Abstract
Background
Antibiotic-resistant Enterobacteriaceae in the gastrointestinal flora can lead to infections with limited therapeutic options. Also, the resistant bacteria can be transferred from colonized persons to others. The present study was conducted to search the fecal carriage rates of (i) Enterobacteriaceae that produce extended-spectrum β-lactamase (ESBL-E) and/or (ii) plasmid-mediated AmpC β-lactamase (pAmpC-E), (iii) ciprofloxacin-resistant Enterobacteriaceae (CIP-RE), and (iv) carbapenem-intermediate or -resistant Enterobacteriaceae (CIRE) in Northern Cyprus.
Methods
A total of 500 community-dwellers were recruited from consecutive admissions to the clinical laboratories of four hospitals. One rectal swab or stool sample was collected from each participant. A questionnaire was applied to evaluate possible risk factors associated with intestinal colonization of resistant bacteria. The samples were cultured on antibiotic containing media to screen for resistant bacteria colonization. The bacterial colonies that grew on the plates were subjected to further phenotypic tests to confirm the resistance.
Results
Of 500 volunteers, ESBL-E, pAmpC-E, CIP-RE and CIRE carriage were detected in 107 (21.4%), 15 (3.0%), 51 (10.2%) and six (1.2%) participants, respectively. Escherichia coli was the most commonly recovered species among Enterobacteriaceae isolates. A significant proportion of ESBL-producing E. coli isolates (n = 22/107; 20.6%) was found to be co-resistant to CIP (p = 0.000, OR 3.21, 95% CI 1.76–5.87). In this study, higher socioeconomic status (CIP-RE: p = 0.024, OR 1.96, 95% CI 1.09–3.53), presence of gastrointestinal symptoms (CIRE: p = 0.033; OR 6.79, 95% CI 1.34–34.39), antibiotic use (ESBL-E: p = 0.031; OR 1.67, 95% CI 1.04–2.67; and CIRE: p = 0.033; OR 6.40, 95% CI 1.16–35.39), and travelling abroad (pAmpC-E: p = 0.010; OR 4.12, 95% CI 1.45–11.66) were indentified as risk factors.
Conclusion
The study indicates that resistant Enterobacteriaceae isolates are carried by humans in the community. To prevent further spread of resistance, rational use of antibiotics should be encouraged, and antibiotic resistance should be carefully monitored in Northern Cyprus.
Similar content being viewed by others
Background
Enterobacteriaceae are gram-negative bacteria that are members of normal intestinal flora. Also, these bacteria are the most common cause of infections in hospital and community settings [1]. More importantly, increasing rates of antibiotic resistance in Enterobacteriaceae are reported globally [2].
Extended-spectrum β-lactamases (ESBLs) are the primary reason of acquired drug resistance seen in gram-negative bacteria [3]. ESBLs have been a global concern, and the resistance rates have increased [4]. ESBL enzymes can hydrolyze many β-lactam antibiotics except for cephamycins and carbapenems [5]. Apart from ESBLs, production of AmpC β-lactamases is the other mechanism that confers resistance to broad-spectrum cephalosporins [6]. Importantly, these enzymes also have the ability to hydrolyze cephamycins [7].
Carbapenems are the antibiotics of last resort that are used for the treatment of multidrug-resistant Enterobacteriaceae (MDR-E) infections [8]. However, carbapenem-intermediate or -resistant Enterobacteriaceae (CIRE) have spread across the globe [9]. Carbapenem resistance in Enterobacteriaceae occur mainly due to the production of carbapenemase enzymes in bacteria [10]. Carbapenemase production, except for OXA-48, is associated with increased resistance against most of the β-lactams; while activity of the enzymes on carbapenems is variable [11].
Fluoroquinolones (FQs) are commonly used for the therapy of various infections [12]. These antibiotics are also one of the treatment options for ESBL-positive infections [13]. However, FQ resistance is reported at high rates among ESBL-producing Enterobacteriaceae (ESBL-E) species [14].
The intestinal tract is the primary source of Enterobacteriaceae, and moreover, transfer of antibiotic resistance genes occur here, and resistant bacteria proliferate as a result of antibiotic therapy [15]. Therefore, resistant enteric bacteria in the gut flora can lead to infections with limited therapeutic options. Furthermore, resistance genes of the intestinal microbiota can spread from one person to another, and to the environment [16].
Fecal carriage of ESBL-E, plasmid-mediated AmpC β-lactamase-producing Enterobacteriaceae (pAmpC-E), FQ-resistant Escherichia coli, or carbapenem-resistant Enterobacteriaceae (CRE) has been demonstrated by a number of research studies [4, 6, 17,18,19,20]. Moreover, several factors including previous exposure to antibiotics have been associated with an increased risk of fecal carriage of resistant Enterobacteriaceae [4, 20,21,22].
In the literature, there is no published data on the intestinal colonization of resistant enteric bacteria in Northern Cyprus. Nevertheless, several studies indicated high rates of antibiotic resistance in Enterobacteriaceae. Indeed, the percentages of ESBL-producing E. coli were detected to be 53 and 44% in the urine samples of hospitalized and outpatients, respectively. Importantly, FQ resistance among ESBL-producing E. coli isolates was found to be 78 and 79% in the samples of inpatients and outpatients, respectively. ESBL-positive E. coli isolates remained susceptible to imipenem (IMP) and meropenem (MEM), however ertapenem (ERT) resistance was noted to be 6% in hospitalized patients, and 11% in outpatients [23]. Another hospital-based study in Northern Cyprus documented the rate of ESBL to be 16.7% in Klebsiella pneumoniae isolates. Moreover, FQ resistance was reported between 16.8 and 20.1%, whereas IMP, MEM and ERT resistance rates were found to be 0.0, 1.0, and 4.6%, respectively, among K. pneumoniae isolates [24].
