regards to age (p = 0.01) and pregnancy (p = 0.002) (Table 3). Similar results were observed with the multivariate analysis, where both age (p = 0.004) and pregnancy (p = 0.04) were found to be independently associated with C. albicans isolated from vaginal swabs. Candida albicans are more likely to occur in pregnant women (adjusted odds ratio 1.9; 95% CI 1.0–3.5, p = 0.002) and in younger women, as C. albicans is less likely to occur with increasing age (adjusted odds ratio 0.94; 95% CI 0.90–0.98, p = 0.01).
Fluconazole susceptibility pattern
The overall drug susceptibility pattern of Candida isolates against fluconazole is shown in Table 4. Fluconazole resistance was low in C. albicans isolates, but high in all non-albicans Candida isolates: C. glabrata (74%), C. krusei (100%) and C. parapsilosis (100%). There was no significant association between fluconazole susceptibility of C. albicans with demographic factors or coinfections.
Relationship between Candida species, STIs and BV
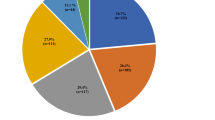
Multiple infections were common: two or more concurrent infections were detected in 86 (34%) women (Fig. 1). Vaginal infections caused by Candida alone occurred in 30/110 (27%) women. Concurrent infections occurred in 80//110 (73%) of women with Candida and included those with STIs (n = 42, 38%) including T. vaginalis (n = 30, 27%), Chlamydia trachomatis (n = 7, 6.4%) and N. gonorrhoeae (n = 5, 4.5%). In addition, BV co-occurred with Candida in 15% of the study population. Concurrent infections with BV (n = 86/98, 88%) included T. vaginalis (n = 24, 24%), Chlamydia trachomatis (n = 19, 19%) and N. gonorrhoeae (n = 5, 5.0%). However, neither Candida spp., C. trachomatis, N. gonorrhoeae nor T. vaginalis was detected in 52 women (21%).
Discussion
Vaginal discharge syndrome (VDS) is a common cause of gynaecological visits among women in sub-Saharan Africa [1, 26, 27]. Most studies report only on specific microbiological aetiology of this condition, usually STIs, but only few have studied microbial aetiology comprehensively. Consequently, VVC and antifungal resistance is often not reported [1, 15, 17, 28]. This study provides a comprehensive analysis of microbial aetiology of VDS in Namibian women; it highlights a high prevalence of Candida species, including fluconazole-resistant non-albicans Candida species, and concurrence with STIs and BV.
Candida species were detected in 110 (43%) of swabs collected from women with VDS in our study making it the most common microbial aetiology. Studies from sub-Saharan Africa reported a wide range of Candida prevalence in women with VDS: 21% in Rwanda [29], 25% in Ethiopia [30], 26% in Mauritania [31], 29% in Senegal and Gabon [15, 32], 38% in Cameroon [33], 39% in Benin [34], 45% in South Africa [35], 49% Burkina Faso [36], 55% in Nigeria [37] and 66% in Tanzania [38]. The geographic differences in the reported prevalence described in different settings might be owing to environmental, behavioural, socioeconomic factors, as well differences in study methodologies [30].
In this cross-sectional study, no significant association with older age, pregnancy or HIV seropositive status was observed although these are known risk factors for VVC [39,40,41,42,43]. However, we did observe a significant association between the presence of Candida infection and detection of Chlamydia trachomatis, but not with the other STIs, where C. trachomatis was less likely to be detected in women with Candida (COR, 0.30, p = 0.006). A study by Kruppa and colleagues demonstrated a novel interaction between Chlamydia trachomatis and C. albicans via the binding of elementary bodies of Chlamydia trachomatis to C. albicans yeast and hyphal forms. This binding was shown to considerably decrease the capacity of Chlamydia trachomatis to infect human cervical epithelial cells, thereby decreasing its disease progression [44]. In contrast, another study illustrated that biofilms related to VVC may act as a reservoir for Chlamydia trachomatis [3]. STIs have been suggested in other studies as risk factor for VVC; however, this relationship should be further confirmed [45, 46].
