Abstract
Background
Concerns about the sustainability of commercially available batteries have driven the development of post-lithium systems. While previous studies on Magnesium batteries have explored both the potential environmental footprint of battery production and their possible use in stationary applications, their environmental impact in electromobility remains unexplored. This study provides an initial prospective evaluation of the environmental performance of a theoretical Mg–S battery for potential use in electric vehicles (EVs). Utilizing life cycle assessment (LCA) methodology, various scenarios are analyzed and compared to conventional systems. The analysis focuses on potential environmental impacts, including climate change, resource criticality, acidification of the biosphere, and particulate matter emissions.
Results
In the battery pack level, the Magnesium anode and its respective supply chain have been identified as main drivers of environmental burdens. Additional concerns arise from the uneven geographical distribution of Mg production, which leads to dependency on few producers. In terms of resource criticality, the Mg–S battery could carry significant advantages over benchmark systems. A look into the use-phase via theoretical implementation in an electric vehicle (EV) also suggests that the Magnesium based EV could perform on a comparable level to an LIB EV, also outperforming conventional ICEVs in several impact categories.
Conclusions
This study is based on optimistic assumptions, acknowledging several remaining technical challenges for the Mg battery. Consequently, the results are indicative and carry a significant degree of uncertainty. Nonetheless, they suggest that the Mg–S system shows promising environmental sustainability performance, comparable to other reference systems.
Similar content being viewed by others
Introduction
The battery market is anticipated to expand significantly over the next few decades as a consequence of growing interest in renewable energy technologies and electric vehicles, which has brought the need for advanced storage systems to the forefront [1]. The installed capacity of storage systems is predicted to increase from 278 GWh in 2021 to over 6300 GWh in 2040 for electromobility and from ~ 56 GWh in 2021 to ~ 426 GWh in 2040 for stationary applications [2].
So far, lead acid (PbA) batteries and lithium-ion batteries (LIB) have been the most widespread technologies in the market [2, 3], owning advantages and drawbacks over other systems, as well as concerns regarding their sustainability. Among the latter, use of toxic materials [4], scarce and critical resources [5,6,7] and the use of child and forced labor [8], among others, have brought these systems under scrutiny.
Different strategies have been developed to mitigate some of these issues. An example is the establishment of a recycling industry to increase resource efficiency, successfully implemented for PbA batteries [9, 10] and with several initiatives focused on LIBs [11,12,13,14,15]. Another example is the development of new alternative systems, such as post-lithium batteries [16], seeking to use less critical resources in their construction. Sodium-(Na) and magnesium-(Mg)-based batteries [17, 18] can be found within this category, among which the magnesium–sulfur (Mg–S) battery stands out due to its promising theoretical capacity, cost-efficiency, safety profile [19]. Beyond the numerous technical challenges that these technologies still need to overcome, the widespread implementation of these systems must also adhere to environmental, economic, and social sustainability standards. To ensure this, technology monitoring is needed throughout the different stages of development, from initial concept design to final commercialization. This process should evaluate the potential implications of raw materials extraction, manufacturing, use-phase and end-of-life (EoL) management of the system. This type of analysis is typically conducted employing the life cycle assessment (LCA) methodology, a systematic approach that evaluates the potential environmental impacts over the life cycle of a product [20]. For emerging systems, however, considerable data limitations and large uncertainties exist, hindering the conduction of typically retrospective LCAs. Instead, an anticipatory or prospective LCA (P-LCA) [21] can be used to support the technology development process from its early stages when design flexibility for impact mitigation exists. Prospective LCA is especially characterized by embracing uncertainty throughout the analysis of potential technology development scenarios and supply chain transformations [21].
Little details are known yet about the potential environmental implications of commercializing an Mg–S battery. In the following, and to the best of the author’s knowledge, the first assessment of a theoretical Mg–S battery for use in electro mobility is presented to fill in this gap, addressing the growing attention that Magnesium batteries are receiving and to evaluate their sustainability character. This evaluation shall provide insight into its environmental hotspots and the role that the successful development of this technology could play in decarbonizing the transport sector. Conducting these initial evaluations was possible with the first tested pouch-cell prototype built within the framework of the Mag–S project, funded by the German Federal Ministry of Education [22]. The analysis here conducted gives continuity to previous studies [23,24,25] that have already evaluated the potential environmental impacts of hypothetical Mg–S battery production and use in stationary applications.
Previous research on magnesium batteries
Over the last decade, significant progress has been made in the field of Mg batteries. A thorough overview [19] described their advantages over conventional systems, for instance the bivalency of the Mg ion, the high volumetric storage capacity of the anode and the non-toxic and abundant nature of Mg. Some of the recently listed advances in the field are mainly associated with the development of electrode materials and suitable electrolytes. The authors also discuss the scientific and technical challenges that this type of batteries is facing, such as lack of suitable cathode materials and over-potentials, among others. The study of Mg–S batteries has been part of comprehensive reviews about the state-of-the-art, [26,27,28,29] with critical issues such as compatibility of electrodes and electrolyte remaining. A few examples of R&D to overcome technical challenges [30,31,32,33,34,35,36,37,38,39] have focused on the development of cathode materials and electrolytes. Li et al. [40] conducted research on Mg alloys for the anode as a potential tool for improved energy densities. Häcker et al. [41, 42] and Attias et al. [43] have investigated the interactions at the battery interfaces and the impact of temperature on the rates of self-discharge and sulfide formation.
Regarding sustainability assessments, the first LCA [23] on Mg battery production was based on a prototype cell [22] followed up by a study based on a theoretical redesign of such prototype [24]. This redesign focused on reducing battery weight, potentially leading to significant decrease of its environmental impacts, down to a level comparable to commercial technologies. This study was further expanded by Pinto-Bautista et al. [25], who analyzed the potential impacts of the use-phase in stationary applications. The results reaffirmed the promising environmental performance of the Mg–S battery and provided insight into the influence that its technical performance may have on its environmental footprint. For instance, it was found that the charge/discharge efficiency bears larger influence on the results than other parameters such as energy density or calendrical life. Nonetheless, while these studies centered on battery production and potential use in stationary applications, data gaps about the role that the Magnesium battery could play in electromobility remain. The work presented in the following aims to reduce some of these gaps.
Life cycle environmental analysis
To determine the potential environmental performance of a Mg–S battery pack for electromobility, a prospective life cycle assessment (LCA) is conducted following the guidelines defined in the ISO standards 14,040/14,044 [44, 45] and the International Reference Life Cycle Data System ILCD handbook [46]. Four steps are executed in an iterative and interdependent manner during the conduction of this LCA: goal and scope definition, inventory analysis, life cycle impact assessment and interpretation of the results.