In the light of above facts, the present study aimed to determine the fecal carriage rates of ESBL-E, pAmpC-E, ciprofloxacin-resistant Enterobacteriaceae (CIP-RE), and CIRE among community-dwelling healthy individuals in Northern Cyprus. Additionally, possible risk factors associated with intestinal colonization of ESBL-E, pAmpC-E, CIP-RE and CIRE were evaluated. To our knowledge, this is the first study that presents the fecal carriage rates of resistant Enterobacteriaceae and associated risk factors in Northern Cyprus.
Methods
Study population
Between September and December 2017, volunteers for this study were recruited from consecutive admissions to the routine clinical laboratories of Near East University Hospital (Nicosia), Nicosia Dr. Burhan Nalbantoglu State Hospital, Kyrenia Dr. Akcicek Hospital and Famagusta State Hospital. Subjects who were referred to the laboratories for stool examination as a part of routine health check-up were informed about the study. The inclusion criteria were being older than 18 years of age, living in Northern Cyprus for at least one year, and not being hospitalized. Eventually, a total of 500 community-dwelling individuals who met these criteria were enrolled in this study.
Ethical approval
The ethical approval for the study was obtained from the Near East University Research Assessment Committee (Project no: YDU/2016/37–296). Written informed consent was collected from each participant.
Sample and data collection
From each participant, one rectal swab or stool sample was collected, therefore a total of 500 specimens were included in the study. During the sample collection, a questionnaire was applied to each participant in order to assess the possible risk factors for fecal carriage of resistant Enterobacteriaceae. In the questionnaire, demographic and socioeconomic data (age, gender, level of education, and economic status) were recorded. Next, the participants were asked whether they had at least one gastrointestinal symptom (GIS) (diarrhea, constipation, abdominal cramps, nausea or bloating) during the sample collection. Participants also provided information on history of antibiotic use and travelling abroad within six months from sample collection. In the data, there were missing information regarding the antibiotic names, therefore, these were excluded from the analysis to rule out inaccurate evaluation.
Phenotypic screening for ESBL-E, pAmpC-E, CIP-RE, and CIRE isolates
Fecal swabs or stool samples (200 mg) were initially suspended in 2 ml of sterile 0.9% saline. Next, an aliquot of each sample were plated onto four sets of MacConkey media (Merck, Germany): (i) control plate without antibiotics, (ii) biplate agar containing cefotaxime (CTX) (Sigma, USA) and ceftazidime (CAZ) (Sigma, USA) at 1 mg/L concentration to screen for ESBL- and/or pAmpC-producing isolates [17], (iii) medium containing ciprofloxacin (CIP) (Sigma, USA) at 1 mg/L to screen for FQ-resistant isolates [18], and (iv) medium supplemented with 1 mg/L ertapenem (ERT) (Sigma, USA) to screen for carbapenem-resistant strains [25, 26]. All of the plates were incubated overnight at 37 °C.
Phenotypic confirmatory tests and identification of ESBL-E, pAmpC-E, CIP-RE, and CIRE isolates
Bacterial isolates that grew on CTX and/or CAZ containing media were further tested for the presence of ESBL- and/or pAmpC-production. Confirmation of ESBL-producing isolates was done by using the combined disc test, according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [27]. CTX (30 μg) and CAZ (30 μg) discs with or without clavulonic acid (CLA) (10 μg) were used. An increase of ≥5 mm in the zone diameter of CTX/CLA or CAZ/CLA compared to the antibiotics alone was considered positive for ESBL production [27]. For the initial screening of pAmpC activity, all the isolates that grew in CTX and/or CAZ containing media were tested using cefoxitin (FOX) (30 μg) discs [6]. The isolates with reduced susceptibility (zone diameter of < 18 mm) to FOX [27] were further analyzed by combined disk (CD) method. CTX (30 μg) and CAZ (30 μg) discs were used with or without cloxacillin (750 μg), an inhibitor of pAmpC. An increase of ≥5 mm of the zone diameter in the presence of the inhibitor was interpreted as a positive pAmpC test [17].
In order to confirm FQ resistance, bacterial strains recovered from CIP containing plates were subjected to disc diffusion test using CIP (5 μg), ofloxacin (OFX) (5 μg), norfloxacin (NOR) (10 μg), levofloxacin (LVX) (5 μg) and gemifloxacin (GEM) (5 μg) discs. Inhibition zones of ≤15 mm for CIP and GEM; ≤12 mm for OFX and NOR; and ≤ 13 mm for LVX discs were interpreted as resistant [27].
The isolates that grew in ERT plates were evaluated by disc diffusion test using ERT (10 μg), MEM (10 μg) and IMP (10 μg) discs. Inhibition zones of ≤18 mm for ERT disc; and ≤ 19 mm for IMP and MEM discs indicated resistance [27]. The isolates with confirmed resistance to carbapenems were further tested for carbapenemase activity. Presence of metallo-β-lactamase (MBL) was assessed by combined disc method using ERT (10 μg) disc alone and with 0.1 M EDTA (10 μl). An increase of ≥5 mm in the zone diameter of ERT/EDTA compared to ERT alone was recorded as MBL-positive [28]. Klebsiella pneumoniae carbapenemase (KPC) detection was done by double disc synergy test (DDST) using ERT (10 μg) and boronic acid (300 μg) discs. An increase in the zone diameter of ERT towards boronic acid disc was interpreted as a positive result for KPC [29]. All antibiotic discs used in this study were purchased from Bioanalyse (Turkey).