In our study, like most other studies looking at the species distribution of Candida, the most prevalent Candida species isolated was C. albicans, followed by C. glabrata [47, 48]. Candida glabrata is the most relevant non-albicans Candida species, owing to its ability to develop acquired resistance subsequent to exposure to azole antifungals [11, 12, 49]. In our study, low rate of fluconazole resistance was found in C. albicans isolates (< 5%), but most non-albicans Candida isolates (n = 19, 17%) were fluconazole resistant. These findings are similar to reports from Africa and other parts of the world [33, 48]. Antifungal resistance of Candida spp. is a mounting problem universally [49,50,51]. High rates of fluconazole resistance has been demonstrated in several countries including China [52], Iran [53], Ethiopia [39], Peshawar [54], Brazil [55], Cameroon [33] and Uganda [48], to mention but a few. The use of azole antifungals may stimulate the selection of resistant subpopulations of Candida by shifting colonisation to more intrinsically resistant species, especially C. krusei or C. glabrata [56]. In our study, non-albicans species that were not susceptible to fluconazole were detected in 24/253 (9.5%) women with VDS, which is 22% of all women with Candida infection, highlighting the challenge in management of Candida species in the syndromic management context. Since pregnancy predisposes women to VVC, which in turn could increase the risk for poor pregnancy outcomes, it is reassuring that the pregnant women in this study all had C. albicans infection, and not fluconazole-resistant non-albicans Candida, and could therefore be adequately treated [57]. We observed an association between older age and the isolation of non-albicans species vs. C. albicans [4]. Similarly, some studies show that non-albicans species, such C. glabrata, are associated with older age when compared to C. albicans, which may be due to the exposure of several risk factors such as the use of hormonal contraceptives and broad spectrum antifungals [58, 59].
Our study demonstrates the complex microbial aetiology of VDS. Several women in this study experienced more than one infection, and some up to three infections at once, which may lead to overlapping diagnosis and conditions. Hence, these findings questions whether empirical approach for the management of VDS based on symptoms is appropriate or not [2].
This study has several limitations. First, limited demographic and clinical information was available from study participants due to collecting specimens submitted to the laboratory. Reliance on the information provided by the requesting clinician might have resulted in some misclassification. Second, the culture and molecular methods used target Candida species that cause vaginal infections; other Candida species might have been missed by these assays.
Conclusion
This study highlights a high prevalence of VVC in women with VDS in Namibia. The high frequency of non-albicans Candida species that are resistant to fluconazole is a great concern and may contribute to poor treatment outcomes. Access to microbiological testing for Candida species in the context of syndromic management is warranted.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AOR:
-
Adjusted odds ratio
- AMR:
-
Antimicrobial resistance
- AST:
-
Antimicrobial susceptibility testing
- ATCC:
-
American Type Culture Collection
- BV:
-
Bacterial vaginosis
- CDC:
-
Centres of Disease Control and Prevention
- CI:
-
Confidence interval
- COR:
-
Crude odds ratio
- DNA:
-
Deoxyribonucleic acid
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- HIV:
-
Human immunodeficiency virus
- IQR:
-
Interquartile range
- LMICs:
-
Low- to middle-income countries
- MIC:
-
Minimum inhibitory concentration
- PCR:
-
Polymerase chain reaction
- RVVC:
-
Recurrent vulvovaginal candidiasis
- SDA:
-
Sabouraud dextrose agar
- STI:
-
Sexually transmitted infection
- VDS:
-
Vaginal discharge syndrome
- VVC:
-
Vulvovaginal candidiasis
- WHO:
-
World Health Organization
References
Chirenje ZM, Dhibi N, Handsfield HH, Gonese E, Tippett Barr B, Gwanzura L, et al. The etiology of vaginal discharge syndrome in Zimbabwe: results from the Zimbabwe STI etiology study. Sex Transm Dis. 2018;45(6):422–8.
