Abstract
Background
More than 30% of the world’s population are anemic with serious medical and economic consequences. Red blood cell transfusion is the mainstay to correct anemia, but it is also one of the top five overused procedures and carries its own risk and cost burden. Patient blood management (PBM) is a patient-centered and multidisciplinary approach to manage anemia, minimize iatrogenic blood loss, and harness tolerance to anemia in an effort to improve patient outcome. Despite resolution 63.12 of the World Health Organization in 2010 endorsing PBM and current guidelines which include evidence-based recommendations on the use of diagnostic/therapeutic resources to provide better health care, many hospitals have yet to implement PBM in routine clinical practice.
Method and results
A number of experienced clinicians developed the following “Simplified International Recommendations for Patient Blood Management.” We propose a series of simple, cost-effective, best-practice, feasible, and evidence-based measures that will enable any hospital to reduce both anemia prevalence on the day of intervention/surgery and anemia-related unnecessary transfusion in surgical and medical patients, including obstetrics and gynecology.
Similar content being viewed by others
Background
Patient blood management (PBM), refers to “the timely application of evidence based medical and surgical concepts designed to maintain hemoglobin concentration, optimize hemostasis and minimize blood loss in an effort to improve patient outcome” (Society for the Advancement of Blood Management (SABM) 2014). Thereby, PBM focuses on preservation of patient’s own blood. It questions the dogma of red blood cell (RBC) transfusion as the primary strategy for the treatment of anemia but also supports the use of appropriate transfusion practice in the hospitalized patient.
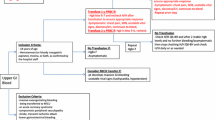
PBM should be performed by an institutionally empowered multidisciplinary team considering three most important principles: first, management of the patient’s anemia, which mainly involves early detection of anemia and utilizing nutritional and pharmaceutical treatments to support erythropoiesis, if it is not mainly genetic-related; second, the use of interdisciplinary blood conservation measures to reduce iatrogenic blood loss, including prevention and proactive management of coagulopathy, precise anesthetic and surgical techniques, intra- and postoperative autologous blood conservation techniques, and minimization of phlebotomy volume and frequency; and third, harness tolerance to anemia and patient-centered decision-making to allow optimal blood use involving thorough communication with the patient about the risks and benefits of the various potential interventions (Society for the Advancement of Blood Management (SABM) 2014; Goodnough et al. 2013; Spahn and Goodnough 2013).
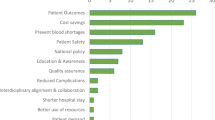
Considerable scientific evidence indicates that PBM reduces perioperative blood loss and transfusion needs (Leahy et al. 2017; Gross et al. 2015; Goodnough et al. 2014a; Goodnough et al. 2014b; Moskowitz et al. 2010; Oliver et al. 2014; Theusinger et al. 2014; Leahy et al. 2014; Roubinian et al. 2014; Meybohm et al. 2016a), perioperative morbidity (Leahy et al. 2017; Gross et al. 2015; Moskowitz et al. 2010; Meybohm et al. 2016a), mortality (Leahy et al. 2017; Goodnough et al. 2014a; Moskowitz et al. 2010), length of hospital stay (Goodnough et al. 2014a; Moskowitz et al. 2010), and costs (Leahy et al. 2017; Trentino et al. 2015). In this respect, the WHO has officially been urging member states to implement PBM since 2010 (WHA63.12). There exist also a number of guidelines and standards from professional associations providing detailed evidence-based information and recommendations on PBM in Australia, the USA, and in several European countries (Society for the Advancement of Blood Management (SABM) 2014; National Blood Authority Australia; Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee 2014; American Society of Anesthesiologists Task Force on Perioperative Blood Management 2015; Government of Western Australia Department of Health; Kozek-Langenecker et al. 2013a; Leal-Noval et al. 2013; Kozek-Langenecker et al. 2013b; Hunt et al. 2015; AABB 2014; NHS Blood and Transplant; AABB 2015; Klein et al. 2016). However, many barriers limit the translation of PBM into clinical practice worldwide (Munoz et al. 2015; Fischer et al. 2015; Vamvakas 2013; Mbanya 2012). These include clear guidance on clinical pathways, lack of knowledge, lack of interdisciplinary commitment, lack of resources, and concerns about risks. In a recent paper (Meybohm et al. 2017), we provided comprehensive bundles of PBM components encompassing more than 100 different PBM measures to facilitate a stepwise implementation process of the most feasible measures.