Identification of ESBL-E, pAmpC-Ε, CIP-RE, and CIRE isolates was done by using the BD Phoenix 100 system (software version 6.01A). The flow chart of the study protocol is given in the Fig. 1.
Flow chart of the study protocol. Abbreviations: ESBL, extended-spectrum β-lactamase; pAmpC, plasmid-mediated AmpC β-lactamase; FQ, fluoroquinolone; MC agar, MacConkey agar; CTX, cefotaxime; CAZ, ceftazidime; CIP, ciprofloxacin; ERT, ertapenem; CD test, combined disc test; DD test, disk diffusion test; FOX, cefoxitin; OFX, ofloxacin; NOR, norfloxacin; LVX, levofloxacin, GEM, gemifloxacin, MEM, meropenem; IMP, imipenem; MBL, metallo-β-lactamase; DDST test, double disc synergy test; KPC, Klebsiella pneumoniae carbapenemase
Statistical analysis
Descriptive statistics of the variables in the questionnaire were calculated. For categorical variables, frequency and percentage information were given while for the continuous variables arithmetic mean, standard deviation, median, minimum and maximum were calculated. Depending on the sample sizes, either Pearson Chi-square or Fisher’s exact test was applied for evaluating the associations between categorical variables. For risk assessments of independent variables, odds ratios (OR) with 95% confidence intervals (95% CI) were calculated. All statistical calculations were performed with IBM SPSS statistics package for Macintosh (Demo version 22.0; Armonk, NY: IBM Corp.). Level of significance was accepted as 0.05.
Results
General characteristics of the study population
A total of 500 volunteers were recruited from Near East University Hospital (n = 49; 9.8%), Nicosia Dr. Burhan Nalbantoglu State Hospital (n = 39; 7.8%), Kyrenia Dr. Akcicek Hospital (n = 383; 76.6%) and Famagusta State Hospital (n = 29; 5.8%). Fecal swab (n = 448; 89.6%) or stool (n = 52; 10.4%) samples were collected from the participants and included for microbiological analysis. Out of 500 individuals, 320 (64.0%) were male, and 180 (36.0%) were female. The mean and median age of the study population were 32.92 ± 9.97 and 31.00 (19.00–78.00), respectively. Distribution of the participants according to the age groups was 238 (48.2%) for age 19–30, and 256 (51.8%) for age 31 and above. One hundred and eighteen (23.6%) of the individuals had a university or higher degree diploma. Number of the participants with middle or high income was 194 (39.4%).
Out of 500 study participants, 67 (13.4%) presented with one or more GIS at the time of sample collection. One hundred and twenty-four (24.8%) individuals declared that they used antibiotic within six months from the study. Ninety-two (18.4%) persons travelled out of Northern Cyprus within the last six months. Of 85 individuals who provided information on the country visited, 11 (12.9%) travelled to countries in Asia (n = 8) or Africa (n = 3), while 74 (87.1%) visited other regions (Turkey, n = 65; Europe, n = 8; Australia, n = 1) in the six months before the study.
Gastrointestinal colonization with ESBL-E, pAmpC-E, CIP-RE, and CIRE
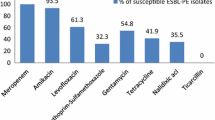
In the present study, 107 (21.4%) of 500 individuals were found to be colonized with ESBL-E. E. coli accounted for the most ESBL cases (n = 101/107; 94.4%), while K. pneumoniae (n = 3/107; 2.8%), Enterobacter cloacae (n = 1/107; 0.9%), Enterobacter aerogenes (n = 1/107; 0.9%), and Providencia rettgeri (n = 1/107; 0.9%) were detected at low rates. Fifteen (3.0%) of 500 participants were colonized with pAmpC-E, and the isolates were identified as E. coli (n = 13/15; 86.7%) and K. pneumoniae (n = 2/15; 13.3%). Fecal carriage rate of CIP-RE was detected to be 10.2% (n = 51/500), and all of the 51 (100%) isolates were defined as E. coli. Among CIP-resistant E. coli strains, number of the isolates which were resistant to OFX, NOR, LVX and GEM was 35 (68.6%), 36 (70.6%), 31 (60.8%) and 22 (43.1%), respectively. In the study, six (1.2%) out of 500 participants were colonized with CIRE. These isolates were identified as E. coli (n = 2/6; 33.3%), K. pneumoniae (n = 2/6; 33.3%), K. oxytoca (n = 1/6; 16.7%) and E. aerogenes (n = 1/6; 16.7%). One of the E. coli isolates was ERT-resistant, MEM-susceptible and IMP-intermediate. This isolate was found to harbour KPC type carbapenemase by DDST. K. oxytoca isolate showed resistance against ERT, MEM and IMP. In this isolate, MBL phenotype was detected by CD test. Other four isolates were intermediately resistant to ERT, and susceptible to both MEM and IMP. Distribution of bacterial species among ESBL-E, pAmpC-E, CIP-RE and CIRE isolates is shown in Table 1.
