Abstract
Background
Minimally invasive surgery is becoming more common and transfemoral transcatheter aortic valve replacement is offered to older patients with multiple comorbidities. Sternotomy is not required but patients must lie flat and still for up to 2–3 h. This procedure is increasingly being performed under conscious sedation with supplementary oxygen, but hypoxia and agitation are commonly observed.
Methods
In this randomised controlled trial, we hypothesised that high-flow nasal oxygen would provide superior oxygenation as compared with our standard practice, 2 l min−1 oxygen by dry nasal specs. This was administered using the Optiflow THRIVE Nasal High Flow delivery system (Fisher and Paykel, Auckland, New Zealand) at a flow rate of 50 l min−1 and FiO2 0.3. The primary endpoint was the change in arterial partial pressure of oxygen (pO2) during the procedure. Secondary outcomes included the incidence of oxygen desaturation, airway interventions, the number of times the patient reached for the oxygen delivery device, incidence of cerebral desaturation, peri-operative oxygen therapy duration, hospital length of stay and patient satisfaction scores.
Results
A total of 72 patients were recruited. There was no difference in change in pO2 from baseline using high-flow compared with standard oxygen therapy: median [IQR] increase from 12.10 (10.05–15.22 [7.2–29.8]) to 13.69 (10.85–18.38 [8.5–32.3]) kPa vs. decrease from 15.45 (12.17–19.33 [9.2–22.8]) to 14.20 (11.80–19.40 [9.7–35.1]) kPa, respectively. The percentage change in pO2 after 30 min was also not significantly different between the two groups (p = 0.171). There was a lower incidence of oxygen desaturation in the high-flow group (p = 0.027). Patients in the high-flow group assigned a significantly higher comfort score to their treatment (p ≤ 0.001).
Conclusion
This study has demonstrated that high flow, compared with standard oxygen therapy, does not improve arterial oxygenation over the course of the procedure. There are suggestions that it may improve the secondary outcomes studied.
Trial registration
International Standard Randomised Controlled Trial Number (ISRCTN) 13,804,861. Registered on 15 April 2019. https://doi.org/10.1186/ISRCTN13804861
Similar content being viewed by others
Background
Transfemoral transcatheter aortic valve replacement (TAVR) has become an established therapy for high-risk patients with severe aortic stenosis (Rosengart et al. 2008). General anaesthesia was the preferred anaesthetic technique in the early days of TAVR, but it has largely been replaced by local anaesthesia and conscious sedation (Miles et al. 2016). Patients undergoing TAVR often have multiple cardiorespiratory risk factors that put them at an increased risk of hypoxia with complications, such as confusion or an exacerbation of pulmonary hypertension (Balanika et al. 2014), especially as they are required to lie flat and still for an extended period of time (Mayr et al. 2015). Lying flat may be difficult for patients with impaired cardiac function as cardiac failure causes pulmonary edema and dyspnea. Also, patients with respiratory disease or patients who are obese may not be able to lie flat and may become hypoxic. In addition, the deployment of the new aortic valve may require rapid cardiac pacing and standstill with no cardiac output which may impair oxygen delivery to the body and make the patient restless (Mayr et al. 2016).
Various sedation techniques have been described, with most supplying oxygen via either nasal cannulae or face mask (Mayr et al. 2015). High-flow nasal oxygen (HFNT) allows the delivery of heated and humidified oxygen at an inspiratory fraction of oxygen (FiO2) of between 0.21 to 1.0, in a flow rate of up to 60 l min−1 (Drake 2018). Emerging evidence shows that HFNT is effective in various clinical settings such as in the treatment of acute heart failure (Kang et al. 2019; Carratalá Perales et al. 2011; Roca et al. 2013), after cardiac surgery (Corley et al. 2011; Parke et al. 2013) and during procedural sedation (Ben-Menachem et al. 2020; Lucangelo et al. 2012; Lin et al. 2019; Mazzeffi et al. 2020; Schumann et al. 2016; Yi et al. 2020). Besides providing a higher degree of patient comfort by supplying warmed and humidified gas to maintain muco-ciliary functions (Nishimura 2015), HFNT increases pharyngeal pressure by 3 cmH20 and generates an effect similar to continuous airway positive pressure (CPAP) (Nishimura 2016).