Goal and scope definition
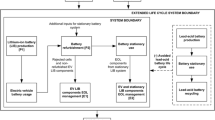
The goal of the study is to estimate the environmental footprint of an electric vehicle equipped with an Mg–S battery pack and to compare its performance with benchmark technologies such as an LIB electric vehicle (LIB EV) and an Internal Combustion Engine Vehicle (ICEV). The system boundaries include raw material extraction, system manufacturing, and use-phase, as illustrated in Fig. 1. Lack of data impedes the inclusion of EoL management and/or recycling within the boundaries. The study is divided in two parts to ease the discussion of environmental hotspots of the battery pack as well as the comparison of technologies in electromobility. First, an assessment of battery manufacturing (cradle-to-gate analysis) is conducted using a functional unit (FU) of production of a 40 kWh battery pack. Second, an assessment of vehicle production and use (cradle-to-use analysis) is conducted with the FU being production of a 40 kWh electric vehicle driven 150,000 km over a lifetime of 10 years. This lifetime also corresponds to the calendar life for the battery; therefore, no replacements are considered during the use-phase.
Modelling of the battery system
The Mg battery system studied here is namely the MgS-Evo2 presented by Montenegro et al. [24]. The anode is made of a 100 µm thick magnesium foil. The cathode is composed of sulfur (50 wt.%), carbon black (40 wt.%) and carboxymethyl-cellulose/styrene–butadiene–rubber binder (CMC/SBR) (10 wt.%), applied onto an aluminum foil [22]. The electrolyte is a solution of magnesium tetrakis hexafluoroisopropyloxy borate, also known as Mg[B(hfip)4]2, and dimethoxyethane (DME), and was first described by Zhao-Karger et al. [34].
This battery is at a stage of basic property research and electrochemical development, and thus can be characterized with a Battery Component Readiness Level (BC-RL) of three [47]. Several technical hurdles must be addressed to achieve satisfactory performance, including limited cycle stability, inadequate sulfur stabilization, and quick self-discharge rates. Its performance is also hindered by potential hysteresis between charge and discharge cycles and the degradation of the electrolyte and the magnesium anode due to polysulfide dissolution [30]. Nonetheless, promising results were observed during the first testing phase of the cell [22].
This study relies on optimistic performance values of the Mg–S battery pack, assuming that further research and innovation will push the Mg–S system to a BC-RL of 9, comparable to that of NMC chemistries. The system presented here is a hypothetical model that combines characteristics from the redesigned prototype and from LIBs. Specifically, the energy density reflects a reassessment of the initial value suggested for the prototype cell, considering the effects of mass reduction resulting from the proposed redesign [24]. In the pack level, a similar composition to that of an LIB pack has been assumed. Table 1 shows the material and mass composition of the model Mg–S battery as well as that of a state-of-the-art LIB (NMC811) as described by Chordia et al. [48], which has been used as benchmark due to the availability of data sets that allow its modelling. The composition at the pack level for both systems is based on the battery pack for electric vehicles (EV) presented by Ellingsen et al. [49], readjusted for 40 kWh of storage capacity. Regarding system efficiency, a previous study [25] assumed charge/discharge (i.e., roundtrip) efficiency of 90% for the Mg–S battery under the presumption that this technology would likely underperform the LIB, for which an efficiency of 95% is a commonplace value. This difference at the pack level is also reflected at vehicle level, where battery efficiency has an influence on the overall EV efficiency, altogether with the efficiencies of power electronics, motor, drivetrain and accessory loads. When all other factors remain the same, a simple division of the battery efficiencies will indicate performance ratio between two vehicles using different types of batteries. Assuming 90% and 95% roundtrip efficiency for the Mg–S battery and LIB, respectively, means that the Mg–S EV performs approximately with 95% of the energy efficiency of LIB EV. Therefore, a factor of 0.95 has been introduced in the calculation of the energy efficiency of Mg–S vehicle (Table 1). For the LIB vehicle, this factor is 1, which corresponds to the energy efficiency directly reported by the manufacturer of an LIB EV. It should be noted that in a practical application, the power electronics of an Mg–S battery could be different from those of an LIB, potentially leading to additional differences in efficiency, which have been neglected here. Finally, it is assumed that both batteries have a calendar life of 10 years, a common and conservative value for LIBs [50], and a cyclability which is sufficient to meet the lifetime of the intended application, avoiding the need of battery replacements over this time.
Use-phase modelling
The implementation of the battery pack has been studied throughout the modelling of a full vehicle, which integrates, aside of battery pack, production of glider and of the charging infrastructure. Two electric vehicles (Mg–S EV, LIB EV) have been modelled with use-phase impacts related to production of electricity to power the vehicle, wear of components and vehicle/road maintenance. In addition, a comparison with an ICEV powered with diesel illustrates the potential advantages and disadvantages of one type of technology over the other. It is assumed that the EVs are operated in Germany between the years 2025–2035, thus the forecast of the German electricity mix for 2030 [51] has been considered as reference to charge the electric vehicles.
Due to its market popularity, the medium sized Nissan Leaf has been selected as the model EV. Table 2 shows the technical specifications as originally presented by the manufacturer, including the energy efficiency of three driving behaviors (city, highway and combined) and under mild weather conditions [52]. These values have been reported in accordance with the Worldwide Harmonized Light Vehicle Test procedure (WLTP) [53]. It must be noted that, in practice, energy consumption may differ from the values established by the WLTP when the individual driving profiles, charging behaviors or vehicle related parameters, such as tire pressure, do not match those defined for the test. In addition, the excessive use of ancillaries or extreme weather conditions can also reduce the autonomy of the vehicle [54]. The vehicle is originally commercialized with a 40 kWh lithium ion manganese oxide (LMO) battery pack which, in this study, has been substituted with a model Mg–S battery pack as well as with a state-of-the-art NMC 811 LIB pack. The curb weight is subsequently readjusted to account for the energy density of each battery pack, leading to a total weight of approximately 1529 kg and 1561 kg for the Mg–S and the LIB EVs, respectively. Accordingly, energy consumption must be recalculated considering the weight differences via a correction factor of 5.6 Wh*km−1 per 100 kg of additional weight following the recommendations from Ellingsen et al. [49].
The Volkswagen Golf 2020 has been chosen as benchmark ICEV given its comparable weight and size to that of the EV, also falling into the medium-size category. Table 3 shows the curb weight and fuel consumption reported in the technical specifications from the manufacturer and under the same standardized testing regulation (WLTP) [55]. In contrast to EVs, the overall energy efficiency of ICEVs is higher when driving in highway conditions. Regarding tailpipe emissions, these are assumed compliant with the currently enforced European emission standard Euro 6 for a medium sized passenger car, which determines the maximum amount of specific pollutants that can be released into the atmosphere and which has been established since 2015 [56].