The aim of this current opinion-based document is to provide clinicians with a working template to start or improve the implementation of PBM practices, specifically through the implementation of simplified recommendations developed with reference to existing best practice guidelines and appropriate key literature on blood transfusion.
Methods
Experts in PBM were consulted in order to provide consensus on key simple recommendations for the implementation of PBM based on their current practice and experience in Australia, Europe, and the USA. The selection of the authors was intended but covered experts who have been involved in developing a European Guide on Good Practices for Patient Blood Management (P.M., K.Z.), international consensus on the perioperative management of anemia and iron deficiency (P.M., A.A.K., M.M., T.R., A.S., D.R.S.), National Institute for Health and Care Excellence Guidance on blood transfusion (M.F.M.) (Padhi et al. 2015), and landmark papers in the field of PBM (B.F., L.T.G., A.S.). Level of evidence was assigned to each recommendation (level A, multiple randomized clinical trials or meta-analyses; level B, single randomized trial or non-randomized studies; level C, consensus opinion of experts, case studies, or standard-of-care). All authors were asked to vote point-by-point for each recommendation (agreed/not agreed/abstention). If a statement did not achieve 80% or more of the votes, the statement underwent further revision and then entered again into the online voting process. The consensus of recommendation is shown in Table 1.
We would like to stress that this paper contains the authors’ independent opinions based on experience, as well as evidence-based practices supported by clinical studies. No pharmaceutical company has funded the development or writing of the manuscript.
Simplified International Recommendations for the Implementation of Patient Blood Management (SIR4PBM)
Comprehensive project management
Each hospital should appoint key leaders for PBM project management (this could be a physician, a nurse, or the institution’s patient safety officer), who should have a central role in charge of communication, education, and documentation. Communication should involve the following stakeholders: chief medical officer, chief executive officer, surgeons, anesthesiologists, intensive care specialists, nurses, transfusion medicine specialists, transfusion committee, gastroenterologists, hematologists, cardiologist, general practitioners, finance administrative and quality management personnel, central laboratory, information technology department, and patients’ representatives (level C).
PBM-related metrics and blood usage should be collected to monitor implementation success and allow identification of potential areas for improvement. PBM-related metrics should include proportion of patients who are anemic and receive treatment, use of blood conservation techniques, and use of hemostatic products and blood components. Data should be audited at ward, specialty, and divisional level with regular feedback to all hospital staff. Further consideration should be given for these metrics to be utilized as a quality improvement marker for appraisal of staff groups (level C).
Education program
In clinical areas, such as preoperative clinic/preadmission testing units, operating room, and intensive care unit, PBM education initiatives need to be addressed with physicians and nurses. Standard operating procedures, clinical protocols, visual aids, and checklists are crucial. They should include outpatient and preoperative protocols and ward-based transfusion algorithms. Also, training on the use of available massive hemorrhage protocols should be provided (level C). These massive hemorrhage protocols including specific coagulation and transfusion algorithms for postpartum, trauma, transplant, or cardiac surgery should be in place and encourage early detection, definitive intervention, and treatment of acute hemorrhage (Kozek-Langenecker et al. 2013a; Hunt et al. 2015; Kozek-Langenecker 2014; Spahn et al. 2013; Rossaint et al. 2013) (level C). A mandatory online PBM e-learning course may further underpin education initiatives (Meybohm et al. 2016b; National Service Scotland; BloodSafe eLearning Australia) (level C). Ideally, education on PBM should be initiated among undergraduates at medical schools and continued at hospital level (level C).