Of 500 samples tested, 22 (4.4%) ESBL-producing E. coli isolates were found to be co-resistant to CIP. Among these, two (0.4%) E. coli isolates were additionally pAmpC producers. Association of CIP resistance and ESBL-production (n = 22/107; 20.6%) in E. coli strains was found to be statistically significant (p = 0.000, OR 3.21, 95% CI 1.76–5.87). In the study, another pAmpC-positive E. coli isolate (n = 1; 0.2%) showed resistance to CIP. Also, one (0.2%) pAmpC-positive E. coli isolate was intermediately resistant to CIP. One (0.2%) E. coli and one (0.2%) K. pneumoniae isolate were ESBL-positive and ERT-intermediate (MER- and IMP-susceptible). One (0.2%) K. pneumoniae isolate was both ESBL- and pAmpC-positive, CIP-intermediate, and also ERT-intermediate (MER- and IMP-susceptible). Furthermore, a sample grew two isolates: one (0.2%) CIP-resistant E. coli, and one (0.2%) carbapenem-resistant K. oxytoca which was resistant to ERT, MEM and IMP.
Association of fecal carriage of ESBL-E, pAmpC-E, CIP-RE and CIRE with risk factors
Statistical analysis revealed that age, gender and education did not significantly affect colonization with ESBL-E, pAmpC-E, CIP-RE or CIRE (p > 0.05). A statistical correlation was found between the socioeconomic status and fecal carriage of CIP-RE. Prevalence of CIP-RE carriage was higher in the participants with middle or high income (n = 27/192; 14.1%) compared to those with low income (n = 23/298; 7.7%) (p = 0.024, OR 1.96, 95% CI 1.09–3.53) (Table 2).
In the study, presence of any GIS at the time of sample collection was statistically correlated with fecal carriage of CIRE. Prevalence of CIRE carriers having current GIS (n = 3/65; 4.6%) was higher than those without symptoms (n = 3/424; 0.7%) (p = 0.033; OR 6.79, 95% CI 1.34–34.39). Statistical analysis showed that, colonization with ESBL-E or CIRE was significantly affected by antibiotic use in the last six months. Rate of ESBL-E colonization in the individuals that used antibiotic (n = 35/123; 28.5%) was higher than those that were not exposed to antibiotics (n = 72/374; 19.3%) (p = 0.031; OR 1.67, 95% CI 1.04–2.67). Likewise, prevalence of CIRE carriage in the participants that used antibiotic (n = 4/119; 3.4%) was higher than nonusers (n = 2/370; 0.5%) (p = 0.033; OR 6.40, 95% CI 1.16–35.39). Furthermore, a statistical association was found between travelling abroad in the last six months and colonization with pAmpC-E. In travellers, gastrointestinal pAmpC-E colonization was more common (n = 7/92; 7.6%) than in nontravellers (n = 8/408; 2.0%) (p = 0.010; OR 4.12, 95% CI 1.45–11.66) (Table 2). No statistical significance was detected between travel destination and gut colonization of resistant bacteria (p > 0.05).
Discussion
Colonization of the gastrointestinal tract with resistant Enterobacteriaceae poses a threat not only to the affected individuals, but also to the community, since antibiotic resistance genes of the gut flora can be transferred from person to person, and to the environment [16]. Therefore, it is important to determine the prevalence of intestinal colonization with resistant Enterobacteriaceae in the community. In the present study, 500 healthy individuals were recruited from consecutive admissions to the routine clinical laboratories of four different hospitals. Fecal carriage rates of ESBL-E, AmpC-E, CIP-RE, and CIRE were determined and the associated risk factors were analyzed.
In this study, the prevalence of ESBL-E was found to be 21.4% (Table 1). In two community-based studies in the Netherlands, this rate was reported to be 9.5% [17] and 4.7% [6]. On the other hand, a recent study from Turkey reported higher rate of ESBL-E carriage (34.3%) in the community [30]. These findings are not surprising, as the prevalence of ESBL-E carriage in Europe is lower than the other regions [15]. In the present study, rate of pAmpC-E colonization was detected to be lower (3.0%) than ESBL-E. This finding is also consistent with other reports where pAmpC carriage was observed less commonly than ESBL-E [6, 17, 30].
Prevalence of CIP-RE intestinal colonization was detected to be 10.2% in our study (Table 1). This finding is slightly higher compared to a previous study in France, where the rate of CIP-resistant E. coli fecal carriage was reported to be 8.0% among hospitalized patients on admission [31]. On the contrary, Steensels et al. documented higher rate (22.0%) of CIP-resistant E. coli carriage in the patients undergoing prostate biopsy. Use of FQs within the six months before the biopsy was found to be a risk factor, therefore this can explain the increased prevalence of colonization with FQ-resistant strains reported by Steensels et al. [18]. In the present study, due to the missing information, antibiotic names provided by the participants were excluded from the analysis, and thus, no evaluation was done on the association between previous FQ exposure and fecal carriage of CIP-resistant strains.
Rate of CIRE fecal carriage was found to be 1.2% (Table 1). Also, prevalence of CRE colonization was 0.4% (2/500) in this study. These rates are lower than those documented elsewhere. Yamamoto et al. reported that percentage of CRE carriage among hospitalized patients in Japan was 12.2% [20]. Rossini et al. documented the colonization rate of carbapenemase-producing Enterobacteriaceae (CPE) to be 10.2% among inpatients in Italy [32]. Unlike those studies, participants in the present report were community-dwellers and not hospitalized. Indeed, longer hospital stay was shown to be a risk factor for fecal carriage of CRE [20]. Furthermore, carbapenem resistance in K. pneumoniae isolates was documented at low levels previously in Northern Cyprus [24]. Taken together, all these evidence can explain the low CRE and CIRE colonization rates in the present study.
E. coli was the most commonly isolated species in the study. Prevalence of E. coli among ESBL-E, pAmpC-E, CIP-RE and CIRE species was 94.4, 86.7, 100.0, and 33.3%, respectively. This was followed by K. pneumoniae and other Enterobacteriaceae species (Table 1). Similarly in other studies, resistant E. coli strains were found to be responsible for the highest intestinal colonization rates, which was followed by Klebsiella and other enteric bacteria species [4, 20, 30, 33].
