Abstract
Background
Uganda’s energy relies heavily on biomass sources. This dependence on biomass for household and commercial purposes, driven largely by population increase, poses pressure on natural resources, such as forests. This study investigates the usage of some of the country’s largely produced agricultural wastes for the production of biofuels.
Methods
Pineapple peels (PP), banana peels (BP) and water hyacinth (WH_Eichhornia crassipes (Mart.) Solms) were used for generation of both carbonized and uncarbonized briquettes. Physical properties and calorific values for the developed briquettes were determined through thermogravimetric analysis and using a bomb calorimeter.
Results
Pineapple peel carbonized briquettes had the highest calorific value (25.08 MJ/kg), followed by a composite of banana peels and pineapple peels (22.77 MJ/kg). The moisture content for briquettes ranged from 3.9% to 18.65%. Uncarbonized briquettes had higher volatile matter (ranging between 62.83% and 75.1%) compared to carbonized briquettes (ranging between 22.01% and 24.74%). Uncarbonized briquettes had a shorter boiling time (ranging between 27 and 36 min for 2.5 L of water) compared to carbonized briquettes (ranging between 26 and 41 min). Bulk density was highest in uncarbonized BP briquettes (1.089 g/cm3) and compressive strength was highest with carbonized BP + PP (53.22 N/mm2). When using water hyacinth alone, the produced carbonized briquettes show low calorific values (16.22 MJ/kg). However, the calorific values increased when they were mixed with banana (20.79 MJ/kg) or pineapple peels (20.55 MJ/kg).
Conclusions
The findings revealed that agricultural wastes could be used to augment the energy sources pool to protect the environment and create social stability in the community.
Similar content being viewed by others
Background
Uganda's energy needs are skewed toward biomass consumption, which accounts for more than 90% of the country’s needs [1]. Wood accounts for 70% of biomass consumption, charcoal for 16%, and agricultural waste for 4% [1,2,3]. Other energy sources include fossil fuels, which make up 5%, and hydroelectricity from two large dams as well as small hydro projects also amounts to 5%. According to the most recent National Charcoal Survey, the per capita fuelwood consumption and charcoal usage amounted to 240 and 680 kg per year, respectively [4]. Such reliance on biomass fuels, along with Uganda's fast population increase, has put significant constraints on natural resources, particularly forests. Uganda lost an average of 122,000 hectares of forest cover every year beginning 1990, and by 2015, it had lost a total of 3.1 million hectares [5]. This has greatly contributed to the irregular and rampant soil erosion, as well as the non-uniform and erratic rainfall distribution [6], and to social impacts, such as poverty [7]. Uganda’s population has continued to grow rapidly to approximately 43 million people in 2022 [8]. In 2019, over 76% of Uganda’s population did not have access to the national grid, making biomass production of wood fuels a key source of fuel [9]. Wood fuel is also used in small-scale businesses, such as brick and tile manufacture, agro-processing, and seafood processing [7].
Considering that agriculture is still the most widely practiced economic activity in Uganda, by-products and/or wastes can be used for energy generation [2, 10]. The produced energy is employed for cooking while also contributing to agricultural waste reduction and disposal. Leaving these agricultural wastes to degrade is a typical practice, and they are occasionally used to enhance animal diets in subsistence farms [10,11,12,13]. Uganda’s banana fruit processing alone is estimated to generate more than 4.3 million metric tons (MT) of banana waste annually [14]. Kayunga district, one of the leading pineapple producing districts in Uganda, produces approximately 15,960 tons of pineapple in a single season [15], of which 60% is waste. According to Komakech et al. [16], the collected waste in Kampala comprised 88.5% organics, with an average gross energy content of 17.3 MJ/kg. The estimated energy potential of crop residues in Sub-Saharan Africa reaches 3317 PJ per year, while that of Uganda is 150 PJ per year [17, 18]. Uganda’s crop energy potential is relatively high compared to that of Cameroon (106 PJ per year) [19], but lower than that of Ethiopia (750 PJ per year) [20] and South Africa (104–238 PJ per year) [21]. Biomass is converted into products with high-quality energy briquettes [22,23,24,25]. Therefore, pineapple and banana waste can be sustainably used through briquetting conversion [10]. Bananas and pineapples are normally seasonal, meaning that feedstocks may not be available during the production off season. Therefore, a sustainable source of feedstocks for producing briquettes, such as water hyacinth, needs to be considered for briquette production in periods when banana and pineapple wastes are unavailable. Water hyacinth is a water weed which can interfere with human activities, adversely affecting flora and fauna in lakes and rivers and, hence, is considered a noxious weed [26, 27]. Water hyacinth invaded Lake Victoria in the 1980s and, by 1998, they had attained peak coverage of approximately 2000 ha of the lake waters of Uganda [28]. Control interventions significantly reduced the weed's coverage to non-nuisance levels (< 10 ha) by 1999. Although resurgence was noticed in 2001, total coverage never reached the infestation levels attained between 1994 and 1998. By March 2012, infestations still occurred in hotspot bays, including MacDonald (52.1 ha), Fielding (38.0 ha), Bunjako (33.7 ha), Murchison (17.1 ha), Lwera (8.5 ha), Napoleon Gulf (2.9 ha), Berkeley (2.1 ha), Ssesse Islands (47.8 ha), and on River Kagera [27]. Floating mats of water hyacinth are physically harvested off the water using boats and transported to nearby disposal sites by trucks. Water hyacinth has been evaluated for production of briquettes [29, 30], biofuels [31] and biogas [32,33,34,35,36]. Hence, it can be used as a co-substrate with banana peels or pineapple peels.