Zemouri C, Wi TE, Kiarie J, Seuc A, Mogasale V, Latif A, et al. The performance of the vaginal discharge syndromic management in treating vaginal and cervical infection: a systematic review and meta-analysis. PLoS ONE. 2016;11(10):e0163365.
Filardo S, Di Pietro M, Tranquilli G, Sessa R. Biofilm in genital ecosystem: a potential risk factor for Chlamydia trachomatis infection. Can J Infect Dis Med Microbiol. 2019;2019:1672109.
Makanjuola O, Bongomin F, Fayemiwo SA. An update on the roles of non-albicans Candida species in vulvovaginitis. J Fungi (Basel). 2018;4(4):121.
Kalia N, Singh J, Kaur M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann Clin Microbiol Antimicrob. 2020;19(1):5.
Djomand G, Schlefer M, Gutreuter S, Tobias S, Patel R, DeLuca N, et al. Prevalence and correlates of genital infections among newly diagnosed human immunodeficiency virus-infected adults entering human immunodeficiency virus care in Windhoek. Namibia Sex Transm Dis. 2016;43(11):698–705.
Kimberly A. Workowski GAB. Sexually transmitted diseases treatment guideline, 2015. Atlanta, GA 30329-4027. Centers for Disease Control and Prevention, Services USDoHaH; 2015 5 June 2015. Contract No.: 3.
Dunaiski CM, Denning DW. Estimated burden of fungal infections in Namibia. J Fungi (Basel). 2019;5(3):75.
Schwartz IS, Boyles TH, Kenyon CR, Hoving JC, Brown GD, Denning DW. The estimated burden of fungal disease in South Africa. S Afr Med J. 2019;109(11):885–92.
Monroy-Perez E, Paniagua-Contreras GL, Rodriguez-Purata P, Vaca-Paniagua F, Vazquez-Villasenor M, Diaz-Velasquez C, et al. High virulence and antifungal resistance in clinical strains of Candida albicans. Can J Infect Dis Med Microbiol. 2016;2016:5930489.
Healey KR, Jimenez Ortigosa C, Shor E, Perlin DS. Genetic drivers of multidrug resistance in Candida glabrata. Front Microbiol. 2016;7:1995.
Chen Y, Wu Y, Lulou K, Yao D, Ying C. Multilocus sequence typing and antifungal susceptibility of vaginal and non-vaginal Candida glabrata isolates from China. Front Microbiol. 2022;13:808890.
Frobenius W, Bogdan C. Diagnostic value of vaginal discharge, wet mount and vaginal pH—an update on the basics of gynecologic infectiology. Geburtshilfe Frauenheilkd. 2015;75(4):355–66.
Ministry of Health and Social Services N. Namibia standard treatment guidelines 2011. Windhoek: Ministry of Health and Social Services, Namibia; 2011.
Barry MS, Ba Diallo A, Diadhiou M, Mall I, Gassama O, Ndiaye Gueye MD, et al. Accuracy of syndromic management in targeting vaginal and cervical infections among symptomatic women of reproductive age attending primary care clinics in Dakar. Senegal Trop Med Int Health. 2018;23(5):541–8.
Kaida A, Dietrich JJ, Laher F, Beksinska M, Jaggernath M, Bardsley M, et al. A high burden of asymptomatic genital tract infections undermines the syndromic management approach among adolescents and young adults in South Africa: implications for HIV prevention efforts. BMC Infect Dis. 2018;18(1):499.
van der Eem L, Dubbink JH, Struthers HE, McIntyre JA, Ouburg S, Morre SA, et al. Evaluation of syndromic management guidelines for treatment of sexually transmitted infections in South African women. Trop Med Int Health. 2016;21(9):1138–46.
