Abstract
Background
Diligent fluid management is an instrumental part of Enhanced Recovery After Surgery. However, the effect of a ward regimen to limit intravenous fluid administration on outcome remains unclear. We performed a meta-analysis investigating the effect of a restrictive versus a conventional fluid regimen on complications in patients after non-cardiac surgery in the postoperative period on the clinical ward.
Study design
We performed a systematic search in MEDLINE, Embase, Cochrane Library, and CINAHL databases, from the start of indexing until June 2022, with constraints for English language and adult human study participants. Data were combined using classic methods of meta-analyses and were expressed as weighted pooled risk ratio (RR) or odds ratio (OR) with 95% confidence interval (CI). Quality assessment and risk of bias analyses was performed according to PRISMA guidelines.
Results
Seven records, three randomized controlled trials, and four non-randomized studies were included with a total of 883 patients. A restrictive fluid regimen was associated with a reduction in overall complication rate in the RCTs (RR 0.46, 95% CI 0.23 to 0.95; P < .03; I2 = 35%). This reduction in overall complication rate was not consistent in the non-randomized studies (RR 0.74, 95% CI 0.53 to 1.03; P 0.07; I2 = 45%). No significant association was found for mortality using a restrictive fluid regimen (RCTs OR 0.51, 95% CI 0.05 to 4.90; P = 0.56; I2 = 0%, non-randomized studies OR 0.30, 95% CI 0.06 to 1.46; P = 0.14; I2 = 0%). A restrictive fluid regimen is significantly associated with a reduction in postoperative length of stay in the non-randomized studies (MD − 1.81 days, 95% CI − 3.27 to − 0.35; P = 0.01; I2 = 0%) but not in the RCTs (MD 0.60 days, 95% CI − 0.75 to 1.95; P = 0.38). Risk of bias was moderate to high. Methodological quality was very low to moderate.
Conclusion
This meta-analysis suggests restrictive fluid therapy on the ward may be associated with an effect on postoperative complication rate. However, the quality of evidence was moderate to low, the sample size was small, and the data came from both RCTs and non-randomized studies.
Similar content being viewed by others
Introduction
Currently, one in every four patients undergoing surgery suffer from one or more complications (Eappen et al. 2013). Besides the burden of complications on patients, the economic impact is overwhelming. Complications increase length of hospital stay and treatment cost up to 150% (Khan et al. 2006).
Hypervolemia as well as hypovolemia has been shown to lead to major complications, such as pneumonia and anastomotic leakage after surgery, and can even contribute to death (Chappell et al. 2008). Fluid balance is known as an independent predictor of outcome after surgery in several studies, (Thacker et al. 2016; Shin 2018) especially in colorectal surgery (Brandstrup et al. 2003; MacKay et al. 2006). Over time fluid regimens have shifted from liberal to restrictive, including goal-directed fluid therapy (GDT), to limit positive perioperative fluid balances (Chappell et al. 2008; Pearse et al. 2014; Holte and Kehlet 2006). In GDT, stroke volume (SV) or cardiac output (CO) is optimized by titrating fluid and cardiovascular stimulants. The goal is to keep the patient normovolemic and to prevent hyper- or hypovolemia. Although more extensive studies are still needed, current evidence suggests that intraoperative GDT decreases morbidity after major surgery (Som et al. 2017; Rollins and Lobo 2016). Diligent fluid management is an instrumental part of Enhanced Recovery After Surgery (ERAS) pathways (Gustafsson et al. 2013). However, it is hard to assess the impact of each item of the ERAS bundle separately (Jurt et al. 2017).
Implementation of GDT is a time-intensive and financially costly investment. Although patients spent most of the time on the ward, application of fluid optimization strategies are mostly limited to theatres, post-anesthesia care units (PACU), and intensive care units (ICU). Therefore, a reduction of the amount of fluid given to patients on the ward might contribute substantially to outcome. This systematic review aims to meta-analyze the available evidence for a restrictive fluid regimen on the ward.
Methods
We performed a systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Moher et al. 2009). Details of the protocol for this systematic review were registered on PROSPERO and are accessible athttp://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017075304.