In the present study, co-existence of resistance was observed in Enterobacteriaceae isolates. Notably, 20.6% (n = 22/107) of ESBL-producing E. coli strains were found to be co-resistant to CIP, and a significant association was observed between CIP resistance and ESBL-production (p = 0.000). Previously, de Lastours et al. demonstrated that fecal carriage of ESBL-E was significantly related to colonization with CIP-resistant E. coli among hospitalized patients at admission [31]. Vervoort et al. reported that 63% of ESBL-E species that colonized the patients with antibiotic-associated diarrhea showed high-level FQ-resistance [14]. Co-existence of ESBL production and CIP resistance could be attributed to the transfer of resistance genes through plasmids. Studies have shown that plasmid-mediated quinolone resistance (PMQR) genes, qnr and aac(6′)-lb-cr, were detected in ESBL-producing isolates with reduced susceptibility to FQs. Moreover, the qnr genes were documented to be common in ESBL-E. Therefore, these findings suggest the possibility of co-transmission of ESBL and FQ resistance via plasmids [34].
In this study, two (0.4%) CIP-resistant ESBL-harbouring E. coli isolates were co-producers of pAmpC. This finding supports previous studies that documented fecal carriage of both ESBL- and AmpC-producing isolates [17, 35]. No carbapenem resistance was observed in any of ESBL-producing isolates. However, one ESBL-positive E. coli and one ESBL-positive K. pneumoniae isolate were intermediately resistant to ERT. Also one ESBL-harbouring K. pneumoniae isolate was co-producer of pAmpC and intermediately resistant to CIP and ERT. Previously, co-existence of ESBL and carbapenemase with or without AmpC production was documented by different studies [36, 37]. In our study, one E. coli and one K. oxytoca isolate with KPC and MBL-type carbapenemase, respectively, were detected, and this result is consistent with the findings reported elsewhere [38]. Reduced carbapenem susceptibility among CIRE (n = 6/500; 1.2%) isolates was mostly detected against ERT (resistant = 2/6, intermediate = 4/6, susceptible = 0/6), while number of the isolates that were susceptible to IMP (resistant = 1/6, intermediate = 1/6, susceptible = 4/6) and MEM (resistant = 1/6, intermediate = 0/6, susceptible = 5/6) was higher. This result is compatible with a previous report from Northern Cyprus where the resistance rate of ERT (4.6%) was higher than those of IMP (0.0%) and MEM (1.0%) in K. pneumoniae isolates [24].
In order to assess the possible risk factors associated with the fecal carriage of resistant Enterobacteriaceae, a questionnaire was applied to each participant. Statistical analysis showed that, age and gender were not significant determinants of ESBL-E, pAmpC, CIP-RE and CIRE carriage (p > 0.05) (Table 2). While this finding is consistent with several reports [4, 17, 20, 39, 40], it also contradicts with a few studies. Duplessis et al. found a statistical correlation between age and fecal carriage of FQ-resistant isolates. The authors stated that age can be suggestive of previous antibiotic use and hospitalization [41]. de Lastours et al. found a significant association between male sex and carriage of FQ-resistant E. coli in the patients treated with FQ [42]. Rossini et al. also documented a significant relation between age, male sex and CPE colonization. The authors attributed this association to the previous admission to intensive care unit (ICU) [32]. Therefore, former antibiotic use or hospital stay appear to be the major contributors to the fecal carriage of resistant bacteria.
In the study, no significant correlation was found between the level of education and fecal carriage of resistant bacteria (p > 0.05) (Table 2), and this result is consistent with previous reports [40]. On the contrary, Luvsansharav et al. documented a statistical association between better education status and CTX-M-type ESBL-E carriage among the individuals in the rural areas of Thailand [21]. Furthermore, we found a significantly higher rate of CIP-RE carriage among the participants with better socioeconomic status (p = 0.024) (Table 2). This can be explained by the fact that the indiviuals with better socioeconomic status can have easier access to health services and also over-the-counter antibiotics [21]. As a result, self-medication is associated with a higher risk of antibiotic overuse and misuse, and therefore, it increases the risk of resistance [43].
Various reports have shown that antibiotic exposure is associated with an increased risk of fecal carriage of resistant bacteria [4, 20,21,22]. Consistently, our study showed that antibiotic use in the last six months was a significant determinant of ESBL-E (p = 0.031) and CIRE (p = 0.033) fecal carriage (Table 2). However, pAmpC-E and CIP-RE carriage were not affected by the previous antibiotic use (p > 0.05), which supports previous reports [17, 39].
International travel has been shown to be a risk factor for acquiring ESBL-E and CIP-RE in rectal flora [2]. In the present study, only pAmpC-E colonization was affected by travelling abroad in the last six months (p = 0.010) (Table 2), which contradicts a previous report [17]. Moreover, a significant association has been demonstrated between travel to Asia, Africa and Northern America and ESBL-E carriage [4]. However, we found no correlation between travelling to Asia or Africa and fecal carriage of resistant bacteria (p > 0.05).