Verwijs MC, Agaba SK, Sumanyi JC, Umulisa MM, Mwambarangwe L, Musengamana V, et al. Targeted point-of-care testing compared with syndromic management of urogenital infections in women (WISH): a cross-sectional screening and diagnostic accuracy study. Lancet Infect Dis. 2019;19(6):658–69.
Mahmoudi Rad M, Zafarghandi A, Amel Zabihi M, Tavallaee M, Mirdamadi Y. Identification of Candida species associated with vulvovaginal candidiasis by multiplex PCR. Infect Dis Obstet Gynecol. 2012;2012:872169.
Arendrup MC, Mouton JW, Lagrou K, Hamal P. J Guinea and the subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID EUCAST antifungal MIC method for yeasts 2017. www.eucast.org.
Jiang C, Dong D, Yu B, Cai G, Wang X, Ji Y, et al. Mechanisms of azole resistance in 52 clinical isolates of Candida tropicalis in China. J Antimicrob Chemother. 2013;68(4):778–85.
Gaydos CA, Beqaj S, Schwebke JR, Lebed J, Smith B, Davis TE, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130(1):181–9.
Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301.
Dols JA, Molenaar D, van der Helm JJ, Caspers MP, de Kat A-B, Schuren FH, et al. Molecular assessment of bacterial vaginosis by Lactobacillus abundance and species diversity. BMC Infect Dis. 2016;16:180.
Pillay A, Radebe F, Fehler G, Htun Y, Ballard RC. Comparison of a TaqMan-based real-time polymerase chain reaction with conventional tests for the detection of Trichomonas vaginalis. Sex Transm Infect. 2007;83(2):126–9.
Hillier SL, Austin M, Macio I, Meyn LA, Badway D, Beigi R. Diagnosis and treatment of vaginal discharge syndromes in community practice settings. Clin Infect Dis. 2021;72(9):1538–43.
Kufa T, Gumede L, Maseko DV, Radebe F, Kularatne R. The demographic and clinical profiles of women presenting with vaginal discharge syndrome at primary care facilities in South Africa: associations with age and implications for management. S Afr Med J. 2018;108(10):876–80.
Morikawa E, Mudau M, Olivier D, de Vos L, Joseph Davey D, Price C, et al. Acceptability and feasibility of integrating point-of-care diagnostic testing of sexually transmitted infections into a South African antenatal care program for HIV-infected pregnant women. Infect Dis Obstet Gynecol. 2018;2018:3946862.
Wall KM, Nyombayire J, Parker R, Ingabire R, Bizimana J, Mukamuyango J, et al. Etiologies of genital inflammation and ulceration in symptomatic Rwandan men and women responding to radio promotions of free screening and treatment services. PLoS ONE. 2021;16(4):e0250044.
Tsega A, Mekonnen F. Prevalence, risk factors and antifungal susceptibility pattern of Candida species among pregnant women at Debre Markos Referral Hospital, Northwest Ethiopia. BMC Pregnancy Childbirth. 2019;19(1):527.
Sy O, Diongue K, Ahmed CB, Ba O, Moulay FC, Lo B, et al. Vulvovaginal candidiasis in pregnant women in the Mere et Enfant Hospital center in Nouakchott, Mauritania. J Mycol Med. 2018;28(2):345–8.
Bignoumba M, Onanga R, Bivigou Mboumba B, Gafou A, Mouanga Ndzime Y, Lendamba RW, et al. Vulvovaginal candidiasis among symptomatic women of childbearing age attended at a medical analysis laboratory in Franceville, Gabon. J Mycol Med. 2019;29(4):317–9.
Kengne M, Shu SV, Nwobegahay JM, Achonduh O. Antifungals susceptibility pattern of Candida spp. isolated from female genital tract at the Yaounde Bethesda Hospital in Cameroon. Pan Afr Med J. 2017;28:294.
Ogouyemi-Hounto A, Adisso S, Djamal J, Sanni R, Amangbegnon R, Biokou-Bankole B, et al. Place of vulvovaginal candidiasis in the lower genital tract infections and associated risk factors among women in Benin. J Mycol Med. 2014;24(2):100–5.