Briquettes are a low-tech, environmentally friendly, and energy-efficient alternatives to wood fuels, which are frequently associated with indoor pollution and its related health issues. A shift to a more sustainable alternative to the prevailing energy sources in Uganda that primarily supports domestic cooking applications is beneficial and crucial in activating and promoting long-term alleviation of environmental degradation and reducing impact on climate change [37, 38]. Briquettes are produced using both low- and high-pressure techniques. They are, hence, affordable to the locals, and can be used to partially substitute the use of firewood and charcoal. In a comparative performance analysis of carbonized briquettes and charcoal fuels in Kampala-urban, Uganda, by Tumutegyerize et al. [39], the gross calorific values of the two fuel types were comparable, in the range of 19.5–27.3 MJ/kg. The same study showed that the boiling times for both fuels were not significantly different. Several studies on the development of carbonized briquettes from agricultural residues have been conducted in Uganda. Katimbo et al. [22], Lubwama and Yiga [23, 24] and Lubwama et al. [25] developed carbonized briquettes from different agricultural residues, including mango waste, rice husks, coffee husks, sugar cane bagasse, and groundnut shells. The results were positive in terms of moisture content (4.6–13%), volatile matter (16–65%), and calorific value (15–24 MJ/kg), hence providing an alternative to using charcoal and firewood. However, seasonal crops such as banana and pineapples tend to generate significant amounts of waste during their peak seasons. Using these wastes for briquette conversion saves the environment by contributing to energy diversity. Combining several materials for briquette production helps to improve strength compared to single-material briquettes [25]. Increasing the amount of empty fruit bunch in briquettes made from water hyacinth and empty fruit bunch increased the calorific value and volatile matter [29]. Composite briquettes developed from different nut shells had high calorific values and densities [40]. Composite briquettes generated from fecal sludge and pineapple peels had a higher volatile matter and calorific value of 39% and 17.92 MJ/kg, respectively, compared to those from fecal sludge alone, with 25.7% and 6.19 MJ/kg [41].
The current study involves the production of briquettes from banana and pineapple peels, blended with water hyacinth. The effect of different biomass combinations on physical and fuel properties were investigated to determine the optimum combination for briquette production. The objective of the study is to test the suitability of banana peels, pineapple peels and water hyacinth as sources of briquettes.
Materials and methods
Material source and preparation
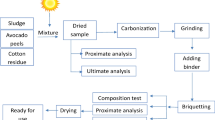
Banana and pineapple peels were collected from local farmers in Kayunga district (0.5817°N, 33.0294°E). Water hyacinth was collected from the Lubigi water stream (0.3472°N, 33.546°E), flowing at the boundaries of Kampala and Wakiso districts. Roots were cut off before being transported to Makerere University Agricultural Research Institute, Kabanyolo (MUARIK) (0.4683°N, 32.6074°E), for drying and briquette production. The collected substrates were sun-dried for 2 weeks to reduce the moisture content to about 15% wb. Every 3 days, samples were taken to the laboratory for thermogravimetric monitoring of the moisture content.
Briquette production
The study considered two kinds of briquettes, carbonized and uncarbonized. Uncarbonized briquette production started after the materials were dried, while materials for carbonized briquettes were carbonized first, as described in the next section.