We designed this randomised controlled study to evaluate clinical outcomes using HFNT in patients undergoing transfemoral TAVR under conscious sedation. The primary hypothesis was that HFNT would improve oxygenation in this group of patients, as measured by arterial partial pressure of oxygen (pO2) during the procedure. Secondary outcomes included the number of episodes of desaturation, the type of airway interventions by the anaesthetist, comfort levels of patients as determined by movement during the procedure, cerebral oxygen saturations, peri-operative oxygen therapy duration, hospital length of stay and patient satisfaction scores.
Methods
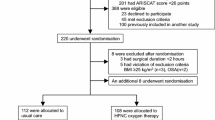
The study protocol was approved by the East of England Cambridge East Ethics Committee and all patients provided written informed consent. This was a single-centre, prospective randomised controlled trial conducted at the Royal Papworth Hospital, Cambridge, UK, a specialist cardiothoracic hospital. Patients with nasal septal defects, those who were participating in another randomised controlled trial and those who were unable to speak English or had special communication needs were not included. Patients undergoing elective TAVR under sedation were randomly allocated in a 1:1 ratio using Sealed Envelope™ (https://www.sealedenvelope.com/) online randomisation (unrestricted) to standard oxygen therapy (SOT) or HFNT. Allocation of treatment arm was done by an investigator before the commencement of the procedure. The transcatheter aortic valve procedure was performed in the cardiac catheter laboratory using a transfemoral approach and percutaneous access via the femoral artery with retrograde valve placement (Fig. 1).
Patients, physicians and nurses caring for patients were not blinded to the study interventions as blinding would not have been possible due to the nature of the intervention. The researcher collecting data including blood gas measurements (primary outcome) and interviewing patients was blinded.
Physiological monitoring according to the Association of Anaesthetists standards was applied to all patients (Klein et al. 2021). Cerebral oximetry was measured using the Masimo O3 regional oximetry (Masimo, Irvine, CA, USA). A peripheral intravenous cannula was sited and an ultrasound-guided ilio-inguinal and fascia iliaca block was performed on the side of the procedure using 2 mg kg−1 of levobupivacaine by the anaesthetist.
Conscious sedation/analgesia is defined as “a drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation” (Miles et al. 2016). In our conscious sedation protocol, a low-dose remifentanil infusion was commenced at 0.05mcg kg−1 min−1 and titrated to achieve a Ramsey sedation scale of 2 to 3 (Ramsay et al. 1974). No other premedication or sedative was given to patients. Oxygen therapy was started after commencement of sedation therapy.
Participants in the HFNT group were given warm and humidified oxygen using the Optiflow THRIVE Nasal High Flow delivery system (Fisher and Paykel, Auckland, New Zealand). Oxygen was administered starting at an FiO2 of 0.3 and flow rate of 50 l min−1. Continuous capnography monitoring was also used throughout the procedure according to Association of Anaesthetists standards using an attachment to the nasal cannulae provided by the manufacturer.
Participants in the SOT group received 2 l min−1 oxygen therapy via standard nasal cannulae (not heated or humidified) and capnography and FiO2 was measured using the gas in-line monitoring system of the anaesthetic machine. The FiO2 using this protocol was 0.3 (the same as the HFNT group). At any stage during the study, the anaesthetist could manage the patient’s airway in any way they deemed to be appropriate in the best interest of the patient. This included changing to an alternative oxygen delivery strategy.
The cardiologist secured further access with a standard arterial sheath in the femoral artery and a venous sheath in the femoral vein. A 14–16-Fr sheath for valve insertion was placed in the contralateral femoral artery. All procedures utilised Edwards SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA) or Medtronic CoreValve Evolut PRO (Medtronic, Minneapolis, MN, USA) heart valve system. Transvenous rapid ventricular pacing using a balloon-tipped pacing catheter inserted via the femoral vein was used to facilitate rapid pacing for valve deployment. Transthoracic echocardiography was used to assess valve function before and after the intervention.