Apalata T, Carr WH, Sturm WA, Longo-Mbenza B, Moodley P. Determinants of symptomatic vulvovaginal candidiasis among human immunodeficiency virus type 1 infected women in rural KwaZulu-Natal, South Africa. Infect Dis Obstet Gynecol. 2014;2014: 387070.
Karou SD, Djigma F, Sagna T, Nadembega C, Zeba M, Kabre A, et al. Antimicrobial resistance of abnormal vaginal discharges microorganisms in Ouagadougou, Burkina Faso. Asian Pac J Trop Biomed. 2012;2(4):294–7.
Emmanuel NN, Romeo O, Mebi AG, Mark OO, Scordino F, Bessy EI, et al. Genotyping and fluconazole susceptibility of Candida albicans strains from patients with vulvovaginal candidiasis in Jos, Nigeria. Asian Pac J Trop Dis. 2012;2(1):48–50.
Mushi MF, Mmole A, Mshana SE. Candida vaginitis among symptomatic pregnant women attending antenatal clinics in Mwanza, Tanzania. BMC Res Notes. 2019;12(1):775.
Bitew A, Abebaw Y. Vulvovaginal candidiasis: species distribution of Candida and their antifungal susceptibility pattern. BMC Womens Health. 2018;18(1):94.
Aniebue UU, Nwankwo TO, Nwafor MI. Vulvovaginal candidiasis in reproductive age women in Enugu Nigeria, clinical versus laboratory-assisted diagnosis. Niger J Clin Pract. 2018;21(8):1017–22.
Mbakwem-Aniebo C, Osadebe AU, Athanasonny E, Okonko IO. Prevalence of Candida spp. and age-related disparities amongst women presenting with vaginitis at the Obstetrics and Gynaecology (O&G) Clinic in a Tertiary hospital in Port Harcourt, Nigeria. Afr Health Sci. 2020;20(1):51–8.
Apalata T, Longo-Mbenza B, Sturm A, Carr W, Moodley P. Factors associated with symptomatic vulvovaginal candidiasis: a study among women attending a primary healthcare clinic in Kwazulu-Natal, South Africa. Ann Med Health Sci Res. 2014;4(3):410–6.
Imadea GE, Musa J, Sagaya AS, Kapigac SH, Sankaled J-L, Idokob J, Kankid P. Association of bacterial vaginosis and other sexually transmitted infections with HIV among pregnant women in Nigeria. Afr J Med Med Sci. 2014;43:23–8.
Kruppa MD, Jacobs J, King-Hook K, Galloway K, Berry A, Kintner J, et al. Binding of elementary bodies by the opportunistic fungal pathogen Candida albicans or soluble beta-glucan, laminarin, inhibits Chlamydia trachomatis infectivity. Front Microbiol. 2018;9:3270.
Melo A, Ossa X, Fetis G, Lazo L, Bustos L, Fonseca-Salamanca F. Concordance between clinical and laboratory diagnosis of abnormal vaginal discharge in Chilean women. Rev Bras Ginecol Obstet. 2021;43(8):600–7.
Rylander E, Berglund AL, Krassny C, Petrini B. Vulvovaginal Candida in a young sexually active population: prevalence and association with oro-genital sex and frequent pain at intercourse. Sex Transm Infect. 2004;80(1):54–7.
Sangare I, Sirima C, Bamba S, Zida A, Cisse M, Bazie WW, et al. Prevalence of vulvovaginal candidiasis in pregnancy at three health centers in Burkina Faso. J Mycol Med. 2018;28(1):186–92.
Mukasa KJ, Herbert I, Daniel A, Sserunkuma KL, Joel B, Frederick B. Antifungal susceptibility patterns of vulvovaginal Candida species among women attending antenatal clinic at Mbarara Regional Referral Hospital, South Western Uganda. Br Microbiol Res J. 2015;5(4):322–31.