Data sources, limitations and other assumptions
Different literature sources and commercial datasheets have been used to build the life cycle inventories. The inventories of the Mg–S cell were extracted from Montenegro et al. [24]. The inventories of the LIB (NMC811) cell were extracted from the work by Chordia et al. [48], noting that the graphite in the anode (originally “market for graphite, battery grade—GLO” as found in Ecoinvent) was substituted with graphite as presented in the life cycle inventories found in Engels et al. [57]. This is due to underestimation of the environmental footprint of graphite in previous studies. Due to lack of data regarding the construction of a Mg–S battery pack, it was assumed that its layout is similar to that of an LIB, thus components such as packaging, battery management system (BMS) and cooling system are the same for both technologies (extracted from Ellingsen et al. [58]). In particular, the cooling system is composed of an aluminium radiator with glycol as coolant medium, whereas the BMS includes module boards, interface system, fasteners and high/low voltage systems. In reality, the layout of the Mg–S battery pack could be different from that of LIBs, for instance, by necessitating a different type of BMS or cooling system. The original mass compositions of the Mg–S and LIB packs were extracted from literature [49] and readjusted according to the respective energy densities used herein. It must be noted that the inventories of the LIB relate to production of cylindrical cells, whereas the Mg battery is composed of pouch cells. This leads to mass imbalances from the BMS and housing, with direct influence in the calculation of energy density of the pack. The sensitivity analysis addresses this issue, estimating the environmental impacts of the Mg battery for a range of values of energy density. It has been assumed that the manufacture of the battery packs takes place in Europe, and therefore, the ´European Network of Transmission Systems Operators for Electricity ENTSO-E’ was used as electricity mix.
The body of the vehicles has been modelled using the commercial database Ecoinvent 3.8, with cutoff data sets for medium sized passenger car production. These include background inventories for glider (chassis, steering, braking and suspension system, tires, cockpit equipment and non-propulsion related electronics) and powertrain. A vehicle lifetime of 150,000 km driven over 10 years has been assumed as this is a commonplace value in literature. It is assumed that the calendar life of the battery is 10 years as well; thus, no battery exchange has been considered during this lifetime. It is likely that the vehicles (ICEV and EVs) and batteries perform acceptably after reaching this lifetime. Environmental credits could thus be obtained from longer battery/vehicle lifetime, recycling or second life of batteries, but these scenarios are out of the scope in this study. The electricity mix forecast for Germany in 2030, namely DE2030, has been modelled according to the report from Agora Energiewende [51] and has the following composition: Wind = 32.4%; PV = 11.57%; Hydro = 3.5%; Biomass = 4.3%; Lignite = 10.1%; Hard coal = 14.4%; Oil = 0.2%; Natural gas = 19.2%; Others = 4.5%. Other use-phase flows such as road construction, vehicle maintenance, brake wear, road wear and tire wear emissions have been considered as presented in the Ecoinvent 3.8 database for transport with a medium sized passenger electric car. The public charging station for EVs has been modelled as presented by Zhang et al. [59] in their assessment of charging infrastructure. It has been assumed that, per electric vehicle, the impacts from 1/3.5 of a complete charging station must be allocated, following the recommendations provided by RISE Viktoria [60] (assumed to be applicable in Germany). The tailpipe emissions during the use-phase of the ICEV have been modelled based on the Life Cycle Inventories of Road and Non-Road Transport Services [56] for a medium-sized diesel fueled passenger car compliant with the Euro 6 emission standard. Additional use-phase impacts of the ICEV arise from the supply chain of the fuel (low-sulphur diesel), the supply chain of the refrigerant and car maintenance have also been extracted from Ecoinvent. No additional fuel station infrastructure for ICEVs was considered as the already existing one is consider sufficient. In the sensitivity analysis, scenarios with other energy sources have been evaluated. An scenario with 100% renewable electricity (50/50 share from onshore wind power and open-ground photovoltaics installed in Germany), which substitutes the baseline 2030 German mix, was modelled with background data sets corresponding to 1–3 MW turbines and 570kWp multi-Si installations as found in Ecoinvent 3.8. Fossil Diesel has been substituted with biodiesel, represented via ´Fatty acid methyl esther’ provided by the global market of vegetable oil as found in Ecoinvent. Other elements in the background from pack and vehicle have also been extracted from Ecoinvent cutoff data set, which considers use of primary materials. The life cycle inventories constructed or edited within this study can be found in the supplementary information.
Life Cycle Impact Assessment
The method provided by the International Reference Life Cycle Data System (ILCD), specifically ILCD Midpoint 2011+, is chosen to conduct the LCIA, as it is representative of the Product Environmental Footprint (PEF) method and thus contains the impact categories recommended by the European Commission in the Product Environmental Footprint Category Rules for rechargeable batteries [61]. These categories are, first, ‘acidification’, which indicates the potential damage to soil and waters from the release of acidifying agents and is measured in molc (moles of charge) H+ eq. Second, ‘Climate change’ or “global warming potential” (GWP), which is related to the emission of CO2 and other greenhouse gases and is measured in kg CO2-eq. Third, ‘mineral, fossil and renewable resource depletion’ (kg Sb eq), which is related to the extraction of abiotic resources. Lastly, ‘particulate matter’ (kg PM2.5 eq), which considers the effects of fine particulates with an aerodynamic diameter of less than 2.5 µm. The specific case of (abiotic) resource depletion has been subject to debate, since some of the existing methodologies sometimes can lead to very different outcomes with each other, hampering the robustness of a study and/or hindering the interpretation process [62]. These differences can be attributed to the use of different perspectives when addressing the concept of criticality, which highlights the importance of selecting a method that suits the goal of the study [63, 64]. A recent study [65] suggests that an adequate reflection of potential resource criticality issues can be achieved by calculating the abiotic depletion impacts via the non-baseline versions of the CML method, developed by the Institute of Environmental Science of Leiden University in the Netherlands [66]. The author concludes that the ultimate reserve version, reflecting long-term criticality issues based on physical reserves, and the economic reserve version, reflecting short-term issues, should be used complementarily in the assessment of batteries for EVs. The above mentioned categories are analyzed in this document, and a complete look of the results in the 16 impact categories of the ILCD method is provided in the supplementary information.
Results and discussion
Battery pack
Figure 2 displays the total impacts of a 40 kWh Mg–S battery pack in several impact categories, breaking down the results into contributions from each battery pack and cell component. The group 'others' relates to contributions from material transportation and construction of infrastructure. Large contributions to the total results originate within the supply chain of Magnesium for the anode, especially critical in the ‘particulate matter’ category. These impacts are associated with the production of Magnesium via the industry standard Pidgeon process. This type of process is highly polluting yet widely popular, especially in China, where over 80% of the global magnesium is produced [67]. The extraction and processing of primary aluminium for the module packaging contributes significantly to climate change, acidification and resource depletion. In the latter category, the construction of the BMS leads to the largest contributions, which mostly arise from the use of Tantalum in the electronic components, material characterized as critical resource.
Figure 3 presents a comparison of the environmental profile of the Mg–S battery pack with an LIB benchmark, specifically an NMC 811. The results suggest potentially better performance from the Mg–S system in most categories, with the exception of ‘particulate matter’, where the contribution of the Magnesium anode leads to higher total impacts. In climate change, the LIB bears larger impacts stemming from the pre-chain of the active material in the electrodes, namely graphite in the anode and nickel/cobalt sulfate in the cathode. It can also be seen that, regardless of the method used for the analysis of resource depletion potential, the Mg–S battery bears about 14–27% of the impacts from the LIB. The LIB performs poorly due to the use of critical Cobalt and Nickel for the synthesis of the active material in the cathode. The high impacts of the LIB in acidification also originate in the supply chain of the active material, with significant contributions from the upstream processes of nickel sulphate production, where large amounts of Sulphur dioxide (acidifying agent) are emitted into the atmosphere. The Mg–S battery benefits instead from the use of less critical active materials.