The primary outcome was the change in arterial oxygenation, as measured by pO2 between the first and second arterial blood gas measurements from the femoral arterial sheath. The first arterial blood gas was obtained as soon as the cardiologist obtained access in the femoral artery and the second was obtained 30 min later. This was the only arterial access in these patients. Both measurements were taken with the patient receiving HFNT or SOT as per group allocation and at the same FiO2. Secondary outcomes include the number of episodes of desaturation, as defined as an SpO2 of < 93% for > 10 s or a decrease of > 5% from the baseline for > 10 s; the type of airway interventions by the anaesthetist; the number of times the patient reached for the oxygen delivery device; cerebral desaturation during valve deployment, as defined as regional SO2 < 50% or decrease in baseline > 20% (Slater et al. 2009); peri-operative oxygen therapy duration; hospital length of stay; and patient satisfaction scores. Blood gas analysis, oxygen device settings, vital signs, cerebral oximetry and sedation score of patients were collected at 30-min intervals after arterial access was obtained.
After the procedure, patients were transferred to the post-anaesthesia recovery unit (PACU) until ready for discharge to the ward. The criteria used for patient discharge was a post-anaesthetic discharge scoring system (PADS) score of > 9. Patients were interviewed within 24 h after the procedure and asked to rate the comfort level of the oxygen delivery device.
We reviewed data in TAVR patients who received sedation with remifentanil from an audit from 2018 and the mean (SD) arterial pO2 was 10.0 (5.2) kPa using SOT. We hypothesised that HFNT would lead to a clinically significant improvement of 35% during the procedure. Following these assumptions, a sample size of 34 patients in each arm would be sufficient to detect a difference between study groups at 80% power and a significance level of 0.05. Assuming a dropout rate of 5%, the final sample size was set at 36 patients per group. Data analysis was performed using an intention-to-treat approach. Categorical variables were compared using Fisher’s exact test. Normality was checked using Shapiro–Wilk test and by assessment of skewness and its standard error. Comparison between groups were analysed using Student’s t-test where assumptions were met, and Wilcoxon rank sum test if the data was not normally distributed. Repeated measurements were analysed with Wilcoxon signed rank test. All tests were two-tailed and statistical significance was accepted at p < 0.05. Statistical analysis was performed using R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 72 patients who underwent transfemoral TAVR between June 2019 and October 2020 were enrolled into the study. None of the participants crossed over to the other treatment arm. Three patients did not complete the procedure: the valve was not deployed in two patients (HFNT group) and one patient passed away as a result of a complication of the procedure (HFNT group). Three patients required conversion to general anaesthesia: one patient in the SOT group developed unstable supraventricular tachycardia and required synchronised DC cardioversion; another in the SOT group had difficulties with valve deployment and required multiple rapid pacing episodes; and one patient in the HFNT group required transesophageal echocardiography. The dose of remifentanil used for sedation during the procedure was similar in both groups, median (IQR [range]) in HFNT group 0.30 (0.24–0.42 [0.14–0.85]) mg vs. SOT group 0.37 (0.26–0.46 [0.10–0.66]) mg, p = 0.454. Procedure time was also similar in both groups, HFNT 117.00 (102.50–131.00 [74.0–151.0]) min vs. SOT 115.50 (101.00–135.50 [73.0–218.0]) min, p = 0.954 (Table 3).
The two groups had similar baseline characteristics (Table 1) except that twice as many patients had undergone previous cardiac surgery in the HFNT group compared with the SOT group (15% vs. 7%, respectively). None of the patients required continuous positive airway pressure therapy and one patient in the HFNT group required home oxygen therapy.
Table 2 and Figs. 2 and 3 illustrate the blood gas analysis results. All patients had their first arterial gas analysis after insertion of the femoral arterial line. This was mean (SD) 51 (16) minutes after the pre-surgery checklist had been completed. pO2 at baseline was lower in the HFNT compared with the SOT group, median [IQR (range)] 12.10 (10.05–15.22 (7.2–29.8)) vs. 15.45 (12.17–19.33 (9.2–22.8)) kPa. The FiO2 was 0.3 in both groups and there was no difference in the percentage change in pO2 after 30 min between the two groups (p = 0.171) (Table 3).