Diagnosis and treatment of preoperative anemia
Preoperative screening includes evaluation and management of anemia. From a practical point of view, patients scheduled for surgical procedures with expected blood loss (>500 ml) or a ≥10% probability of RBC transfusion should be identified and assessed at the earliest opportunity and be screened for iron deficiency and other likely causes of anemia (Government of Western Australia Department of Health; AABB 2014; National Service Scotland; Carless et al. 2010; Goodnough and Schrier 2014; Shah et al. 2015; Muñoz et al. 2017) (level B). Serum ferritin level <30 ng/ml, transferrin saturation <20%, and/or microcytic hypochromic red cells (mean corpuscular volume <80 fl; mean corpuscular hemoglobin <27 pg) are indicative of iron deficiency. In the presence of inflammation or transferrin saturation <20%, a ferritin >100 ng/ml points to functional iron deficiency (iron sequestration). The availability of an easy-to-follow, diagnostic algorithm is desirable (Goodnough and Schrier 2014; Muñoz et al. 2017). Intravenous iron is efficacious and safe (Auerbach and Macdougall 2014) and should be used in patients in whom oral iron is not tolerated or if surgery is planned in less than 4–6 weeks after the diagnosis of iron deficiency (Shander et al. 2014a; Froessler et al. 2016; Bisbe et al. 2011; Munoz et al. 2014) (level B). Erythropoiesis-stimulating agents might be suggested for anemic patients in whom nutritional deficiencies have been ruled out, corrected, or both (Goodnough et al. 2011; Voorn et al. 2016; Weltert et al. 2015) (level B). Elective surgery should be postponed until preoperative anemia has been appropriately classified and treated, if possible (level C).
Reduction of iatrogenic diagnostic-/surgery-related blood loss
Blood loss associated with invasive laboratory testing can either cause or aggravate hospital-acquired anemia which is associated with increased length of stay and complications (Koch et al. 2015). Blood testing should not be “routined” but informed. Reduction of blood drawn for laboratory analyses can be achieved by avoiding unnecessary laboratory tests and lower frequency of sampling (Raad et al. 2016) and using the smallest collection tube size that is practical for the required analysis, which is often pediatric-size bottles (level C). However, there are several difficulties with these bottles (e.g., overfill and underfill of tubes, large IT labels not fitting on small tubes, issues with the bottles not fitting into automated analyzers). Further reduction of phlebotomy-associated blood loss can be achieved by using closed in-line flush blood sampling devices for arterial (and central) lines (Koch et al. 2015; Ranasinghe and Freeman 2014; Fischer et al. 2014; Mukhopadhyay et al. 2010) (level B).
Reduction of surgery-related blood loss starts from the preoperative stage with appropriate cessation strategies for anticoagulation and antiplatelet therapy (level C). Intraoperative approaches such as advanced anesthetic and surgical techniques with meticulous hemostasis including minimally invasive surgery, laparoscopic surgery, judicious use of diathermy dissection, physicians’ mindfulness regarding limiting blood loss, and application of topical hemostatic agents (Shander et al. 2014b; Menkis et al. 2012; Emilia et al. 2011; Anastasiadis et al. 2016) (level B).
Advanced perioperative coagulation monitoring is crucial for avoiding unnecessary blood loss. Adequate coagulation management needs to be a precondition before RBC transfusion is considered. In this respect, the use of a coagulation algorithm is recommended (Weber et al. 2012; Weber et al. 2014), encompassing preoperative assessment (Munoz et al. 2016) and ensuring basic conditions for hemostasis (e.g., temperature, calcium, pH), reversal of anticoagulants, point-of-care diagnostics in bleeding (e.g., coagulopathic) patients (if available), and optimized coagulation management with the use of clotting factor concentrates (Kozek-Langenecker et al. 2013a; Kozek-Langenecker 2014; Meybohm et al. 2013; Weber et al. 2013) (level B). To reduce surgical blood loss, tranexamic acid should be used unless contraindicated (Perel et al. 2013; Ker et al. 2013) (level A).