Search strategy
With support from a clinical librarian, a search in MEDLINE, Embase, Cochrane Library, and CINAHL databases was performed from the start of indexing until June 2022, with constraints for English language and adult human study participants. Duplicate studies were excluded. The full-search strategy is available on PROSPERO (see details regarding the search strategy in Additional file 1).
Study selection
The following eligibility criteria were specified: (1) randomized controlled trials (RCTs) and non-randomized studies, (2) the studied population should include adult patients undergoing elective non-cardiac surgery in the postoperative period on the ward (≥ 2 h after surgery and ≤ 30 days) without further restrictions for type of anesthesia, (3) the intervention should include a restrictive fluid regimen (solely or as part of an ERAS protocol) compared to a conventional fluid regimen, and (4) the studies should report on the incidence of complications. Studies investigating fluid regimens at high care units (e.g., ICU or PACU) were excluded.
Title and abstract of the records were screened for relevance with the use of a systematic review system (Rayyan, Data Analytics (QCRI), Doha, Qatar) (Ouzzani et al. 2016). This system allows for a blinded screening by two independent reviewers (JB and MK). After identification of the records, reference lists were screened for additional relevant records. Two reviewers independently reviewed the full-text records and selected relevant studies based on the inclusion criteria (JB and MK). Discrepancies were resolved by discussion with a third reviewer (MW).
Primary outcome was overall complications. Complications, morbidity, or adverse events were defined as overall complications. Secondary outcomes extracted were the postoperative length of hospital stay (PLOS), mortality (30-day, 90-day, hospital or overall mortality), and severe complications (≥ grade 3 using the Clavien-Dindo classification (Dindo et al. 2004) or major complications/major adverse events).
Data synthesis and analysis
Data were extracted from the articles and appendices by two independent reviewers (JB and MW) using a standardized form. This form was an adapted version of the Data Extraction and Assessment Template from the Cochrane Public Health Group. The variables extracted included the year of publication, type of study, country, number of patients, type of patients, type of surgery, type of hospital, the age of patients, gender, ASA score, the aim of the study, the definition of complications used, the complications looked at in the study, the definition of restricted and conventional fluid regimen, and outcomes reported as mentioned above.
Risk of bias
RCTs were assessed for the risk of bias according to a ten-point checklist (Cochrane). Risk of bias due to missing results in the synthesis was assessed according the Cochrane Handbook Sect. 13.3.3 (Higgins 2008). For non-randomized studies, the Newcastle–Ottawa Scale (NOS) was used to evaluate the risk of bias (Wells 2013). A maximum of nine stars can be awarded. The quality of the body of evidence was assessed employing the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach (Guyatt et al. 2011).
For each study, the risk ratio (RR) for common events (i.e., > 20%) or Peto odds ratio (OR) method for rare events, and the 95% confidence intervals for complications and mortality comparing the restrictive and the standard groups were calculated (Deeks et al. 2005). For PLOS, the mean (SD) or median (IQR) was extracted, and SD was estimated with Wan’s method when not given (Wan et al. n.d.). In the protocol submitted to PROSPERO we intended to use the Mantel–Haenszel method to compute a weighted-pooled odds ratio based on the fixed effects model, however, based on an expected clinical, and methodological diversity we used the random-effects model (Mantel and Haenszel 1959; Borenstein et al. 2010). For data analysis, we used a software program (RevMan, version 5.4; The Cochrane Collaboration). We performed a post hoc trial sequential analysis (TSA) to assess whether our result of this meta-analysis are mathematically supported, with the use of the TSA software package (version 0.9.5.9 beta) (Wetterslev et al. 2008). We calculated information size using O’Brien-Flemming boundaries, setting the risk of a type 1 error at one-sided 5% and power at 80%. We used the data of the included studies to calculate relative risk reduction and incidence of events in our study (RRR 53%, incidence restrictive arm 21%, incidence conventional arm 46%).
Results
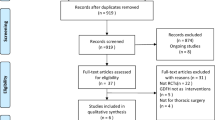
Our systematic search resulted in 4050 relevant records after removal of duplicates. Review of the titles and abstracts excluded 3986 records. After full-text review of 64 records, seven articles were included (PRISMA diagram, see Additional file 2 and 3 for exclusion criteria).