Life cycle impact of electric vehicles
Energy efficiency during vehicle operation is influenced by conditions of the environment such as road type and climate as well as driving style and use of ancillaries. In this analysis, average weather conditions and average driving style have been considered during the use-phase of the vehicles. Figure 4a illustrates the cradle-to-use greenhouse gas emissions of the three vehicles under baseline conditions (German mix for 2030) and for a scenario with renewable electricity (RN) in a 50–50 ratio of wind and solar. The emissions are initially comprised by the impacts from vehicle (glider + powertrain) and battery pack production, as well as from the construction of the EV charging station. In the baseline, it can be seen that the accumulated emissions of the Mg–S–EV would remain higher than those of the ICEV until reaching a point of ~ 26,500 km driven, where the additional emissions from the battery pack are compensated by the less pollutant nature of its use-phase. In the RN scenario, this point is located at ~ 16,000 km. With respect to the LIB EV, the Mg–S EV would carry slightly lower impacts along the entire use-phase, benefiting from a lesser CO2 intensive construction of the battery pack. The accumulated life cycle impacts (Fig. 4b) result in comparable values for both technologies, with almost negligible contributions stemming from constructing EV charging infrastructure. The total cradle-to-use emissions of the Mg–S EV, the LIB EV and the ICE vehicles add up to about ~ 24,800, ~ 26,500 and ~ 37,500 kg of CO2-eq, respectively. When renewable electricity is used to charge the vehicles, the magnitudes add up to about 41–46% of the total impacts from the ICEV. Values in literature range between 28,000 to 60,000 kg of CO2-eq for ICEVs and 13,000 to 32,000 kg of CO2-eq for LIB EVs [49, 68,69,70,71,72,73,74,75], associated to their corresponding system boundaries. The overall balance of CO2 emissions largely depends on the specific conditions and modelling parameters of each study, for instance composition of the energy mix and driving behavior, which lead to a broad range of potential results.
Figure 5 illustrates the aggregated CO2-eq emissions for different driving conditions (average, city and highway). Changes in energy/fuel efficiency of the EVs, mainly associated with braking regeneration, result in better performance when driving in city conditions. Conversely, battery efficiency of EVs decreases at higher driving speeds, which are commonly employed on highways. The ICEV benefits from driving at optimal speeds on highways and is less efficient when driving in city conditions. The impact ratio between ICEV/Mg–S is approximately 1.76 in the city and 1.35 on the highway when using DE2030 electricity. Likewise, the ratios between LIB/Mg–S are approximately 1.07 in the city and 1.06 on the highway for the same electricity mix.
Regarding acidification potential, the results suggest that the Mg–S vehicle could outperform the benchmark, accounting for only about 75–77% of the impacts of the ICEV and the LIB EV (Fig. 6). On one hand, the use-phase of the ICEV contributes largely to its acidification potential. This is mostly related to the emission of Nitrogen oxides produced during fuel combustion, as well as within the supply chain of diesel, where Sulphur dioxide is also released into the atmosphere. EVs do not directly emit such types of emissions. The contributions of the use-phase for both batteries are almost identical, mostly depending on the electricity mix used to charge the batteries However, in the case of the LIB EV, significant emissions of acidifying agents are found within the supply chain of the cathode active material. This is mostly related to nickel sulphate production, as well as during the production of copper and battery-grade graphite for the anode, which are absent in the Mg battery.
Regarding resource depletion potential, Fig. 7 illustrates the abiotic resource depletion potential using different assessment methods. The differences in the results are attributed to the different characterization factors of each method. The CML-baseline method yields much lower values overall, whereas the non-baseline version with an economic focus results in the largest magnitudes. However, regardless of the method, a trend can be observed. In particular, the LIB EV carries the largest impacts, followed by the Mg–S EV in the second place and the ICEV with the lowest total impacts. The EVs have larger impacts than the ICEV due to several reasons. Apart from the battery, the powertrain of EVs contains significant amounts of valuable resources such as copper and neodymium used in the electric motor, which are not present in the powertrain of an ICEV. Traces of gold can also be found in the power electronics such as the inverter, charger, and power distribution units, which have a considerable influence on the results. In addition, the resources employed in the batteries and the minimal contributions from the use-phase contribute to the observed picture. It should be noted that this analysis does not consider criticalities and bottlenecks that could potentially arise from the geographical distribution of such materials.
Regarding particulate matter potential, the Mg–S EV is outperformed by both the ICEV and the LIB EV. The impacts of Mg–S battery production are significantly large, resulting in poorer overall performance. As previously described, the production of Magnesium via the Pidgeon process in China is the main driver of impacts in this category. While the ICEV and LIB perform at very similar levels, the total impacts of the Mg–S EV are about 7% higher (Fig. 8). Other sources of particulate emissions related to the use-phase include tire and road wear, which account for almost half of the total.
Sensitivity analysis
The impacts associated with the use-phase could be influenced by technical performance parameters of the battery, such as energy density and system efficiency. However, these parameters are characterized with large uncertainty during the early phase of technological development. In addition, the future conditions in which the technology could be rolled out may be different than the current ones. For instance, transformations of the supply chain or energy mix influencing the environmental footprint of the technology could be expected during long timespans between conception and market introduction. Thus, it becomes relevant to assess different potential scenarios in which the technology may develop to reveal the sensitivity of its environmental profile to a changing environment.
The theoretical energy density in the baseline analysis (~ 159 Wh/kg) suggested by the developers with an optimistic view could be distant from the technically achievable one. It is of interest to analyze how the environmental profile could look like under inferior performance levels. A recalculation of the results is given considering a decrease in energy density down to 50% of the initial estimation (~ 79.5 Wh/kg), with corresponding readjustment of vehicle weight. Figure 9 displays the cradle-to-use greenhouse gas emissions of the Mg–S EV under this condition. With respect to the ICEV, the breakeven point shifts from 26,500 km driven to about ~ 47,000 km in the DE2030 scenario and from ~ 16,000 km to 33,000 km in the RN scenario.
Beyond the sensitivity of the environmental footprint to energy density, other performance parameters may also have large influence on the results. Performance parameters such as charge/discharge (roundtrip) efficiency, cycle life and calendrical life were evaluated in a previous study [25], which identified battery efficiency as a relevant factor affecting environmental performance. Therefore, sensitivity to overall system efficiency is assessed here for a range of values between 0.95 or 95% efficiency (EFF) with respect to LIB EV (baseline) and 0.8 or 80% with the same reference as a lower limit. In addition, the reference ICEV system is re-evaluated with the adoption of biofuels, considered as a more sustainable alternative to conventional fossil fuels. Two fuel substitution scenarios have been modeled: B20, a blend of 20% biodiesel and 80% fossil-based diesel, and B100, comprising 100% biodiesel. It is noted that biodiesel has a lower energy density compared to regular diesel, with reported values of 99% and 93% for B20 and B100, respectively [76]. This leads to increased fuel consumption, factored into the model. ‘Vegetable oil methyl ester’ extracted from Ecoinvent 3.8 has been used to represent biodiesel. It has been assumed that, when it comes to use-phase emissions, biodiesel powered ICEVs also adhere to the same EURO6 standard as in the base case (fossil diesel) and thus the same admissible limit has been considered. Figure 10 depicts the total impacts for the three different vehicle configurations under the described conditions.