The use of intraoperative autologous blood collection and re-transfusion (cell salvage) should be standardized including indications and contraindications (Carless et al. 2010). The use of (washed) cell recovery is highly recommended in surgical settings where blood loss is routinely or anticipated over 500 ml as it reduces the rate of exposure to allogeneic RBC, risk of infection, and length of hospital stay (Meybohm et al. 2016c) (level A).
Optimal blood component use with patient-centered clinical decision support
In order to optimize utilization of allogeneic blood products/components and to identify the ordering physician, it is beneficial to adopt a physician order entry with a clinical decision support based on electronic medical records (Goodnough et al. 2014a; Oliver et al. 2014) (level B). Thereby, indication for transfusion considering patient-specific factors (e.g., age, diagnosis, co-morbidity, surgical or non-surgical setting), signs/symptoms of acute anemia, laboratory values (e.g., hemoglobin), presence or absence of bleeding, and physiologic factors (e.g., oxygenation, hemodynamic status) can be confirmed with required checkboxes (Goodnough et al. 2016).
Patient’s informed consent should be obtained prior transfusion of allogeneic blood products (components). In emergency cases where it is not possible to obtain consent, patient should be informed as soon as possible after transfusion. A hand-written or computer-generated forms (ideally a separate consent form) should be used that is comprehensive and includes a detailed outline of transfusion benefits, risks, and alternatives (ISBT ethic code for blood donation and transfusion (International Society of Blood Transfusion 2000)). It is necessary to effectively communicate the risks and benefits of the various potential interventions and to decide on the right course of action together with the patient. It may further be recommended that any transfusion of allogeneic blood products (components) should be mentioned in the discharge summary. The patient’s own preferences and values should be considered when developing a medical plan (level C).
Conclusions
To enable any hospital to reduce both anemia prevalence and anemia-related unnecessary blood transfusion, here, we provide simple, cost-effective, best-practice, feasible, and evidence-based measure recommendations developed with reference to existing best practice guidelines. As the level of evidence is still low for some recommendations, we suggest that further studies are needed to elucidate the potential role of these PBM measures.
Abbreviations
- PBM:
-
Patient blood management
- RBC:
-
Red blood cells
References
AABB. Standards for a patient blood management program. 1st ed. 2014.
AABB. AABB and The Joint Commission to Partner on Patient Blood Management Certification. 2015. http://www.aabb.org/press/Pages/pr151203a.aspx. Accessed 09 Sept 2016.
American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology. 2015;122:241–75.
Anastasiadis K, Murkin J, Antonitsis P, Bauer A, Ranucci M, Gygax E, et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg. 2016;22:647–62.
Auerbach M, Macdougall IC. Safety of intravenous iron formulations: facts and folklore. Blood Transfus. 2014;12:296–300.
Bisbe E, Garcia-Erce JA, Diez-Lobo AI, Munoz M, Anaemia Working Group E. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011;107:477–8.
BloodSafe eLearning Australia. https://www.bloodsafelearning.org.au/. Accessed 09 Sept 2016.
Carless PA, Henry DA, Moxey AJ, O’Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010;CD001888.
Emilia M, Luca S, Francesca B, Luca B, Paolo S, Giuseppe F, et al. Topical hemostatic agents in surgical practice. Transfus Apher Sci. 2011;45:305–11.
Fischer DP, Zacharowski KD, Meybohm P. Savoring every drop—vampire or mosquito? Crit Care. 2014;18:306.
Fischer DP, Zacharowski KD, Muller MM, Geisen C, Seifried E, Muller H, et al. Patient blood management implementation strategies and their effect on physicians’ risk perception, clinical knowledge and perioperative practice—the frankfurt experience. Transfus Med Hemother. 2015;42:91–7.
Froessler B, Palm P, Weber I, Hodyl NA, Singh R, Murphy EM. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Ann Surg. 2016;264:41–6.