Study characteristics
Seven studies, three RCTs, and four non-randomized studies with a total of 883 patients were included (Table 1) (de 2009; Lobo et al. 2002; Morgan et al. 2016; Muller et al. 2009; Vermeulen et al. 2009; Walsh et al. 2008; Zargar-Shoshtari et al. 2008). Data of a total of 859 patients were included in this meta-analysis, because of exclusion of patients in the original studies. The studies were published between 2002 and 2016. The studies focused on major gastrointestinal surgery, ranging from colorectal to pancreatic surgery. Study characteristics are shown in Table 1. The number of complications was a primary endpoint in four out of seven studies. All studies looked at infectious and non-infectious complications including wound infection, respiratory complications, and complications related to surgery such as an anastomotic leakage, postoperative ileus, and pancreatic fistula. For details regarding the definition of complication and the complications scored per study see Tables 2 and 3.
Fluid regimens
Intraoperative fluid regimens were similar (de 2009; Lobo et al. 2002; Morgan et al. 2016; Vermeulen et al. 2009) or differed only minimal between the conventional group and restricted group in the studies (Muller et al. 2009). Intraoperative fluid balance significantly differed in one study (Zargar-Shoshtari et al. 2008) of those not describing the intraoperative fluid regimen (Additional file 4) (Walsh et al. 2008; Zargar-Shoshtari et al. 2008). No data for fluid balances were available.
Postoperative conventional fluid therapy ranged between more than two liters a day and non-restricted. The intervention groups were treated with zero to less than 3 l a day. The exact fluid regimens are stated in Table 1. The type of postoperative fluid administered was saline, Ringer’s lactate, and dextrose.
Risk of bias
Risk of bias was high for reporting bias in the three RCTs (see Additional file 5). For non-randomized studies risk of bias was moderate (3–6 stars on NOS, see Additional file 5). Risk of bias due to missing results in a synthesis was assessed. Results are available in Additional file 5. There was no mortality assessed for the separate cohorts in the study of Walsh (Walsh et al. 2008). For the other studies, there were no missing results for synthesis.
Outcomes
A restrictive fluid regimen is associated with a reduction in overall complication rate in RCTs (RR 0.46, 95% CI 0.23 to 0.95; P < 0.03; I2 = 35%) (Fig. 1a). This reduction in overall complication rate was not statistically significant in the non-randomized studies (RR 0.74, 95% CI 0.0.53 to 1.03; P 0.07; I2 = 45%) (Fig. 1b). Mortality was defined as 30-day (Lobo et al. 2002), 90-day (Morgan et al. 2016), in-hospital (Vermeulen et al. 2009; Walsh et al. 2008), or overall mortality (de 2009; Zargar-Shoshtari et al. 2008). A restrictive fluid regimen is not significantly associated with a reduction in mortality in the RCTs (OR 0.51, 95% CI 0.05 to 4.90;P = 0.56; I2 = 0%) or in the non-randomized studies (OR 0.30, 95% CI 0.06 to 1.46; P = 0.14; I2 = 0%) (Additional file 6). A restrictive fluid regimen is significantly associated with a reduction in PLOS in the non-randomized studies (mean difference − 1.81, 95% CI − 3.27 to − 0.35; P = 0.01; I2 = 0%) but not in the RCTs (mean difference 0.60, 95% CI − 0.75 to 1.95; P = 0.38) (see Additional file 7). Severe complications were described in two studies and defined as major complications (Vermeulen et al. 2009) or as a Clavien-Dindo ≥ grade 3 (Muller et al. 2009). Severe complications in a restrictive fluid regimen presented in 3/76 vs 7/75 patients and 1/18 vs 3/25 patients in a conventional fluid regimen. Insufficient data were available to analyze severe complications. The TSA showed the cumulativeZ score crossed the 5% trial sequential monitoring boundaries and therefore is supportive of the meta-analysis (Fig. 2). Furthermore, TSA showed the heterogeneity-adjusted required information size to demonstrate a 53% % relative risk reduction of overall complications (with a proportion of 46% of complications in the conventional fluid regimen group, an alpha of 5%, and a beta of 20%) is 238 patients.