Sixty-seven (13.4%) of the study participants had at least one GIS at the time of sample collection, and the association between having a gastrointestinal complaint and CIRE colonization was found to be statistically significant (p = 0.033) (Table 2). Fecal carriage of resistant bacteria has also been documented in symptomatic patients by other reports. Prevalence of ESBL-E carriage was found to be 10.1% in community-dwelling patients with gastrointestinal complaints in the Netherlands, while no carbapenemase was detected [3]. In Egypt Abdallah et al. reported that 68 and five of 100 patients having community-onset gastrointestinal complaints were colonized with ESBL-E and CPE, respectively [19]. The rates of ESBL-E (25.4%) and CIRE colonization (4.6%) among the symptomatic patients in the present study are higher than in the Netherlands but lower than in Egypt. This result is not unexpected, as the antibiotic use and resistance rates in the Netherlands are low [4] in contrast to the imprudent use of antimicrobials in Egypt [19]. This suggests that, more strict regulations should be applied on antibiotic use in Northern Cyprus.
Our study has two limitations. Firstly, names of antibiotics were excluded from the analysis because of missing information, and thus the association between exposure to specific antibiotics and fecal carriage of resistant Enterobacteriaceae could not be evaluated. Secondly, the molecular analysis of antibiotic resistance genes could not be performed due to insufficient financial resources. In order to detect resistance by phenotypic tests, the standard two-step algorithm was performed consisting of (i) initial screening with antibiotic containing media, and (ii) further evaluation using a confirmatory test [44]. This strategy generally gives accurate results. However, phenotypic detection of ESBL can sometimes be difficult, because presence of ESBL may be masked by co-existing pAmpC β-lactamases and carbapenemases [45]. In the present study, pAmpC (n = 15/500; 3.0%), KPC (n = 1/500; 0.2%) and MBL (n = 1/500; 0.2%) were detected at low levels. Co-production of ESBL was detected in two pAmpC-positive isolates, while KPC- and MBL-positive isolates were found to be ESBL-negative. In these isolates, there is a possibility that presence of ESBL might have been masked by co-existing β-lactamases. Nevertheless, considering the low rates of pAmpC and carbapenemases, number of ESBL-producing isolates that were possibly misdiagnosed as negative is also very low in our study. In order to overcome these problems, phenotypic test results should also be confirmed by genotypic methods that allow molecular identification of resistant strains. Therefore, future studies are essential to elucidate the molecular basis of resistance in bacteria that colonize the gastrointestinal tract of humans in Northern Cyprus.
Conclusion
The fecal carriage rates of ESBL-E, pAmpC-E, CIP-RE and CIRE were found to be 21.4, 3.0, 10.2 and 1.2%, respectively in this study. Notably, CIP resistance was detected in 20.6% of ESBL-producing E. coli isolates. Risk factors associated with the fecal carriage of resistant Enterobacteriaceae were identified as higher socioeconomic status (CIP-RE), presence of any gastrointestinal complaints at the time of sample collection (CIRE), antibiotic use in the last six months (ESBL-E and CIRE), and travelling abroad within six months prior to the study (pAmpC-E).
To our knowledge, this is the first study that demonstrates the fecal carriage of resistant Enterobacteriaceae in Northern Cyprus. The results presented here indicate that the rates of ESBL-E and CIP-RE carriage in the community are particularly alarming. The high levels of resistance suggest that control programmes should be maintained to encourage the rational use of antibiotics, and resistance should be carefully monitored in Northern Cyprus.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- CAZ:
-
Ceftazidime
- CD:
-
Combined disk
- CI:
-
Confidence interval
- CIP:
-
Ciprofloxacin
- CIP-RE:
-
Ciprofloxacin-resistant Enterobacteriaceae
- CIRE:
-
Carbapenem-intermediate or -resistant Enterobacteriaceae
- CLA:
-
Clavulonic acid
- CLSI:
-
Clinical and Laboratory Standards Institute
- CRE:
-
Carbapenem-resistant Enterobacteriaceae
- CTX:
-
Cefotaxime
- DDST:
-
Double disc synergy test
- ERT:
-
Ertapenem
- ESBL:
-
Extended-spectrum β-lactamase
- ESBL-E:
-
ESBL-producing Enterobacteriaceae
- FOX:
-
Cefoxitin
- FQs:
-
Fluoroquinolones
- GEM:
-
Gemifloxacin
- GIS:
-
Gastrointestinal symptom
- ICU:
-
Intensive care unit
- IMP:
-
Imipenem
- KPC:
-
Klebsiella pneumoniae carbapenemase
- LVX:
-
Levofloxacin
- MBL:
-
Metallo-β-lactamase
- MDR-E:
-
Multidrug-resistant Enterobacteriaceae
- MEM:
-
Meropenem
- NOR:
-
Norfloxacin
- OFX:
-
Ofloxacin
- OR:
-
Odds ratio
- pAmpC-E:
-
Plasmid-mediated AmpC β-lactamase-producing Enterobacteriaceae
References
Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18:263–72.
Reuland EA, Sonder GJ, Stolte I, Al Naiemi N, Koek A, Linde GB, et al. Travel to Asia and traveller's diarrhoea with antibiotic treatment are independent risk factors for acquiring ciprofloxacin-resistant and extended spectrum β-lactamase-producing Enterobacteriaceae-a prospective cohort study. Clin Microbiol Infect. 2016;22:731.e1–7.
Reuland EA, Overdevest IT, Al Naiemi N, Kalpoe JS, Rijnsburger MC, Raadsen SA, et al. High prevalence of ESBL-producing Enterobacteriaceae carriage in Dutch community patients with gastrointestinal complaints. Clin Microbiol Infect. 2013;19:542–9.
Reuland EA, Al Naiemi N, Kaiser AM, Heck M, Kluytmans JA, Savelkoul PH, et al. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in Amsterdam. J Antimicrob Chemother. 2016;71:1076–82.