Results of the sensitivity analysis for EVs and ICEV. EFF overall EV efficiency (influenced by charge/discharge efficiency of the battery), Baseline Electricity mix Germany 2030 (EVs) and fossil diesel (ICEV), RN Renewable energy 50/50 solar–wind, B100 Diesel 100% biodiesel, B20 80% biodiesel / 20% fossil diesel, % Energy density percentage of the Mg–S battery energy density in the baseline (158.8 Wh/kg) to account for uncertainty. For the Mg–S EV, results are presented as a range of potential values (striped area) according to system efficiency EFF (0.8–0.95) and energy density (50–100% of baseline value)
A look into the GWP suggests that the EVs outperform the ICEV in any scenario. In addition, the use of renewably sourced electricity to charge the EVs drastically lowers the total impacts. For the Mg–S vehicle, when system efficiency decreases to EFF = 0.8, greenhouse gas emissions increase by approximately 2500 kg CO2-eq (if the baseline electricity mix is used), regardless of energy density. Regarding the ICEV, the use of B20 diesel has little influence on the impacts, but substituting regular diesel with B100 could lead to a reduction of ~ 13% of the total GWP. This can be attributed mostly to the carbon capturing process during the growth of biomass (e.g. rapeseed, soybean, etc.) used for fuel synthesis. With regards to the acidification potential, the impacts of the EVs slightly decrease when using renewable sources instead of the fossil intensive energy mix. For ICEVs, because of the acidifying potential of ammonia used in fertilizers for biomass harvesting, the impacts in this category drastically increase with the use of biodiesel. In resource depletion, little variations can be observed between scenarios. This is partly due to the little contributions that the use-phase has in this category. On top of that, the Mg–S battery pack is, in principle, not resource-critical, retaining this character even when pack size increase as consequence of lower energy densities. ICEVs perform better than the EVs in this category because of their simpler and less resource intensive construction. Similarly, little fluctuations are observed in ´particulate matter´ given that the contributions of the use-phase have origin in several sources beyond energy carrier, thus changes in the supply chain of fuel (ICEV) or energy mix (EVs) have minor effects on the total impacts. The most significant variations can be seen in the B100 scenario for the ICEV, related to land transformations for the harvesting of biomass, and in the poorly performing scenarios of the Mg–S EV, associated with a larger battery pack and consequently higher demand for Magnesium sourced in China.
Discussion
It must be emphasized that the Magnesium battery analyzed herein corresponds to an ideal model based on a prototype cell distant from market readiness. Further research is necessary to overcome the technological constraints that currently limit the performance of this system. The present analysis has an optimist character that neglects some of these limitations relying on technological innovation to materialize the assumptions made. A discussion from a prospective point of view provides only indicative results that are subject to a large degree of uncertainty but that may highlight relevant issues as well as potential advantages and disadvantages of the system.
The analysis of the battery pack indicates that production of magnesium for the anode contributes largely to the total impacts in several categories. This large contribution is associated mostly with the market-dominating route (Pidgeon process) for production which takes place in China. Market expansion achieved by introducing cleaner suppliers as well as by increasing the share of secondary material i.e. recycled Magnesium could partially mitigate these impacts. With respect to resource criticality, the use of abundant materials make the Mg–S battery a promising technology, bearing just about a quarter of the impacts of the reference LIB pack. Nevertheless, despite its widespread abundancy on the Earth’s crust [67], Magnesium is considered a critical material by the European Union [77]. Conventional LCA methods do not reveal this situation since they determine resource criticality based on the estimated reserves on in the Earth’s crust and material extraction rates. However, these methods do not consider the effects from external factors such as international trade regulations, resource distribution, social/political instability, etc. that could impact the supply chain. In particular, the criticality of magnesium arises primarily from the uneven concentration of production plants, which creates dependency on imports. Global production of magnesium is heavily concentrated in China, which controls over 83% of the market [67, 78]. Advantageous local conditions allow for low production costs leading this country to dominate the market [79]. Europe imports about 93% of its total demand from China [77], leading to significant dependency on a single supplier. If the demand for Magnesium were to increase as a consequence of introducing Mg based batteries into the market, potential supply risks may arise. Therefore, strategies to diversify the supply chain such as incentivizing domestic production or recycling may become essential to mitigate this issue [80].
In general, the results suggest that the Mg–S system could play a significant role in the decarbonisation of the transportation sector. Based on optimistic assumptions, this type of battery could perform comparably or even better than the reference systems. Overall superior performance was found in GWP and acidification potentials, attributed mainly to the lesser criticality of the supply chain of electrode active materials. In resource criticality, the Mg–S EV outperforms the LIB EV but entails higher impacts than the ICEV. The manufacturing of an electric vehicle is more resource-intensive than that of an ICEV due to the additional components in the powertrain, on top of the additional resources needed for the battery pack. However, the analysis of the end-of-life phase of the vehicles has been left out of the study due to insufficient knowledge of potential pathways for the processing of the Mg battery. In addition, the cutoff version of Ecoinvent 3.8 has been used to model the elements in the background considering use of primary materials. If recycling and use of secondary materials were to be considered, the impacts of glider and powertrains would be largely compensated for any vehicle and battery recycling would become crucial to quantify the environmental impacts of the EVs. Recycling of LIBs has already been the subject of different studies [11,12,13, 15], which identified large potential for environmental impact mitigation while also indicating that the benefits and economic viability highly depend on the battery composition and valuable elements within it. For magnesium batteries, the abundancy and consequently the low cost of the resources used may pose economic challenges for the establishment of a dedicated recycling industry. Little profit could be made from recovery of basic components, and it is uncertain to which degree the intrinsic environmental impacts of the recycling activity could be mitigated. To make recycling cost-effective, the revenue of material extraction must be higher than the costs of recycling itself, which has been proven already difficult for other emerging markets such as recycling of SIBs, for which costs of pyrolysis- and hydrometallurgy-based recycling are comparable or even higher than the practical revenue of elemental extraction [81, 82]. Due to the uncertainty surrounding the development of the Mg–S system, it is unfeasible to propose a plausible recycling model at this stage. However, a key strategy to enhance the economic viability of recycling would be to consider design for recycling already during the development stages of this technology. This could be achieved, for instance, by using more simple architectures that facilitate material separation and enable low effort and effective recycling. An example of a simpler design can be found in [83], where the authors describe a SIB containing a single current collector, which ultimately allows for high material recovery rates at low costs. In this sense, easy recovery of magnesium foil could partially alleviate the criticality associated to import dependency of this material and associated environmental impacts caused by primary production. Another example of design for recycling is to use water-soluble binders that can be easily removed in contrast to conventional binders, or the exclusion of toxic components, which would simplify the material separation steps [84].