Discussion
In this meta-analysis, we reviewed and compared fluid management strategies in the ward. The main finding is that a restrictive fluid regimen is significantly associated with a reduction in complications in RCTs and a reduction in PLOS in non-randomized studies. However, a restrictive fluid regimen is not significantly associated with a reduction in complications in non-randomized studies or a decrease in mortality in the under 1000 patients studied.
Restricted or patient-tailored fluid therapy is part of ERAS (Gustafsson et al. 2013). It is difficult to assess the effectiveness of each part of the ERAS bundle separately (Jurt et al. 2017). Our finding of an association between a postoperative fluid regimen to prevent hypervolemia and a reduction in complications is in line with large retrospective cohort studies, systematic reviews, and a meta-analysis (Thacker et al. 2016; Holte and Kehlet 2006; Rollins and Lobo 2016; Varadhan and Lobo 2010; Thacker et al. 2014). However, not all trials investigating restrictive fluid therapy show this association (Vermeulen et al. 2009; Srinivasa et al. 2013; van Samkar et al. 2015). One of the included RCTs was terminated prematurely because of frequent protocol violations (e.g., unblinding by personnel not involved in the trial) and insufficient patient inclusion (Vermeulen et al. 2009). Significantly more complications in the restrictive fluid regimen group in an intention-to-treat analysis were seen. Reasons for unblinding were not directly related to hypovolemia. Furthermore, after unmasking the amount of fluid given was not recorded and treatment effect was not measured. For this meta-analysis, we were interested in the effect of adhering to the intervention as described in the study. We chose to include the per-protocol analyses, because the estimation of its effects relates most closely to the implications of our analysis (Higgins 2022). This may be biased as this analysis is restricted to the patients who adhered to the study protocol.
We performed a TSA to determine if the results of this met-analysis are mathematically supported (Wetterslev et al. 2008). Based on these TSA, a required information size of 238 patients in a meta-analysis is necessary to confirm the effect of a restrictive fluid regimen on overall complications on the ward and to exclude early overestimation (Wetterslev et al. 2008). Recently, a large retrospective cohort study showed perioperative fluid volume to be an independent predictor for length of hospital stay (Aga et al. 2016). However, there was no distinction between intra- and postoperative fluid volume.
Despite several studies and the “The British Consensus Guidelines concerning postoperative fluid therapy,” there is no widely accepted appropriate fluid therapy on the ward (Powell-Tuck 2011). A wide range of what is called “restrictive” or “conventional” is presented in the included studies, with an overlap between the definitions and thereby influencing data and interpretation. A previous trial attempted to define fluid restriction as < 1.75 L per day, and liberal as > 2.75 L per day, which seems a fair summary of the averages used in literature (Varadhan and Lobo 2010). All studies included major gastrointestinal surgery, ranging from colonic resections to pancreatic operations, with different underlying conditions. Thus, the wide range of the types of surgical procedures makes the studies more heterogenic. Furthermore, only a small number of studies with a limited number of participants and heterogeneity in the primary endpoint, study design, and definition of fluid regimens could be included in the present meta-analysis. Perioperative complications were a primary endpoint in one RCT and three retrospective studies. Therefore, the included RCTs might be underpowered to assess complications. We used a random-effects model as we expect the effect size varies between studies (Borenstein et al. 2010). However, meta-analysis shows a statistical significant reduction of the complication rate in RCTs and a trend towards significance in non-randomized studies (Fig. 1). The limited number of participants may explain why the effect of a reduction in complication rate did not translate into a reduced mortality and only a decreased PLOS in non-randomized studies. Also, the complications might not be severe enough to lead to a clinically relevant reduction. We included severe complications as a secondary outcome. Only limited data were available. Therefore, we did not perform analyses on these data. Intraoperative fluid regimens were (near-)similar in the included studies, and intraoperative fluid volume was only significantly different in one retrospective study (Zargar-Shoshtari et al. 2008). Therefore, we think intraoperative fluid regimens did not influence our results in this meta-analysis. Last, it is not clear how signs of hypovolemia (e.g., hypotension or thirst) where treated in the included retrospective studies. Assessing the risk of bias resulted in a high or unclear risk of bias for some of the assessed bias domains. To address this issue, we evaluated the quality of evidence using a validated tool supported by the PRISMA statement (Moher et al. 2009; Guyatt et al. 2011). The significance level was the same for all outcomes. Therefore, there is a risk of overestimating the effect due to multiple testing. Furthermore, there is a risk of confounding by induction for the non-randomized studies. Patients may have received more fluids because they developed more complications or may have needed less fluids because they had fewer complications.