Nordmann P, Dortet L, Poirel L. Rapid detection of extended-spectrum-β-lactamase-producing Enterobacteriaceae. J Clin Microbiol. 2012;50:3016–22.
van Hoek AH, Schouls L, van Santen MG, Florijn A, de Greeff SC, van Duijkeren E. Molecular characteristics of extended-spectrum cephalosporin-resistant Enterobacteriaceae from humans in the community. PLoS One. 2015;10:e0129085.
Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22:161–82.
Eser OK, Altun Uludağ H, Ergin A, Boral B, Sener B, Hasçelik G. Carbapenem resistance in ESBL positive Enterobacteriaceae isolates causing invasive infections. Mikrobiyol Bul. 2014;48:59–69.
McLaughlin M, Advincula MR, Malczynski M, Qi C, Bolon M, Scheetz MH. Correlations of antibiotic use and carbapenem resistance in Enterobacteriaceae. Antimicrob Agents Chemother. 2013;57:5131–3.
Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–8.
Gijón D, Curiao T, Baquero F, Coque TM, Cantón R. Fecal carriage of carbapenemase-producing Enterobacteriaceae: a hidden reservoir in hospitalized and nonhospitalized patients. J Clin Microbiol. 2012;50:1558–63.
Claeys KC, Hopkins TL, Vega AD, Heil EL. Fluoroquinolone restriction as an effective antimicrobial stewardship intervention. Curr Infect Dis Rep. 2018;20:7.
Moxon CA, Paulus S. Beta-lactamases in Enterobacteriaceae infections in children. J Inf Secur. 2016;72:S41–9.
Vervoort J, Gazin M, Kazma M, Kotlovsky T, Lammens C, Carmeli Y, et al. High rates of intestinal colonisation with fluoroquinolone-resistant ESBL-harbouring Enterobacteriaceae in hospitalised patients with antibiotic-associated diarrhoea. Eur J Clin Microbiol Infect Dis. 2014;33:2215–21.
Woerther PL, Burdet C, Chachaty E, Andremont A. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev. 2013;26:744–58.
Knudsen PK, Brandtzaeg P, Høiby EA, Bohlin J, Samuelsen Ø, Steinbakk M, et al. Impact of extensive antibiotic treatment on faecal carriage of antibiotic-resistant enterobacteria in children in a low resistance prevalence setting. PLoS One. 2017;12:e0187618.
Reuland EA, Halaby T, Hays JP, de Jongh DM, Snetselaar HD, van Keulen M, et al. Plasmid-mediated AmpC: prevalence in community-acquired isolates in Amsterdam, the Netherlands, and risk factors for carriage. PLoS One. 2015;10:e0113033.
Steensels D, Slabbaert K, De Wever L, Vermeersch P, Van Poppel H, Verhaegen J. Fluoroquinolone-resistant E. coli in intestinal flora of patients undergoing transrectal ultrasound-guided prostate biopsy--should we reassess our practices for antibiotic prophylaxis? Clin Microbiol Infect. 2012;18:575–81.
Abdallah HM, Alnaiemi N, Reuland EA, Wintermans BB, Koek A, Abdelwahab AM, et al. Fecal carriage of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae in Egyptian patients with community-onset gastrointestinal complaints: a hospital -based cross-sectional study. Antimicrob Resist Infect Control. 2017;6:62.
Yamamoto N, Asada R, Kawahara R, Hagiya H, Akeda Y, Shanmugakani RK, et al. Prevalence of, and risk factors for, carriage of carbapenem-resistant Enterobacteriaceae among hospitalized patients in Japan. J Hosp Infect. 2017;97:212–7.
Luvsansharav UO, Hirai I, Nakata A, Imura K, Yamauchi K, Niki M, et al. Prevalence of and risk factors associated with faecal carriage of CTX-M β-lactamase-producing Enterobacteriaceae in rural Thai communities. J Antimicrob Chemother. 2012;67:1769–74.
Torres-Gonzalez P, Cervera-Hernandez ME, Niembro-Ortega MD, Leal-Vega F, Cruz-Hervert LP, García-García L, et al. Factors associated to prevalence and incidence of Carbapenem-resistant Enterobacteriaceae fecal carriage: a cohort study in a Mexican tertiary care hospital. PLoS One. 2015;10:e0139883.
Cantas L, Suer K, Guler E, Imir T. High emergence of ESBL-Producing E. coli cystitis: time to get smarter in Cyprus. Front Microbiol. 2016;6:1446.
Ruh E, Gazi U, Guvenir M, Suer K, Cakır N. Antibiotic resistance rates of Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae isolated from a university-affiliated hospital in North Cyprus. Turk Hij Den Biyol Derg. 2016;73:333–44.
Clinical and Laboratory Standards Institute. M100-S24, Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. Wayne, PA, USA. 2014.
Thomson KS. Extended-spectrum-beta-lactamase, AmpC, and Carbapenemase issues. J Clin Microbiol. 2010;48:1019–25.
Clinical and Laboratory Standards Institute. M100S. Performance standards for antimicrobial susceptibility testing. 26th ed. USA: Wayne, PA; 2016.
Kim HK, Park JS, Sung H, Kim MN. Further modification of the modified Hodge test for detecting Metallo-β-lactamase-producing Carbapenem-resistant Enterobacteriaceae. Ann Lab Med. 2015;35:298–305.
Pasteran F, Mendez T, Guerriero L, Rapoport M, Corso A. Sensitive screening tests for suspected class a carbapenemase production in species of Enterobacteriaceae. J Clin Microbiol. 2009;47:1631–9.