From the sensitivity analysis, it can be inferred that the impacts of the use-phase are heavily influenced by the composition of the electricity mix used to charge batteries. In general, battery efficiency becomes less critical with a higher penetration of renewables, where contributions from the use-phase also diminish. In the case of lower energy densities, despite the additional emissions arising from higher energy demand in heavier vehicles, a very slight increase in the total impacts can be observed. The sensitivity analysis was conducted for average driving behavior and weather conditions, which could be further complemented by considering the effects of other conditions beyond the average, as well as considering the influence of specific events such as cold starts.
Conclusion
In this study, the environmental profile of a theoretical Magnesium–Sulfur (Mg–S) used in electromobility has been evaluated from a prospective view. This analysis has been performed based on a prototype and a series of optimistic assumptions that do not correspond to the performance of the Mg–S battery at its current state. The premise is that, with further research, this technology could eventually overcome the current technical constraints that hamper its performance. With a focus on its environmental sustainability, the potential impacts have been analyzed into detail in four categories of concern: climate change, acidification, particular matter and resource criticality (other categories are presented in the SI). In the analysis of a battery pack, it was found that the Magnesium anode and its respective supply chain are the main drivers of environmental burdens, mostly related to the primary production pathway of Mg that takes place in China. Additional concerns are associated with the uneven geographical distribution of Mg production, which leads to dependency on few producers located mostly in China. Decentralized market expansion and recycling could become vital tasks to promote a more sustainable character of this technology in the case of an eventual rollout. A comparison with an LIB suggests that, when considering resource criticality, the Mg–S battery carries significant advantages, while still performing on a competitive level in other categories. A look into the use-phase via theoretical implementation in an electric vehicle (EV) also suggests that the magnesium-based EV could perform at a comparable level to an LIB EV, also outperforming conventional ICEVs in several categories with the exception of resource depletion. The absence of a battery pack and simpler power train composition grants ICEVs advantages over EVs in this category. The establishment of a battery recycling industry with high recovery rates could mitigate this issue. Lastly, if the technical challenges found at the current stage of development are overcome, the Mg–S battery could become an attractive alternative for the energy and mobility transition. Further research on new materials is still necessary, and a continuous assessment hand-in-hand with technology developers is also needed to ensure the sustainable character of this type of battery.
Availability of data and materials
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Emmerich P, Hülemeier A-G, Jendryczko D et al (2020) Public acceptance of emerging energy technologies in context of the German energy transition. Energy Policy 142:111516. https://doi.org/10.1016/j.enpol.2020.111516
M. Bielewski, A. Pfrang, S. Bobba, A. Kronberga, A. Georgakaki, S. Letout, A. Kuokkanen, A. Mountraki, E. Ince, D. Shtjefni, G. Joanny, O. Eulaerts, M. Grabowska (2022) Clean energy technology observatory: batteries for energy storage in the European Union: 2022 status report on technology development, trends, value chains and markets, Luxembourg
Figgener J, Stenzel P, Kairies K-P et al (2020) The development of stationary battery storage systems in Germany—a market review. J Energy Storage 29:101153. https://doi.org/10.1016/j.est.2019.101153
Zhang J, Chen C, Zhang X et al (2016) Study on the environmental risk assessment of lead-acid batteries. Procedia Environ Sci 31:873–879. https://doi.org/10.1016/j.proenv.2016.02.103
Agusdinata DB, Liu W, Eakin H et al (2018) Socio-environmental impacts of lithium mineral extraction: towards a research agenda. Environ Res Lett 13:123001. https://doi.org/10.1088/1748-9326/aae9b1
Leader A, Gaustad G, Babbitt C (2019) The effect of critical material prices on the competitiveness of clean energy technologies. Mater Renew Sustain Energy. https://doi.org/10.1007/s40243-019-0146-z
Pistoia G, Liaw B (2018) Behaviour of lithium-ion batteries in electric vehicles: battery health, performance, safety, and cost. Green Energy and Technology, 1st edn. Springer International Publishing, Springer, Cham
Faber B., Krause B., De La Sierra R. Center for Effective Global Action Policy Report: Artisanal Mining, Livelihoods, and Child Labor in the Cobalt Supply Chain of the Democratic Republic of Congo, Berkeley
Sun Z, Cao H, Zhang X et al (2017) Spent lead-acid battery recycling in China—a review and sustainable analyses on mass flow of lead. Waste Manage 64:190–201. https://doi.org/10.1016/j.wasman.2017.03.007
Zhang W, Yang J, Wu X et al (2016) A critical review on secondary lead recycling technology and its prospect. Renew Sustain Energy Rev 61:108–122. https://doi.org/10.1016/j.rser.2016.03.046
Ciez RE, Whitacre JF (2019) Examining different recycling processes for lithium-ion batteries. Nat Sustain 2:148–156. https://doi.org/10.1038/s41893-019-0222-5
Mohr M, Peters JF, Baumann M et al (2020) Toward a cell-chemistry specific life cycle assessment of lithium-ion battery recycling processes. J Ind Ecol 24:1310–1322. https://doi.org/10.1111/jiec.13021
Sommerville R, Zhu P, Rajaeifar MA et al (2021) A qualitative assessment of lithium ion battery recycling processes. Resour Conserv Recycl 165:105219. https://doi.org/10.1016/j.resconrec.2020.105219
Zheng X, Zhu Z, Lin X et al (2018) A mini-review on metal recycling from spent lithium ion batteries. Engineering 4:361–370. https://doi.org/10.1016/j.eng.2018.05.018
Zhou L-F, Yang D, Du T et al (2020) The current process for the recycling of spent lithium ion batteries. Front Chem 8:578044. https://doi.org/10.3389/fchem.2020.578044
Choi JW, Aurbach D (2016) Promise and reality of post-lithium-ion batteries with high energy densities. Nat Rev Mater 1:16013. https://doi.org/10.1038/natrevmats.2016.13
Aurbach D, Gofera Y, Lu Z, Schechter A, Chusid O, Gizbar H, Cohen Y, Ashkenazi V, Moshkovich M, Turgeman R, Levi E (2001) A short review on the comparison between Li battery systems and rechargeable magnesium battery technology. J Power Sources 97:28–32
Ellis BL, Nazar LF (2012) Sodium and sodium-ion energy storage batteries. Curr Opin Solid State Mater Sci 16:168–177. https://doi.org/10.1016/j.cossms.2012.04.002
Fichtner M (2019) Magnesium batteries: research and applications. Energy and Environment Series. The Royal Society of Chemistry, Cambridge