Carbonization
Prior to producing briquettes, the dried banana peels, pineapple peels and water hyacinth were carbonized using slow pyrolysis at temperatures of 400 °C [42, 43]. The process was carried out using a locally fabricated carbonizer. The carbonizer was made from a steel drum of 200-L volume capacity, with a height of 1 m and a diameter of 0.5 m. The drum had openings with a diameter of 0.02 m on its surface, used to regulate the amount of air intake during ignition of the feedstocks. The dried substrates were loaded into the drum, ignited using a lighter fire, and the drum was left open for 10 min to allow for escape of volatiles. The top lid was then covered and the holes were covered with mud/clay to seal off air from entering for about 45 min. After the carbonization process, the biochar was removed from the drum and ground into fine particles to allow more contact with the binder.
Starch binder
A paste was prepared using 400 g of cassava flour, mixed with 3 L of cold water at 25 °C and thereafter poured into 4.5 L of boiling water at a temperature of 100 °C. Continuous mixing was carried out to produce a good binder with more water and less stickiness. In the final mixture of the required binder, water could disperse without any clumps of flour, which took 10 min. Cassava is a good binding agent due to its chemical and structural properties—imparts higher drop strength of over 95% onto the briquettes than clay binder material [24]. Cassava starch is also available locally at low prices.
Briquetting
The produced biochar was divided into 1000 g batches and mixed with 50 g of the prepared cassava starch binder for each of the substrate combinations (Table 1), for both carbonized and uncarbonized substrates, and mixed thoroughly to obtain a composite mixture. Banana peels are used for many purposes compared to pineapple peels and water hyacinth; therefore, the mixing ratios were determined based on the relative abundance. Each mixture was filled in the mold with nine slots, each having a 40 mm outer diameter, 15 mm inner diameter, and 52 mm height, and placed onto the hydraulic press machine (Model: HHP-60), for compaction, operating manually. The maximum compaction pressure of 10 MPa was regulated using a pressure gauge inserted at the pressing point of the machine, purposely to prevent the binder from diffusing out of the mold. The extruded briquettes (Fig. 1) were then dried under sunshine. The production process can easily be adopted by the locals, since only human energy was used in the production of briquettes [44]. There is no application of electrical, chemical and thermal energy to raw materials prior to carbonization. In addition, no thermal energy was needed to dry the raw materials and briquettes, because they were sufficiently dried in the sun.
Evaluation of briquettes’ properties
Proximate analysis and thermogravimetric analysis
Physical properties of the produced briquettes, which include moisture content, ash content, fixed carbon, and volatile matter of the substrates were determined, using an Eltra Thermostep non-isothermal Thermogravimetric analyzer (TGA), Haan, Germany, according to ASTM-D7582-15 [24, 45, 46]. This method allows for sequential determination of moisture, volatiles, ash content, and fixed carbon, up to 19 samples in a single analysis. The standard operating procedure (SOP) in the computer was selected, and briquette sample identifications were entered into the software. Briquette samples in the crucible, at a position assigned to the sample ID in the carousel, were weighed by the integrated detection balance. After one sample had been weighed, the carousel automatically rotated to the next position and the next registered sample was then weighed in the crucible until all 19 positions were filled with the samples. TGA experiments’ temperatures varied from room temperature to 920 °C at a heating rate of 16 °C/min. Prior to TGA experimentation, compressed air of high purity (oxygen:nitrogen = 21:79, > 99.5%) was used to clean the crucibles and chamber. The flow rate was maintained at 1 l/min, and the average mass of the samples used was 1.1 g. Thermogravimetric analysis was carried out to determine the weight loss of the briquettes with an increase in temperature. The Eltra Themostep Thermogravimetric analyzer was calibrated using calibration standard coal 92,511–3030 Lot 20131211B, with informative values for moisture wt.% wb, ash content wt.% db and volatile matter wt.% db of 4.0, 6.5 and 35.9, respectively. The moisture content (wt.%, wb), volatile matter (wt.% db), ash content (wt.% db) and fixed carbon (wt.% db) of the samples were calculated using Eqs. (1-4), as described by LECO Corporation [46]:
where a(g) is the initial mass (g), b(g) is the moisture mass (g), c(g) is the volatile mass (g), and d(g) is the ash mass (g).
Calorific value determination
Higher heating values for the produced briquettes were determined using an IKA C 2000 oxygen bomb calorimeter. The briquettes developed from banana peels, pineapple peels and water hyacinth were prepared to fit into the holdings of the calorimeter. Approximately 1 g of the developed briquette was placed in a nickel crucible and fired inside the bomb calorimeter using an ignition wire in the presence of oxygen. The calorimeter produced readings on the screen in about 30 min.