Hazirolan G, Mumcuoglu I, Altan G, Özmen BB, Aksu N, Karahan ZC. Fecal carriage of extended-spectrum beta-lactamase and ampc beta-lactamase-producing enterobacteriaceae in a turkish community. Niger J Clin Pract. 2018;21:81–6.
de Lastours V, Chau F, Tubach F, Pasquet B, Ruppé E, Fantin B. Independent behavior of commensal flora for carriage of fluoroquinolone-resistant bacteria in patients at admission. Antimicrob Agents Chemother. 2010;54:5193–200.
Rossini A, Di Santo SG, Libori MF, Tiracchia V, Balice MP, Salvia A. Risk factors for carbapenemase-producing Enterobacteriaceae colonization of asymptomatic carriers on admission to an Italian rehabilitation hospital. J Hosp Infect. 2016;92:78–81.
Eruz ED, Yalci A, Ozden E, Aslaner H, Ogucu-Durgun S, Koseoglu-Taymur DD, et al. Risk factors for infection development after transrectal prostate biopsy and the role of resistant bacteria in colonic flora. J Infect Dev Ctries. 2017;11:188–91.
Wiener ES, Heil EL, Hynicka LM, Johnson JK. Are fluoroquinolones appropriate for the treatment of extended-Spectrum β-lactamase-producing gram-negative bacilli? J Pharm Technol. 2016;32:16–21.
Dandachi I, Salem Sokhn E, Najem E, Azar E, Daoud Z. Carriage of beta-lactamase-producing Enterobacteriaceae among nursing home residents in North Lebanon. Int J Infect Dis. 2016;45:24–31.
Desta K, Woldeamanuel Y, Azazh A, Mohammod H, Desalegn D, Shimelis D, et al. High gastrointestinal colonization rate with extended-Spectrum β-lactamase-producing Enterobacteriaceae in hospitalized patients: emergence of Carbapenemase-Producing K. pneumoniae in Ethiopia. PLoS One. 2016;11:e0161685.
Parascandalo FA, Zarb P, Tartari E, Lacej D, Bitincka S, Manastirliu O, et al. Carriage of multidrug-resistant organisms in a tertiary university hospital in Albania-a point prevalence survey. Antimicrob Resist Infect Control. 2016;5:29.
Barbarini D, Russello G, Brovarone F, Capatti C, Colla R, Perilli M, et al. Evaluation of carbapenem-resistant Enterobacteriaceae in an Italian setting: report from the trench. Infect Genet Evol. 2015;30:8–14.
Gurnee EA, Ndao IM, Johnson JR, Johnston BD, Gonzalez MD, Burnham CA, et al. Gut colonization of healthy children and their mothers with pathogenic ciprofloxacin-resistant Escherichia coli. J Infect Dis. 2015;212:1862–8.
Wielders CCH, van Hoek AHAM, Hengeveld PD, Veenman C, Dierikx CM, Zomer TP, et al. Extended-spectrum β-lactamase- and pAmpC-producing Enterobacteriaceae among the general population in a livestock-dense area. Clin Microbiol Infect. 2017;23:120.e1–8.
Duplessis CA, Bavaro M, Simons MP, Marguet C, Santomauro M, Auge B, et al. Rectal cultures before transrectal ultrasound-guided prostate biopsy reduce post-prostatic biopsy infection rates. Urology. 2012;79:556–61.
de Lastours V, Chau F, Roy C, Larroque B, Fantin B. Emergence of quinolone resistance in the microbiota of hospitalized patients treated or not with a fluoroquinolone. J Antimicrob Chemother. 2014;69:3393–400.
Kliemann BS, Levin AS, Moura ML, Boszczowski I, Lewis JJ. Socioeconomic determinants of antibiotic consumption in the state of São Paulo, Brazil: the effect of restricting over-the-counter sales. PLoS One. 2016;11:e0167885.
Pereckaite L, Tatarunas V, Giedraitiene A. Current antimicrobial susceptibility testing for beta-lactamase-producing Enterobacteriaceae in clinical settings. J Microbiol Methods. 2018;152:154–64.
Poulou A, Grivakou E, Vrioni G, Koumaki V, Pittaras T, Pournaras S, et al. Modified CLSI extended-spectrum β-lactamase (ESBL) confirmatory test for phenotypic detection of ESBLs among Enterobacteriaceae producing various β-lactamases. J Clin Microbiol. 2014;52:1483–9.
Acknowledgements
The authors are grateful to the Turkish Republic of Northern Cyprus Ministry of Health, Near East University Hospital, Nicosia Dr. Burhan Nalbantoglu State Hospital, Kyrenia Dr. Akcicek Hospital and Famagusta State Hospital for their collaboration in the study. The authors would like to thank Assist. Prof. Ozgur Tosun from Near East University Faculty of Medicine, Department of Biostatistics for performing the statistical analysis.
Funding
The study was financially supported by Near East University Scientific Research Projects Coordination Unit (No: 2016–04020). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
ER conceived the study. JZ, KH, AF and EG collected the samples and data. ER, JZ, KH and AF carried out interpretation of test results. ER was a major contributor in writing the manuscript. UG, ZE and KS critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical approval for the study was obtained from the Near East University Research Assessment Committee (Project no: YDU/2016/37–296). Written informed consent was collected from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ruh, E., Zakka, J., Hoti, K. et al. Extended-spectrum β-lactamase, plasmid-mediated AmpC β-lactamase, fluoroquinolone resistance, and decreased susceptibility to carbapenems in Enterobacteriaceae: fecal carriage rates and associated risk factors in the community of Northern Cyprus. Antimicrob Resist Infect Control 8, 98 (2019). https://doi.org/10.1186/s13756-019-0548-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-019-0548-9