Overall, it seems that a restrictive fluid regimen may help in improving post-surgical patient outcome. Still, some challenges for prescription of fluids apply. A fluid balance is difficult to obtain (Walsh et al. 2008; Boersema et al. 2014). Studies show that weighing is often neglected and fluid charts are not registered correctly (Walsh et al. 2008; Boersema et al. 2014). Prescriptions were not based on the patient’s current status, and junior doctors ordered the majority of prescriptions as they mostly care for patients on the ward (Walsh et al. 2008; Nadler et al. 2014; Lobo et al. 2001). A small audit in 2015 showed that 60% of the patients were not treated according to protocol (Birk 2015). Following the studies mentioned earlier, only 40% of patients were weighed, and fluid charts were inaccurate. Moreover, several large-scale surveys found that protocols or guidelines for fluid therapy or even standard hemodynamic monitoring are not present in around 75% of hospitals (Holte and Kehlet 2006; Geerts 2009). Different studies suggest fewer complications arise when comparing a restricted fluid regimen to conventional therapy (MacKay et al. 2006; Lobo et al. 2002; Muller et al. 2010; Gonzalez-Fajardo et al. 2009). Our analysis shows a significantly lower risk ratio for complications with restricted use of fluids postoperatively in RCTs. We can therefore only assume that there is a serious gap in our hemodynamic assessment capabilities on the ward. Wearable technology, non-invasive cardiac output monitoring, and even sophisticated algorithms are now becoming available and could be a potential solution for this shortcoming (Michard 2017). However, currently, there are no data available to support the use of these techniques, and implementation can be expensive.
Our meta-analysis suggests that reduction of complications with more diligent fluid therapy on the ward may be feasible. Individualized fluid prescription in a more structured approach may be beneficial, based on protocols addressing both the volume status and the clinical response of the patient (Myles et al. 2017). The studies describing the effect of a restrictive fluid regimen on complication rate showed an overall significant difference in the meta-analysis. We performed a TSA for the RCT’s with primary outcome overall complications and found an information size of 238 patients to be necessary for a meta-analysis to draw more solid conclusions.
Conclusions
Our study suggests a restrictive approach towards intravenous fluid use on the ward following major gastrointestinal surgery may be associated with a statistically significant effect on complication rate in RCTs and postoperative length of stay in non-randomized studies. However, the quality of evidence was moderate to low, the sample size was small, and the data came from both RCTs and non-randomized studies. The association did not translate into reduced mortality.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Aga Z, Machina M, McCluskey SA. Greater intravenous fluid volumes are associated with prolonged recovery after colorectal surgery: a retrospective cohort study. Br J Anaesth. 2016;116(6):804–10.
Birk B MM, Stevens J, Sayegh M. Standards for intravenous fluid therapy – audit of maintenance fluids in surgical inpatients. Abstract IFAD 2015, Antwerp, Belgium. Available at: http://www.ifad.eu/abstract-presentations-ii/. Accessed Sept 12, 2017.
Boersema GS, van der Laan L, Wijsman JH. A close look at postoperative fluid management and electrolyte disorders after gastrointestinal surgery in a teaching hospital where patients are treated according to the ERAS protocol. Surg Today. 2014;44(11):2052–7.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111.
Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641–8.
Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109(4):723–40.
de Aguilar-Nascimento JE, Diniz BN, do Carmo AV, Silveira EA, Silva RM. Clinical benefits after the implementation of a protocol of restricted perioperative intravenous crystalloid fluids in major abdominal operations. World J Surg. 2009;33(5):925–30.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–93.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Eappen S, Lane BH, Rosenberg B, Lipsitz SA, Sadoff D, Matheson D, et al. Relationship between occurrence of surgical complications and hospital finances. JAMA. 2013;309(15):1599–606.
Geerts BF. Haemodynamic assessment in dutch Intensive Care Units. In: Maas J, editor. Neth J Crit Care. 2009;13:178–84.
Gonzalez-Fajardo JA, Mengibar L, Brizuela JA, Castrodeza J, Vaquero-Puerta C. Effect of postoperative restrictive fluid therapy in the recovery of patients with abdominal vascular surgery. Eur J Vasc Endovasc Surg. 2009;37(5):538–43.
Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J Surg. 2013;37(2):259–84.
Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2.
Higgins JPT. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. The Cochrane Collaboration. 2008. Updated Sept 2008.
Higgins JPT SJ, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Cochrane, 2022. Available from www.training.cochrane.org/handbook. Updated Feb 2022
Holte K, Kehlet H. Fluid therapy and surgical outcomes in elective surgery: a need for reassessment in fast-track surgery. J Am Coll Surg. 2006;202(6):971–89.
Jurt J, Slieker J, Frauche P, Addor V, Solà J, Demartines N, et al. Enhanced recovery after surgery: can we rely on the key factors or do we need the bel ensemble? World J Surg. 2017;41(10):2464–70.
Khan NA, Quan H, Bugar JM, Lemaire JB, Brant R, Ghali WA. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med. 2006;21(2):177–80.
Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359(9320):1812–8.
Lobo DN, Dube MG, Neal KR, Simpson J, Rowlands BJ, Allison SP. Problems with solutions: drowning in the brine of an inadequate knowledge base. Clin Nutr (Edinburgh, Scotland). 2001;20(2):125–30.
MacKay G, Fearon K, McConnachie A, Serpell MG, Molloy RG, O’Dwyer PJ. Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. Br J Surg. 2006;93(12):1469–74.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48.
Michard F, Gan TJ, Kehlet H. Digital innovations and emerging technologies for enhanced recovery programmes. Br J Anaesth. 2017;119(1):31–9.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Morgan KA, Lancaster WP, Walters ML, Owczarski SM, Clark CA, McSwain JR, et al. Enhanced recovery after surgery protocols are valuable in pancreas surgery patients. J Am Coll Surg. 2016;222(4):658–64.
Muller L, Louart G, Bousquet PJ, Candela D, Zoric L, de La Coussaye JE, et al. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med. 2010;36(3):496–503.
Muller S, Zalunardo MP, Hubner M, Clavien PA, Demartines N. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology. 2009;136(3):842-7.e1.
Myles PS, Andrews S, Nicholson J, Lobo DN, Mythen M. Contemporary approaches to perioperative IV fluid therapy. World J Surg. 2017;41(10):2457–63.
Nadler A, Pearsall EA, Victor JC, Aarts MA, Okrainec A, McLeod RS. Understanding surgical residents’ postoperative practices and barriers and enablers to the implementation of an Enhanced Recovery After Surgery (ERAS) Guideline. J Surg Educ. 2014;71(4):632–8.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311(21):2181–90.
Powell-Tuck JGP, Lobo DN, Allison SP, Carlson GL. British Consensus Guidelines on intravenous fluid therapy for adult surgical patients. On behalf of BAPEN Medical - a core group of BAPEN, the Association for Clinical Biochemistry, the Association of Surgeons of Great Britain and Ireland, the Society of Academic and Research Surgery, the Renal Association and the Intensive Care Society. 2011. https://www.bapen.org.uk/pdfs/bapen_pubs/giftasup.pdf.
Rollins KE, Lobo DN. Intraoperative goal-directed fluid therapy in elective major abdominal surgery: a meta-analysis of randomized controlled trials. Ann Surg. 2016;263(3):465–76.