Goodnough LT, Schrier SL. Evaluation and management of anemia in the elderly. Am J Hematol. 2014;89:88–96.
Goodnough LT, Maniatis A, Earnshaw P, Benoni G, Beris P, Bisbe E, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22.
Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet. 2013;381:1845–54.
Goodnough LT, Shieh L, Hadhazy E, Cheng N, Khari P, Maggio P. Improved blood utilization using real-time clinical decision support. Transfusion. 2014a;54:1358–65.
Goodnough LT, Maggio P, Hadhazy E, Shieh L, Hernandez-Boussard T, Khari P, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion. 2014b;54:2753–9.
Goodnough LT, Baker SA, Shah N. How I use clinical decision support to improve red blood cell utliization. Transfusion. 2016;56(10):2406–11.
Government of Western Australia Department of Health. Patient blood management guidelines and standards. http://ww2.health.wa.gov.au/Articles/N_R/Patient-blood-management-guidelines-and-standards. Accessed 09 Sept 2016.
Gross I, Seifert B, Hofmann A, Spahn DR. Patient blood management in cardiac surgery results in fewer transfusions and better outcome. Transfusion. 2015;55:1075–81.
Hunt BJ, Allard S, Keeling D, Norfolk D, Stanworth SJ, Pendry K, et al. A practical guideline for the haematological management of major haemorrhage. Br J Haematol. 2015;70:788–803.
International Society of Blood Transfusion. The ISBT code of ethics. 2000. http://www.isbtweb.org/fileadmin/user_upload/_About_ISBT/ISBT_Code_of_Ethics_English.pdf. Accessed 12 Sept 2016.
Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee. Patient blood management—an evidence-based approach to patient care. 2014. http://www.transfusionguidelines.org.uk/uk-transfusion-committees/national-blood-transfusion-committee/patient-blood-management. Accessed 09 Sept 2016.
Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100:1271–9.
Klein AA, Arnold P, Bingham RM, Brohi K, Clark R, Collis R, et al. AAGBI guidelines: the use of blood components and their alternatives 2016. Anaesthesia. 2016;71:829–42.
Koch CG, Reineks EZ, Tang AS, Hixson ED, Phillips S, Sabik 3rd JF, et al. Contemporary bloodletting in cardiac surgical care. Ann Thorac Surg. 2015;99:779–84.
Kozek-Langenecker SA. Coagulation and transfusion in the postoperative bleeding patient. Curr Opin Crit Care. 2014;20:460–6.
Kozek-Langenecker SA, Afshari A, Albaladejo P, Santullano CA, De Robertis E, Filipescu DC, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013a;30:270–382.
Kozek-Langenecker S, Bettelheim P, Giurea A, Halbmayer W, Haushofer A, Holzer P, et al. Interdisciplinary recommendations for the management of anaemia (patient blood management). 2013b. http://www.oegari.at/web_files/dateiarchiv/editor/interdisciplinary_recommendations_for_the_management_of_anaemia_2013.pdf. Accessed 06 Sept 2016.
Leahy MF, Roberts H, Mukhtar SA, Farmer S, Tovey J, Jewlachow V, et al. A pragmatic approach to embedding patient blood management in a tertiary hospital. Transfusion. 2014;54:1133–45.
Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, Hamdorf J, Gallagher T, Koay A, Geelhoed GC, Farmer SL. Improved outcomes and reduced costs associated with a health-system-wide patient bloodmanagement program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017. doi:10.1111/trf.14006. [Epub ahead of print].
Leal-Noval SR, Munoz M, Asuero M, Contreras E, Garcia-Erce JA, Llau JV, et al. Spanish consensus statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document”. Blood Transfus. 2013;11:585–610.
Mbanya D. Barriers and enablers to introducing comprehensive patient blood management in the hospital. Biologicals. 2012;40:205–8.
Menkis AH, Martin J, Cheng DC, Fitzgerald DC, Freedman JJ, Gao C, et al. Drug, devices, technologies, and techniques for blood management in minimally invasive and conventional cardiothoracic surgery: a consensus statement from the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) 2011. Innovations (Phila). 2012;7:229–41.