Nathan C, Coles S (2020) Life Cycle Assessment and judgement nanoethics 14:271–283. https://doi.org/10.1007/s11569-020-00376-2
Thonemann N, Schulte A, Maga D (2020) How to conduct prospective Life Cycle Assessment for emerging technologies? A systematic review and methodological guidance. Sustainability 12:1192. https://doi.org/10.3390/su12031192
Wagner N, Fichtner M, Bremes H.G, Zwanziger I, Wolter C, Remmlinger J (2018) Entwicklung und Herstellung von wiederaufladbaren Magnesium-Schwefel Batterien “MagS” : Schlussbericht an die Technische Informationsbibliothek (TIB) (Development and production of rechargeable magnesium-sulfur batteries “MagS”: Final report to the Technical Information Library), Bonn
Montenegro CT, Peters JF, Zhao-Karger Z et al (2019) CHAPTER 13. Life cycle analysis of a magnesium-sulfur battery. In: Fichtner M (ed) Magnesium batteries: research and applications. The Royal Society of Chemistry, Cambridge, pp 309–330
Montenegro CT, Peters JF, Baumann M et al (2021) Environmental assessment of a new generation battery: the magnesium-sulfur system. Journal of Energy Storage 35:102053. https://doi.org/10.1016/j.est.2020.102053
Pinto-Bautista S, Weil M, Baumann M et al (2021) Prospective Life Cycle Assessment of a model magnesium battery. Energy Tech 9:2000964. https://doi.org/10.1002/ente.202000964
Fan C, Wei Q, Zhang L et al (2022) Research Progress of Magnesium Sulfur Batteries. In: Cabeza LF (ed) Encyclopedia of energy storage. Elsevier, Oxford, pp 158–170
Rashad M, Asif M, Ali Z (2020) Quest for magnesium-sulfur batteries: current challenges in electrolytes and cathode materials developments. Coord Chem Rev 415:213312. https://doi.org/10.1016/j.ccr.2020.213312
Wang P, Buchmeiser MR (2019) Rechargeable magnesium-sulfur battery technology: state of the art and key challenges. Adv Funct Mater 29:1905248. https://doi.org/10.1002/adfm.201905248
Zhao-Karger Z, Fichtner M (2017) Magnesium-sulfur battery: its beginning and recent progress. MRS Communications 7:770–784. https://doi.org/10.1557/mrc.2017.101
Zhao-Karger Z, Fichtner M (2018) Beyond intercalation chemistry for rechargeable Mg batteries: a short review and perspective. Front Chem 6:656. https://doi.org/10.3389/fchem.2018.00656
Zhao-Karger Z, Gil Bardaji ME, Fuhr O et al (2017) A new class of non-corrosive, highly efficient electrolytes for rechargeable magnesium batteries. J Mater Chem A 5:10815–10820. https://doi.org/10.1039/C7TA02237A
Zhao-Karger Z, Lin X-M, Bonatto Minella C et al (2016) Selenium and selenium-sulfur cathode materials for high-energy rechargeable magnesium batteries. J Power Sources 323:213–219. https://doi.org/10.1016/j.jpowsour.2016.05.034
Zhao-Karger Z, Zhao X, Wang Di et al (2015) Performance improvement of magnesium sulfur batteries with modified non-nucleophilic electrolytes. Adv Energy Mater 5:1401155. https://doi.org/10.1002/aenm.201401155
Zhao-Karger Z, Liu R, Dai W et al (2018) Toward highly reversible magnesium-sulfur batteries with efficient and practical Mg[B(hfip) 4 ] 2 electrolyte. ACS Energy Lett 3:2005–2013. https://doi.org/10.1021/acsenergylett.8b01061
Vinayan BP, Zhao-Karger Z, Diemant T et al (2016) Performance study of magnesium-sulfur battery using a graphene based sulfur composite cathode electrode and a non-nucleophilic Mg electrolyte. Nanoscale 8:3296–3306. https://doi.org/10.1039/c5nr04383b
Vinayan BP, Euchner H, Zhao-Karger Z et al (2019) Insights into the electrochemical processes of rechargeable magnesium–sulfur batteries with a new cathode design. J Mater Chem A 7:25490–25502. https://doi.org/10.1039/C9TA09155F
Wang P, Kappler J, Sievert B et al (2020) Characteristics of magnesium-sulfur batteries based on a sulfurized poly(acrylonitrile) composite and a fluorinated electrolyte. Electrochim Acta 361:137024. https://doi.org/10.1016/j.electacta.2020.137024
Wang P, Küster K, Starke U et al (2021) Performance enhancement of rechargeable magnesium–sulfur batteries based on a sulfurized poly(acrylonitrile) composite and a lithium salt. J Power Sources 515:230604. https://doi.org/10.1016/j.jpowsour.2021.230604
Lee B, Choi J, Na S et al (2019) Critical role of elemental copper for enhancing conversion kinetics of sulphur cathodes in rechargeable magnesium batteries. Appl Surf Sci 484:933–940. https://doi.org/10.1016/j.apsusc.2019.04.143
Li R, Liu Q, Zhang R et al (2022) Achieving high-energy-density magnesium/sulfur battery via a passivation-free Mg-Li alloy anode. Energy Storage Mater 50:380–386. https://doi.org/10.1016/j.ensm.2022.05.039
Häcker J, Danner C, Sievert B et al (2020) Investigation of magnesium-sulfur batteries using electrochemical impedance spectroscopy. Electrochim Acta 338:135787. https://doi.org/10.1016/j.electacta.2020.135787
Häcker J, Nguyen DH, Rommel T et al (2022) Operando UV/vis spectroscopy providing insights into the sulfur and polysulfide dissolution in magnesium-sulfur batteries. ACS Energy Lett 7:1–9. https://doi.org/10.1021/acsenergylett.1c02152
Attias R, Salama M, Hirsch B et al (2019) Anode-electrolyte interfaces in secondary magnesium batteries. Joule 3:27–52. https://doi.org/10.1016/j.joule.2018.10.028
ISO (2006) Environmental management: Life Cycle Assessment—principles and framework, ISO 14040, London
ISO (2006) Environmental management: Life Cycle Assessment—requirements and guidelines, ISO 14044, London
European Commission (2010) ILCD Handbook, international reference life cycle data system—general guide on LCA—detailed guidance. Publications Office of the European Union, Luxembourg
Greenwood M, Wrogemann JM, Schmuch R et al (2022) The battery component readiness level (BC-RL) framework: a technology-specific development framework. J Power Sour Adv 14:100089. https://doi.org/10.1016/j.powera.2022.100089
Chordia M, Nordelöf A, Ellingsen LA-W (2021) Environmental life cycle implications of upscaling lithium-ion battery production. Int J Life Cycle Assess 26:2024–2039. https://doi.org/10.1007/s11367-021-01976-0
Ellingsen LA-W, Singh B, Strømman AH (2016) The size and range effect: lifecycle greenhouse gas emissions of electric vehicles. Environ Res Lett 11:54010. https://doi.org/10.1088/1748-9326/11/5/054010
Baumann M, Peters JF, Weil M et al (2017) CO 2 Footprint and Life-Cycle costs of electrochemical energy storage for stationary grid applications. Energy Tech 5:1071–1083. https://doi.org/10.1002/ente.201600622
Hein F, Peter F (2020) Die Energiewende im Stromsektor: Stand der Dinge 2019 (The energy ransition in the electricity sector: current status in 2019). Agora Energiewende. Berlin
(2022) Electric Vehicle Database: Nissan Leaf. https://ev-database.org/car/1656/Nissan-Leaf. Accessed 29 Jan 2024
United Nations (2018) United Nations Global Technical Regulation on Worldwide harmonized Light vehicles Test Procedures (WLTP), New York
Reick B, Konzept A, Kaufmann A et al (2021) Influence of charging losses on energy consumption and CO2 emissions of battery-electric vehicles. Vehicles 3:736–748. https://doi.org/10.3390/vehicles3040043