Percentage change in arterial partial pressure of oxygen (pO2) and arterial partial pressure of carbon dioxide (pCO2) in both high-flow nasal oxygen (HFNT) and standard oxygen therapy (SOT) groups from 0 to 30 min during transfemoral transcatheter aortic valve implantation (TAVR) in patients randomly allocated to HFNT or SOT
Other study secondary outcomes are shown in Table 3. There was a statistically significantly reduced incidence of oxygen desaturation in the HFNT group (p = 0.027). Furthermore, five patients in the SOT group required escalation of oxygen delivery device from nasal cannula to a face mask, versus none in the HFNT group. Four patients in the HFNT group demonstrated cerebral desaturation during valve deployment (11.8%) compared with nine in the SOT group (25.0%), p = 0.221. Furthermore, three patients in the SOT group experienced syncope compared with none in the HFNT group. Two patients in the standard group were observed to be restless and agitated, as compared with one in the HFNT group.
Patients described HFNT as being more comfortable, with fewer patients reaching for the oxygen delivery device (p = 0.003) and giving it a significantly higher comfort score when interviewed after the procedure (p ≤ 0.001). There was no significant difference between the two groups in oxygen requirements in PACU (p = 0.123) or duration of hospital stay (p = 0.936). There were no postoperative complications related to the use of either oxygen delivery device. No patient died in either group during the first 30 days after TAVR.
Discussion
We have shown no difference in change in arterial oxygenation over the course of the TAVR procedure in patients who received HFNT or SOT while sedated. Regarding secondary outcomes, fewer patients who received HFNT desaturated. Patients who received HFNT reported feeling more comfortable.
The treatment for aortic stenosis is aortic valve replacement, which has traditionally required sternotomy and cardiopulmonary bypass. Minimally invasive TAVR without sternotomy has become the standard of care for patients with aortic stenosis at increased surgical risk (Baumgartner et al. 2018). Nevertheless, TAVR remains a high-risk procedure when applied to patients who are poor surgical candidates. Around 3250 TAVR cases were performed across centres in the UK in the year 2016 (Ludman 2019) and this is increasing yearly. Transfemoral TAVR is increasingly being done while patients receive conscious sedation (Mayr et al. 2015), with observational study data showing benefits such as an improvement in hemodynamic stability and shorter hospital and ICU stay (Ehret et al. 2017). Hence, it is important to review our anaesthetic practice.
Hypoxia is likely to be more common in patients undergoing transfemoral TAVR due to both patient- and procedure-related factors. Patients are often elderly and have multiple comorbidities (Trauzeddel et al. 2021). Up to a third of them have chronic lung disease (Howard et al. 2019). Furthermore, the procedure takes up to 2 h and patients are required to lie still in the supine position on a reasonably hard and narrow table. The reduction in functional residual capacity further predisposes them to hypoxia (Coonan and Hope 1983), which is often worsened by the need for procedural sedation. As a result of oxygen desaturation, patients regularly become restless and agitated. Therefore, we decided to study whether HFNT would increase arterial pO2 and reduce the incidence of intra-operative adverse events.
One of the mechanisms by which HFNT is proposed to improve oxygenation is by alveolar recruitment (Drake 2018). This results from the generation of positive airway pressure, although the magnitude of this effect is dependent on the patient having their mouth closed (Chanques et al. 2013). We did not notice the expected improvements in blood gases despite high oxygen flows. This may be because patients did not have their mouths closed or this is not seen in sedated patients for another reason. In procedures such as bronchoscopy (Ben-Menachem et al. 2020; Lucangelo et al. 2012), gastroscopy (Lin et al. 2019; Mazzeffi et al. 2020) and endoscopic retrograde cholangiopancreatography (Schumann et al. 2016) in which an improvement in oxygenation with the use of HFNT has been shown, the presence of an endoscope may have produced the same effect as having the mouth closed.