Meybohm P, Zacharowski K, Weber CF. Point-of-care coagulation management in intensive care medicine. Crit Care. 2013;17:218.
Meybohm P, Herrmann E, Steinbicker AU, Wittmann M, Gruenewald M, Fischer D, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome. A prospective, multicenter cohort study with a noninferiority design. Ann Surg. 2016a;264:203–11.
Meybohm P, Regaei A, Mueller M, Seifried E, Geisen C, Fischer D, et al. Patient blood manager—elearning. http://www.patientbloodmanager.de/. Accessed 09 Sept 2016.
Meybohm P, Choorapoikayil S, Wessels A, Herrmann E, Zacharowski K, Spahn DR. Washed cell salvage in surgical patients. A review and meta-analysis of prospective randomized trials under PRISMA. Medicine (Baltimore). 2016c;95:e4490.
Meybohm P, Richards T, Isbister J, Hofmann A, Shander A, Goodnough LT, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev. 2017;31:62–71.
Moskowitz DM, McCullough JN, Shander A, Klein JJ, Bodian CA, Goldweit RS, et al. The impact of blood conservation on outcomes in cardiac surgery: is it safe and effective? Ann Thorac Surg. 2010;90:451–8.
Mukhopadhyay A, Yip HS, Prabhuswamy D, Chan YH, Phua J, Lim TK, et al. The use of a blood conservation device to reduce red blood cell transfusion requirements: a before and after study. Crit Care. 2010;14:R7.
Munoz M, Gomez-Ramirez S, Cuenca J, Garcia-Erce JA, Iglesias-Aparicio D, Haman-Alcober S, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54:289–99.
Munoz M, Gomez-Ramirez S, Kozek-Langeneker S, Shander A, Richards T, Pavia J, et al. ‘Fit to fly’: overcoming barriers to preoperative haemoglobin optimization in surgical patientsdagger. Br J Anaesth. 2015;115:15–24.
Munoz M, Gomez-Ramirez S, Kozek-Langeneker S. Pre-operative haematological assessment in patients scheduled for major surgery. Anaesthesia. 2016;71 Suppl 1:19–28.
Muñoz M, Acheson AG, Auerbach M, Besser M, Habler O, Kehlet H, Liumbruno GM, Lasocki S, Meybohm P, Rao Baikady R, Richards T, Shander A, So-Osman C, Spahn DR, Klein AA. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. 2017;72(2):233–247.
National Blood Authority Australia. Patient blood management guidelines. https://www.nba.gov.au/patient-blood-management-pbm. Accessed 09 Sept 2016.
National Service Scotland. Learn blood transfusion. http://www.learnbloodtransfusion.org.uk/. Accessed 04 Sept 2016.
NHS Blood and Transplant. Patient Blood Management. http://hospital.blood.co.uk/patient-services/patient-blood-management/. Accessed 09 Sept 2016.
Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion. 2014;54:2617–24.
Padhi S, Kemmis-Betty S, Rajesh S, Hill J, Murphy MF, Guideline Development G. Blood transfusion: summary of NICE guidance. BMJ. 2015;351:h5832.
Perel P, Ker K, Morales Uribe CH, Roberts I. Tranexamic acid for reducing mortality in emergency and urgent surgery. Cochrane Database Syst Rev. 2013;CD010245.
Raad S, Elliott R, Dickerson E, Khan B, Diab K. Reduction of laboratory utilization in the intensive care unit. J Intensive Care Med. 2016. [Epub ahead of print].
Ranasinghe T, Freeman WD. ‘ICU vampirism’—time for judicious blood draws in critically ill patients. Br J Haematol. 2014;164:302–3.
Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, et al. The STOP the bleeding campaign. Crit Care. 2013;17:136.
Roubinian NH, Escobar GJ, Liu V, Swain BE, Gardner MN, Kipnis P, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion. 2014;54:2678–86.