Arendrup MC, Patterson TF. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216(suppl_3):S445–51.
Beardsley J, Halliday CL, Chen SC, Sorrell TC. Responding to the emergence of antifungal drug resistance: perspectives from the bench and the bedside. Fut Microbiol. 2018;13(10):1175–91.
Berkow EL, Lockhart SR. Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist. 2017;10:237–45.
Xiao M, Chen SC, Kong F, Xu XL, Yan L, Kong HS, et al. Distribution and antifungal susceptibility of Candida species causing candidemia in China: an update from the CHIF-NET study. J Infect Dis. 2020;221(Suppl 2):S139–47.
Arastehfar A, Kargar ML, Mohammadi SR, Roudbary M, Ghods N, Haghighi L, et al. A high rate of recurrent vulvovaginal candidiasis and therapeutic failure of azole derivatives among Iranian women. Front Microbiol. 2021;12:655069.
Khan M, Ahmed J, Gul A, Ikram A, Lalani FK. Antifungal susceptibility testing of vulvovaginal Candida species among women attending antenatal clinic in tertiary care hospitals of Peshawar. Infect Drug Resist. 2018;11:447–56.
Brandolt TM, Klafke GB, Goncalves CV, Bitencourt LR, Martinez AM, Mendes JF, et al. Prevalence of Candida spp. in cervical-vaginal samples and the in vitro susceptibility of isolates. Braz J Microbiol. 2017;48(1):145–50.
Waikhom SD, Afeke I, Kwawu GS, Mbroh HK, Osei GY, Louis B, et al. Prevalence of vulvovaginal candidiasis among pregnant women in the Ho municipality, Ghana: species identification and antifungal susceptibility of Candida isolates. BMC Pregnancy Childbirth. 2020;20(1):266.
Tellapragada C, Eshwara VK, Bhat P, Kamath A, Aletty S, Mukhopadhyay C. Screening of vulvovaginal infections during pregnancy in resource constrained settings: implications on preterm delivery. J Infect Public Health. 2017;10(4):431–7.
Arfiputri DS, Hidayati AN, Handayani S, Ervianti E. Risk factors of vulvovaginal candidiasis in dermato-venereology outpatients clinic of Soetomo Heneral Hospital, Surabaya, Indonesia. Afr J Infect Dis. 2018;12(1 Suppl):90–4.
Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36(2):288–305.
Acknowledgements
The author hereby acknowledges the Department of Microbiology, Namibia Institute of Pathology for providing specimens and data required to perform study. In addition, the author also recognizes the Department of Health and Applied Sciences, Faculty of Health and Applied Sciences, Namibia University of Science and Technology, and the Department of Medical Microbiology, Faculty of Health Sciences, University of Pretoria for providing the facilities and support required to perform the experimental work.
Funding
CMD has received funding from the University of Pretoria Doctoral Commonwealth Scholarship (20200846). MMK, HJ and RPP did not receive funding for preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
CD was responsible for the design of the study, acquisition of data, conducting the experimental work, analysis of data, writing the article and final approval of manuscript. RP, MK and HJ were responsible for supervising the design and implementation of the study, substantively revising the article and approving the submitted version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was obtained from the Human Research Ethics Committee of the University of the Pretoria (Ref: 518/2020), the Namibia University of Science and Technology Faculty of Health and Applied Sciences Research Ethics Board (Ref: FHAS 11/2020) and regulatory approval from the Namibian Ministry of Health and Social Services (Ref: 17/3/3/CMD). As approved by the Ethics committees, anonymised swabs were used and no individual informed consent was obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dunaiski, C.M., Kock, M.M., Jung, H. et al. Importance of Candida infection and fluconazole resistance in women with vaginal discharge syndrome in Namibia. Antimicrob Resist Infect Control 11, 104 (2022). https://doi.org/10.1186/s13756-022-01143-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-022-01143-6