High-flow nasal therapy did not have a significant effect on carbon dioxide partial pressure in our study, with percentage change in pCO2 levels increasing similarly over time in both the HFNT and SOT groups. Achieving a sufficiently high flow rate is critical to reduce the anatomic dead space in order to maximise CO2 clearance (Möller et al. 2017). Douglas et al. noted that increasing the HFNT flow rate from 40 to 60 l min−1 brought the pCO2 down to within the normal range (Douglas et al. 2018), suggesting that a higher O2 flow rate than that used in our study (50 l min−1) may have a greater effect on CO2 clearance.
In contrast to the lower rates of desaturation in our HFNT patient group, this was not proven in morbidly obese patients undergoing colonoscopy (Riccio et al. 2019). However, there is increasing evidence that the high concentration oxygen therapy used in this study may be detrimental by causing hypoventilation and, hence, hypercapnia (Pilcher et al. 2017). This suggests that hypoventilation may be the cause of hypoxia in morbidly obese patients receiving HFNT. In contrast, our patient cohort has a much lower BMI of 27.6 (5.2) kg m−2 (mean (SD)), suggesting that HFNT may be less useful in morbidly obese patients.
Patient comfort level and satisfaction score in many of these studies produced mixed results (Roca et al. 2010; Maggiore et al. 2014), with many suggesting no added benefit of HFNT (Ben-Menachem et al. 2020; Lucangelo et al. 2012; Yilmazel Ucar et al. 2020). This is in contrast to our study which suggests that HFNT has an added advantage of improving the comfort level in patients. This could have been due to the humidification effect of HFNT as seen by the lower incidence of throat dryness in the HFNT group.
HFNT has been shown to have beneficial effects in cardiac patients both in the setting of the treatment of acute heart failure (Kang et al. 2019; Carratalá Perales et al. 2011; Roca et al. 2013) and in the treatment of postoperative hypoxemia after cardiac surgery. In a recent case report, HFNT was used to treat desaturation in a patient undergoing percutaneous balloon aortic valvuloplasty under sedation (Sakazaki et al. 2019). In addition to respiratory benefits, HFNT also resulted in hemodynamic improvements in patients with heart failure (Roca et al. 2013). This is a potential added benefit for patients undergoing transfemoral TAVR, many of whom have heart failure due to severe aortic stenosis.
Limitations
We have shown no difference in arterial oxygenation in patients who received HFNT or SOT during sedation for TAVR. This may have been because we did not recruit enough patients to see a true difference as the difference was smaller than that used in our power calculation (35%). The study was not powered for the secondary outcomes so these are exploratory and must be interpreted with caution and future studies should look to confirm their findings. Also, our groups differed with respect to baseline pO2; we are not sure of the reason for this but it is like to be a random finding. The pO2 tended to decrease slightly 30 min later in the SOT group and increase in the HFNT but this was not statistically significant when comparing the two groups—this could also be due to type-2 error.
Secondly, arterial blood gases were only able to pick up pO2 and pCO2 values at certain time-points and we only managed to capture data at two time-points in most patients. The first arterial gas analysis was obtained at a mean (SD) of 51 (16) minutes after the patient had been checked in, allowing for only one more arterial gas analysis before the end of the procedure.
Also, this study investigated HFNT using a single delivery device at a FiO2 of 0.3 and flow rate of 50 l min−1. Differences might be seen with alternative HFNT delivery devices or different FiO2 and flow rates, as shown in studies demonstrating the beneficial effects of a higher flow rate on CO2 clearance (Mauri et al. 2017).
Conclusions
We have shown that HFNT, compared with SOT, did not improve arterial oxygenation in patients undergoing transfemoral TAVR under sedation over the course of the procedure. There are suggestions that it may improve the secondary outcomes studied, which are hypothesis-generating for future studies.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CPAP:
-
Continuous airway positive pressure
- FiO2 :
-
Inspiratory fraction of oxygen
- HFNT:
-
High-flow nasal oxygen
- PACU:
-
Post-anaesthesia recovery unit
- PADS:
-
Post-anaesthetic discharge scoring system
- pCO2 :
-
Arterial partial pressure of carbon dioxide
- pO2 :
-
Arterial partial pressure of oxygen
- SOT:
-
Standard oxygen therapy
- TAVR:
-
Transfemoral transcatheter aortic valve replacement
References
Balanika M, Smyrli A, Samanidis G, et al. Anesthetic management of patients undergoing transcatheter aortic valve implantation. J Cardiothorac Vasc Anesth. 2014;28:285–9.
Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Rev Esp Cardiol. 2018;71:110.
Ben-Menachem E, McKenzie J, O’Sullivan C, Havryk AP. High-flow nasal oxygen versus standard oxygen during flexible bronchoscopy in lung transplant patients: a randomized controlled trial. Journal of Bronchology and Interventional Pulmonology. 2020;27:259–65.
Carratalá Perales JM, Llorens P, Brouzet B, et al. High-Flow therapy via nasal cannula in acute heart failure. Rev Esp Cardiol. 2011;64:723–5.
Chanques G, Riboulet F, Molinari N, et al. Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol. 2013;79:1344–55.
Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107:998–1004.
Coonan TJ, Hope CE. Cardio-respiratory effects of change of body position. Can Anaesth Soc J. 1983;30:424–38.
Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018;15:145–55.
Douglas N, Ng I, Nazeem F, et al. A randomised controlled trial comparing high-flow nasal oxygen with standard management for conscious sedation during bronchoscopy. Anaesthesia. 2018;73:169–76.
Ehret C, Rossaint R, Foldenauer AC, et al. Is local anaesthesia a favourable approach for transcatheter aortic valve implantation? A systematic review and meta-analysis comparing local and general anaesthesia. BMJ Open. 2017;7:e016321.
Howard C, Jullian L, Joshi M, Noshirwani A, Bashir M, Harky A. TAVI and the future of aortic valve replacement. J Card Surg. 2019;34:1577–90.
Kang MG, Kim K, Ju S, et al. Clinical efficacy of high-flow oxygen therapy through nasal cannula in patients with acute heart failure. Journal of Thoracic Dieases. 2019;11:410–7.
Klein AA, Meek T, Allcock E, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2021. Anaesthesia. 2021;76:1212–23.
Ludman PF. UK TAVI Registry Heart. 2019;105:s2–5.
Lucangelo U, Vassallo FG, Marras E, et al. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit Care Res Prac. 2012;2012:506382.
Lin Y, Zhang X, Li L, et al. High-flow nasal cannula oxygen therapy and hypoxia during gastroscopy with propofol sedation: a randomized multicenter clinical trial. Gastrointest Endosc. 2019;90:591–601.
Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195:1207–15.
Mayr NP, Michel J, Bleiziffer S, Tassani P, Martin K. Sedation or general anesthesia for transcatheter aortic valve implantation (TAVI). Journal ofThoracic Disease. 2015;7:1518–26.
Mayr NP, Hapfelmeier A, Martin K, et al. Comparison of sedation and general anaesthesia for transcatheter aortic valve implantation on cerebral oxygen saturation and neurocognitive outcome†. Br J Anaesth. 2016;116:90–9.
Mazzeffi MA, Petrick KM, Magder L, et al. High-flow nasal cannula oxygen in patients having anesthesia for advanced esophagogastroduodenoscopy: HIFLOW-ENDO, a randomized clinical trial. Anesth Analg. 2020;132:743–51.
Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. American Journal of Respiratory and Critical Care Medicine. 2014;190:282–8.
Miles LF, Joshi KR, Ogilvie EH, et al. General anaesthesia vs. conscious sedation for transfemoral aortic valve implantation: a single UK centre before-and-after study. Anaesthesia. 2016;71:892–900.
Möller W, Feng S, Domanski U, et al. Nasal high flow reduces dead space. J Appl Physiol. 2017;122:191–7.
Nishimura M. High-flow nasal cannula oxygen therapy in adults. J Intensive Care. 2015;3:15.
Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61:529–41.
Parke R, McGuinness S, Dixon R, Jull A. Open-label, phase II study of routine high-flow nasal oxygen therapy in cardiac surgical patients. Br J Anaesth. 2013;111:925–31.
Pilcher J, Richards M, Eastlake L, et al. High flow or titrated oxygen for obese medical inpatients: a randomised crossover trial. Med J Aust. 2017;207:430–4.
Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2:656–9.
Riccio CA, Sarmiento S, Minhajuddin A, Nasir D, Fox AA. High-flow versus standard nasal cannula in morbidly obese patients during colonoscopy: A prospective, randomized clinical trial. J Clin Anesth. 2019;54:19–24.
Rosengart TK, Feldman T, Borger MA, et al. Percutaneous and minimally invasive valve procedures. Circulation. 2008;117:1750–67.
Roca O, Pérez-Terán P, Masclans JR, et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. J Crit Care. 2013;28:741–6.
Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55:408–13.
Schumann R, Natov NS, Rocuts-Martinez KA, et al. High-flow nasal oxygen availability for sedation decreases the use of general anesthesia during endoscopic retrograde cholangiopancreatography and endoscopic ultrasound. World J Gastroenterol. 2016;22:10398–405.
Sakazaki R, Suzuki T, Ikeda N. High-flow nasal cannula oxygen supported-transesophageal echocardiography under sedation in a respiratory compromised patient. J Cardiothorac Vasc Anesth. 2019;33:255–6.
Slater JP, Guarino T, Stack J, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36–44.
Trauzeddel RF, Nordine M, Balanika M, et al. Current anesthetic care of patients undergoing transcatheter aortic valve replacement in Europe: results of an online survey. J Cardiothorac Vasc Anesth. 2021;35:1737–46.
Yi P, Li Q, Yang Z, Cao L, Hu X, Gu H. High-flow nasal cannula improves clinical efficacy of airway management in patients undergoing awake craniotomy. BMC Anesthesiol. 2020;20:156.
Yilmazel Ucar E, Araz Ö, Kerget B, Akgun M, Saglam L. Comparison of high-flow and conventional nasal cannula oxygen in patients undergoing endobronchial ultrasonography. Intern Med J. 2020;56:4413.
Acknowledgements
Not applicable.
Funding
The study was funded by an unrestricted educational grant provided by Fisher and Paykal Healthcare. Fischer and Paykal Healthcare had no involvement in the design, conduct, or reporting of the trial or the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
A.A. Klein was responsible for study design, protocol writing, ethical approval, patient recruitment, trial conduct, sample collection, data analysis, interpretation and manuscript writing. S. Scheuermann was responsible for study design, protocol writing, ethical approval, patient recruitment, trial conduct, sample collection, data analysis, interpretation and manuscript writing. A. Tan was responsible for patient recruitment, sample collection, trial conduct, data analysis, interpretation and manuscript writing. P. Govender was responsible for patient recruitment, trial conduct, sample collection, data analysis, interpretation and manuscript writing. S. George was responsible for study design, trial conduct, sample collection, data analysis, interpretation and manuscript writing. F. Falter was responsible for trial conduct, sample collection, data analysis, interpretation and manuscript writing. G. Martinez was responsible for trial conduct, sample collection, data analysis, interpretation and manuscript writing. M. McKie was responsible for data analysis, interpretation and manuscript writing. J. Pack was responsible for trial conduct, data analysis, interpretation and manuscript writing. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the East of England Cambridge East Ethics Committee and all patients provided written informed consent. The study was registered before recruitment started (International Standard Randomised Controlled Trial Number (ISRCTN) 13804861).
Consent for publication
Not applicable.
Competing interests
AK and SS have received educational grant funding or honoraria from Fisher and Paykel, Auckland, New Zealand. AK or his institution has received educational grant funding, honoraria or travel funding from Pharmacosmos, Nordic Pharma, Hemonetics and Masimo. AK is the Editor-in-Chief of Anaesthesia. FF or his institution has received educational funding from Werfen and grant funding and honoraria from Abbott Point of Care. No other conflicting interests were declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Scheuermann, S., Tan, A., Govender, P. et al. High-flow nasal oxygen vs. standard oxygen therapy for patients undergoing transcatheter aortic valve replacement with conscious sedation: a randomised controlled trial. Perioper Med 12, 11 (2023). https://doi.org/10.1186/s13741-023-00300-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13741-023-00300-8