Shah N, Andrews J, Goodnough LT. Transfusions for anemia in adult and pediatric patients with malignancies. Blood Rev. 2015;29:291–9.
Shander A, Goodnough LT, Javidroozi M, Auerbach M, Carson J, Ershler WB, et al. Iron deficiency anemia—bridging the knowledge and practice gap. Transfus Med Rev. 2014a;28:156–66.
Shander A, Kaplan LJ, Harris MT, Gross I, Nagarsheth NP, Nemeth J, et al. Topical hemostatic therapy in surgery: bridging the knowledge and practice gap. J Am Coll Surg. 2014b;219:570–9. e4.
Society for the Advancement of Blood Management (SABM). SABM administrative and clinical standards for patient blood management programs. https://www.sabm.org/publications - adminstandards 2014. Accessed 09 Sept 2016.
Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet. 2013;381:1855–65.
Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17:R76.
Theusinger OM, Kind SL, Seifert B, Borgeat L, Gerber C, Spahn DR. Patient blood management in orthopaedic surgery: a four-year follow-up of transfusion requirements and blood loss from 2008 to 2011 at the Balgrist University Hospital in Zurich, Switzerland. Blood Transfus. 2014;12:195–203.
Trentino KM, Farmer SL, Swain SG, Burrows SA, Hofmann A, Ienco R, et al. Increased hospital costs associated with red blood cell transfusion. Transfusion. 2015;55:1082–9.
Vamvakas EC. Reasons for moving toward a patient-centric paradigm of clinical transfusion medicine practice. Transfusion. 2013;53:888–901.
Voorn VM, van der Hout A, So-Osman C, Vliet Vlieland TP, Nelissen RG, van den Akker-van Marle ME, et al. Erythropoietin to reduce allogeneic red blood cell transfusion in patients undergoing total hip or knee arthroplasty. Vox Sang. 2016;111(3):219–25.
Weber CF, Gorlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531–47.
Weber CF, Klages M, Zacharowski K. Perioperative coagulation management during cardiac surgery. Curr Opin Anaesthesiol. 2013;26:60–4.
Weber CF, Zacharowski K, Meybohm P, Adam EH, Hofer S, Brun K, et al. Hemotherapy algorithms for coagulopathic cardiac surgery patients. Clin Lab. 2014;60:1059–63.
Weltert L, Rondinelli B, Bello R, Falco M, Bellisario A, Maselli D, et al. A single dose of erythropoietin reduces perioperative transfusions in cardiac surgery: results of a prospective single-blind randomized controlled trial. Transfusion. 2015;55:1644–54.
Acknowledgements
None.
Funding
There is no funding for this study.
Availability of data and materials
Not applicable.
Authors’ contributions
PM, KZ, and DS developed the first draft of this manuscript. All authors contributed to improve this draft. All authors read and approved the final manuscript.