Shin CH, Long DR, McLean D, Grabitz SD, Ladha K, Timm FP, Thevathasan T, Pieretti A, Ferrone C, Hoeft A, Scheeren TWL, Thompson BT, Kurth T, Eikermann M. Effects of Intraoperative Fluid Management on Postoperative Outcomes: A Hospital Registry Study. Ann Surg. 2018;267(6):1084–92. https://doi.org/10.1097/SLA.0000000000002220.
Som A, Maitra S, Bhattacharjee S, Baidya DK. Goal directed fluid therapy decreases postoperative morbidity but not mortality in major non-cardiac surgery: a meta-analysis and trial sequential analysis of randomized controlled trials. J Anesth. 2017;31(1):66–81.
Srinivasa S, Taylor MH, Singh PP, Yu TC, Soop M, Hill AG. Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br J Surg. 2013;100(1):66–74.
Thacker JK, Mountford WK, Ernst FR, Krukas MR, Mythen MM. Perioperative fluid utilization variability and association with outcomes: considerations for enhanced recovery efforts in sample US surgical populations. Ann Surg. 2016;263(3):502–10.
Thacker JK, Mountford WK, Mythen M, Krukas MR, Ernst FR. Increased risk of post-operative ileus with excess fluid on the day of colon surgery: results from 524 hospitals in the United States. J Am Coll Surg. 2014;219(4):e33. https://doi.org/10.1016/j.jamcollsurg.2014.07.473.
van Samkar G, Eshuis WJ, Bennink RJ, van Gulik TM, Dijkgraaf MG, Preckel B, et al. Intraoperative fluid restriction in pancreatic surgery: a double blinded randomised controlled trial. PLoS One. 2015;10(10):e0140294.
Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc. 2010;69(4):488–98.
Vermeulen H, Hofland J, Legemate DA, Ubbink DT. Intravenous fluid restriction after major abdominal surgery: a randomized blinded clinical trial. Trials. 2009;10:50.
Walsh SR, Cook EJ, Bentley R, Farooq N, Gardner-Thorpe J, Tang T, et al. Perioperative fluid management: prospective audit. Int J Clin Pract. 2008;62(3):492–7.
Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. https://doi.org/10.1186/1471-2288-14-135.
Wells GSB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013.
Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75.
Zargar-Shoshtari K, Connolly AB, Israel LH, Hill AG. Fast-track surgery may reduce complications following major colonic surgery. Dis Colon Rectum. 2008;51(11):1633–40.
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Study conception: BG, MM, DV; Study design: JB, MW, AV, BG, DV; Data collection: JB, MW, MK; Analysis of the data: JB, MW, AV, BG, DV; Interpretation of the data: JB, MW, BG, MM, AV, MH, DV; Drafting the manuscript: JB, MW, DV; Critical revision of the manuscript for important intellectual content: All authors. All authors read and approved the final manuscript. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Denise P. Veelo reported receipt of grants and consultancy fees from Edwards Lifesciences, and research grants from Philips and Hemologic. Dr. Markus W. Hollmann reported serving as executive section editor of pharmacology for Anaesthesia & Analgesia and as section editor of anaesthesiology for the Journal of Clinical Medicine and receipt of speakers fees from CSL Behring and Eurocept BV and consultancy fees from Eurocept BV. Dr. Michael G. Mythen is a paid consultant for Edwards Lifesciences and Deltex Medical. His University chair is sponsored by Smiths Medical Ltd. He has received honoraria for speaking or travel expenses from Baxter, BBraun, Fresenius Kabi, Hospira, and LidCo, and is a board member of the Bloomsbury Innovation Group. The other authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search documents.
Additional file 2.
PRISMA flow diagram.
Additional file 3.
Full text screening—exclusion criteria.
Additional file 4.
Intraoperative fluid regimens.
Additional file 5.
Risk of bias summary.
Additional file 6.
Mortality.
Additional file 7.
PLOS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bosboom, J.J., Wijnberge, M., Geerts, B.F. et al. Restrictive versus conventional ward fluid therapy in non-cardiac surgery patients and the effect on postoperative complications: a meta-analysis. Perioper Med 12, 52 (2023). https://doi.org/10.1186/s13741-023-00337-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13741-023-00337-9