(2020) Wiki Automotive Catalog: 2020 Volkswagen Golf VIII 2.0 TDI (150 Hp) DSG. https://www.auto-data.net/en/volkswagen-golf-viii-2.0-tdi-150hp-dsg-38155. Accessed 29 Jan 2024
Stolz P, Messmer A, Frischknecht R (2016) Life Cycle inventories of road and non-road transport services, Uster
Engels P, Cerdas F, Dettmer T et al (2022) Life cycle assessment of natural graphite production for lithium-ion battery anodes based on industrial primary data. J Clean Prod 336:130474. https://doi.org/10.1016/j.jclepro.2022.130474
Ellingsen LA-W, Majeau-Bettez G, Singh B et al (2014) Life Cycle Assessment of a lithium-ion battery vehicle pack. J Ind Ecol 18:113–124. https://doi.org/10.1111/jiec.12072
Zhang Z, Sun X, Ding N et al (2019) Life cycle environmental assessment of charging infrastructure for electric vehicles in China. J Clean Prod 227:932–941. https://doi.org/10.1016/j.jclepro.2019.04.167
van Loon P, Olsson L, Klintbom P (2018) LCA Guidelines for Electric Vehicles: How to determine the environmental impact of electric passenger cars and compare them against conventional internal-combustion vehicles, Göteborg
European Commission (2021) PEFCR—product environmental footprint category rules for high specific energy rechargeable batteries for mobile applications
Peters J, Weil M (2016) A critical assessment of the resource depletion potential of current and future lithium-ion batteries. Resources 5:46. https://doi.org/10.3390/resources5040046
Bachmann T, Hackenhaar I, Horn R et al. (2021) ORIENTING—D1.4 Critical evaluation of material criticality and product related circularity approaches. https://doi.org/10.13140/RG.2.2.29900.08323
Berger M, Sonderegger T, Alvarenga R et al (2020) Mineral resources in life cycle impact assessment: part II – recommendations on application-dependent use of existing methods and on future method development needs. Int J Life Cycle Assess 25:798–813. https://doi.org/10.1007/s11367-020-01737-5
Mats Zackrisson (2021) Life cycle assessment of electric vehicle batteries and new technologies. Doctoral Thesis in Production Engineering. KTH Royal Institute of Technology, Stockholm
Guinee JB (2002) Handbook on life cycle assessment operational guide to the ISO standards. Int J Life Cycle Assess 7:311–313. https://doi.org/10.1007/BF02978897
U.S. Geological Survey (2022) Mineral commodity summaries 2022. U.S. Geological Survey, Reston
Cox B, Bauer C, Mendoza Beltran A et al (2020) Life cycle environmental and cost comparison of current and future passenger cars under different energy scenarios. Appl Energy 269:115021. https://doi.org/10.1016/j.apenergy.2020.115021
Helmers E, Dietz J, Weiss M (2020) Suplemmentary information: sensitivity analysis in the life-cycle assessment of electric vs combustion engine cars under approximate real world conditions. Sustainability 12:1241
Evrard E, Davis J, Hagdahl KH, Palm R, Lindholm J, Dahllöf L (2021) Carbon footprint report Volvo-C40-Recharge
European Commission (2020) Determining the environmental impacts of conventional and alternatively fuelled vehicles through LCA. Final Report for the European Commission, DG Climate Action. European Comissions. DG Climate Action, Brussels
Bieker G. A global comparison of the life-cycle greenhouse gas emissions of combustion engine and electric passenger cars. Berlin: The International Council on Clean Transportation
Kukreja B (2018) Life cycle analysis of electric vehicles: quantifying the impact, Vancouver
Steinfort T, Bothe D (2020) Cradle-to-Grave Life-Cycle assessment in the mobility sector: a meta-analysis of LCA studies on alternative powertrain technologies, Frankfurt
Transport & Environment (2020) T&E´s analysis of electric car life cycle CO2 emissions. Briefing, Brussels
Putzig M, John G, Kristen M et al. (2021) Alternative fuels data center: fuel properties comparison, Washington
Blengini GA, El Latunussa C, Eynard U et al. (2020) Study on the EU's list of critical raw materials (2020): Final report. Luxembourg: Publications Office of the European Union
BGR, Bundesanstalt für Geowissenschaften und Rohstoffe (2020) Magnesium (Metall)—Rohstoffwirtschaftliche Steckbriefe (Raw materials industry profiles), Hannover, Germany
Cherubini F, Raugei M, Ulgiati S (2008) LCA of magnesium production. Resour Conserv Recycl 52:1093–1100. https://doi.org/10.1016/j.resconrec.2008.05.001
Li Z, Wang L, Bautista SP et al (2023) Electrochemistry of elements|chemistry, electrochemistry, and electrochemical applications of magnesium. In: Li Z (ed) Reference module in chemistry, molecular sciences and chemical engineering. Elsevier
Zhao Y, Kang Y, Wozny J et al (2023) Recycling of sodium-ion batteries. Nat Rev Mater 8:623–634. https://doi.org/10.1038/s41578-023-00574-w
Peters JF, Baumann M, Binder JR et al (2021) On the environmental competitiveness of sodium-ion batteries under a full life cycle perspective – a cell-chemistry specific modelling approach. Sustain Energy Fuels 5:6414–6429. https://doi.org/10.1039/D1SE01292D
Liu T, Zhang Y, Chen C et al (2019) Sustainability-inspired cell design for a fully recyclable sodium ion battery. Nat Commun 10:1965. https://doi.org/10.1038/s41467-019-09933-0
Trivedi S, Pamidi V, Fichtner M et al (2022) Ionically conducting inorganic binders: a paradigm shift in electrochemical energy storage. Green Chem 24:5620–5631. https://doi.org/10.1039/D2GC01389D
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work contributes to the research performed at CELEST (Center for Electrochemical Energy Storage Ulm-Karlsruhe). The authors acknowledge the financial support by the German Research Foundation (DFG) under Project ID 390874152 (POLiS Cluster of Excellence, EXC 2154). The funding by the European Union’s Horizon 2020 Research and Innovation Program under Grant Agreement No. 875126 (StoRIES) supports the presented scientific work.
Author information
Authors and Affiliations
Contributions
The manuscript is written by SPB under the guidance of MW and MB. The manuscript has been reviewed by MW and MB, who also supported the concept development of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13705_2024_475_MOESM1_ESM.pdf
Supplementary Material. It contains a detailed look at the results in several environmental impact categories, beyond those presented in the main body of the manuscript. The life cycle inventories developed or adapted for this work have also been included. Other inventories directly extracted from secondary sources have been indicated and referenced, and thus can be found in the respective literature.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pinto-Bautista, S., Baumann, M. & Weil, M. Prospective life cycle assessment of an electric vehicle equipped with a model magnesium battery. Energ Sustain Soc 14, 44 (2024). https://doi.org/10.1186/s13705-024-00475-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13705-024-00475-y