Competing interests
P.M. and K.Z. received grants by B. Braun Melsungen, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of Frankfurt’s Patient Blood Management Program in four German university hospitals. P.M. received honoraria for scientific lectures from B.Braun Melsungen, Ferring, CSL Behring and from Pharmacosmos. K.Z. received grants from B.Braun Melsungen, CSL Behring, Fresenius Kabi, Vifor Pharma, and the European Union. A.K. have received funding and/or honoraria from Pharmacosmos and Vifor Pharma. M.M. has received honoraria for consultancy or lectures and/or travel support from Stryker Ibérica (Spain), Wellspect HealthCare (Sweden), Ferrer Pharma (Spain), Roche (Spain), Vifor Pharma (Spain and Switzerland), PharmaCosmos (Denmark), and Zambon (Spain) but not for this work. UCL and the research program lead by T.R. has received research funding from a variety of sources including government, charity, and industry sources for research into anemia, blood transfusion and iron therapy including NIHR HTA, NHMRC, Health Foundation, Gideon Richter, Vifor Pharma Ltd, and Pharmocosmos. T.R. has also been an invited speaker at conferences and provided consultancy to government and industry on anemia, blood transfusion and iron therapy in the last 5 years. This does not alter our adherence to the journal policies on sharing data and materials. A.S. received research grants from CSL Behring, Gauss Surgical, Masimo, and HbO2 Therapeutics; he has also received honoraria from CSL Behring, Masimo, and Merck and acted as a consultant for CSL Behring, Gauss Surgical, Masimo Corporation, and Vifor Pharma. D.S.’s academic department is receiving grant support from the Swiss National Science Foundation, Berne, Switzerland; the Ministry of Health (Gesundheitsdirektion) of the Canton of Zurich, Switzerland, for highly specialized medicine; the Swiss Society of Anesthesiology and Reanimation, Berne, Switzerland; the Swiss Foundation for Anesthesia Research, Zurich, Switzerland; Bundesprogramm Chancengleichheit, Berne, Switzerland; CSL Behring, Berne, Switzerland; and Vifor SA, Villars-sur-Glâne, Switzerland. D.S. was the chairman of the ABC Faculty and is the co-chairman of the ABC-Trauma Faculty, which both are managed by Physicians World Europe GmbH, Mannheim, Germany, and sponsored by unrestricted educational grants from Novo Nordisk Health Care AG, Zurich, Switzerland; CSL Behring GmbH, Marburg, Germany; and LFB Biomédicaments, Courtaboeuf Cedex, France. In the past 5 years, D.S. has received honoraria or travel support for consulting or lecturing from the following companies: Abbott AG, Baar, Switzerland; AMGEN GmbH, Munich, Germany; AstraZeneca AG, Zug, Switzerland; Baxter AG, Volketswil, Switzerland; Baxter S.p.A., Roma, Italy; Bayer (Schweiz) AG, Zürich, Switzerland; Bayer Pharma AG, Berlin, Germany; B. Braun Melsungen AG, Melsungen, Germany; Boehringer Ingelheim (Schweiz) GmbH, Basel, Switzerland; Bristol-Myers-Squibb, Rueil-Malmaison Cedex, France, and Baar, Switzerland; CSL Behring GmbH, Hattersheim am Main, Germany, and Berne, Switzerland; Curacyte AG, Munich, Germany; Daiichi Sankyo (Schweiz) AG, Thalwil, Switzerland; Ethicon Biosurgery, Sommerville, NJ, USA; Fresenius SE, Bad Homburg v.d.H., Germany; Galenica AG, Bern, Switzerland (including Vifor SA, Villars-sur-Glâne, Switzerland); GlaxoSmithKline GmbH & Co. KG, Hamburg, Germany; Janssen-Cilag AG, Baar, Switzerland; Janssen-Cilag EMEA, Beerse, Belgium; Merck Sharp & Dohme AG, Luzern, Switzerland; Novo Nordisk A/S, Bagsvärd, Denmark; Octapharma AG, Lachen, Switzerland; Organon AG, Pfäffikon/SZ, Switzerland; Oxygen Biotherapeutics, Costa Mesa, CA; PAION Deutschland GmbH, Aachen, Germany; Photonics Healthcare B.V., Utrecht, the Netherlands; ratiopharm Arzneimittel Vertriebs-GmbH, Vienna, Austria; Roche Diagnostics International Ltd, Reinach, Switzerland; Roche Pharma (Schweiz) AG, Reinach, Switzerland; Schering-Plough International, Inc., Kenilworth, NJ, USA; Tem International GmbH, Munich, Germany; Verum Diagnostica GmbH, Munich, Germany; Vifor Pharma Deutschland GmbH, Munich, Germany; Vifor Pharma Österreich GmbH, Vienna, Austria; and Vifor (International) AG, St. Gallen, Switzerland. The remaining authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Meybohm, P., Froessler, B., Goodnough, L.T. et al. “Simplified International Recommendations for the Implementation of Patient Blood Management” (SIR4PBM). Perioper Med 6, 5 (2017). https://doi.org/10.1186/s13741-017-0061-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13741-017-0061-8