Mechanical properties
Bulk density
The bulk density of briquettes was calculated from the ratio of mass to volume of the briquettes according to [42]. The mass of each briquette was determined by weighing, using a digital weighing scale (OHAUS digital scale pan model PA114 (USA) serial number B45138480). The volume was determined by measuring the diameter, height, and central hole diameter at different points using a vernier caliper, and the final volume of the briquette was obtained based on the formula for determining the volume of a hollow cylinder given in the following equation:
where Vb = calculated briquette volume (cm3), R2 and r2 denotes the outer and inner radii (cm) of the briquette, respectively. Eventually, the bulk density was calculated using the following equation:
where ρb = calculated bulk density of the briquette (g/cm3), mb = measured mass of the briquette.
Compressive strength
Compressive strength is the maximum load a briquette can withstand before breaking or cracking. High compressive values are an indicator of high crush resistance, an aspect that is important during the handling and transportation of the briquettes. Compressive strength was determined by means of a universal testing machine (UTM-Tinius Olsen H50KT, USA), connected to a computer to enable real-time data logging, following a testing procedure according to [43, 44]. The briquettes were placed in between the two-machine sample holding plates and a uniform load (50 kN) was applied perpendicularly to the axis of the tested briquette at a rate of 1 mm/s until failure. The maximum force before failure (N) and compressive stress (N/mm2) were recorded by the machine. A stress–strain curve was also plotted for each tested sample using Qwat software installed on the computer.
Water boiling test
The water boiling test was employed to determine the efficiency of the developed briquettes for the cooking function (Fig. 2). The test was carried out using 250 g of briquettes to boil 2.5 L of water on an improved stove. The boiling temperatures were monitored using a thermometer, until the temperatures were constant. The time taken to reach the constant temperature was recorded using a stop watch.
Statistical analysis
Substrate characteristics and briquette quality attributes for different substrate combinations were statistically analyzed using R Software. One-way ANOVA, with Tukey's post-hoc analysis, was employed to assess the differences between mean values of different briquette qualities for these combinations. A paired sample t test was used between uncarbonized and carbonized briquette samples to determine the effect of carbonization. All tests for significance were conducted at a 0.05 significance level.
Results and discussion
Physical properties for briquettes
Moisture content
The mean moisture content was determined for the briquettes produced from different substrate combinations (Table 2). The moisture content for each nominal category (uncarbonized and carbonized) was significantly different (p ≤ 0.05). The single substrate-based briquette samples had lower moisture contents compared to the composite ones. The moisture content of briquette samples was within the recommended range (5–15%) [25] for good and high quality briquettes, except for composite uncarbonized briquette samples, pineapple peels and water hyacinth briquette samples. A paired statistical t test showed a significant difference (p = 0.003) in the moisture content between uncarbonized and carbonized briquettes. The moisture content was lower in carbonized briquettes than in uncarbonized briquettes, because the hydrophilic hydroxyl group is destructed [47]. The carbonization process helps in reducing absorption of moisture, which is a necessary aspect for increased shelf life and storage of briquettes, preventing rotting and decomposition [42, 43]. High moisture content in briquettes results in swelling and disintegration [47], and it interferes with thermo-chemical conversion processes. During combustion, a section of the energy is used to evaporate the water, leading to low heating values and, thus, reducing the overall energy efficiency of briquettes [48]. In addition, high moisture content in biomass fuels leads to increased production of green-house gases due to incomplete combustion [49].
Volatile matter
The volatile matter for both uncarbonized and carbonized briquette samples were significantly different (p ≤ 0.05) (Table 3). For uncarbonized samples, the banana peels briquettes had the highest volatile matter (75.1 ± 1.066%), while for carbonized samples the composite of the three substrates (banana peels, pineapple peels and water hyacinth) in proportions of 1:2:2 had the highest volatiles (24.74 ± 0.965%), and water hyacinth the lowest (22.01 ± 0.432%). Carbonized sample briquettes had a lower volatile matter percentage compared to the uncarbonized sample briquettes. A paired sample statistical t test showed a significant difference (p = 0.001) between uncarbonized and carbonized briquette sample pairs. These results were in agreement with the results obtained by Deshannavar et al. [49] on rice husk and rice husk char. Low volatile matter implies that the ignitability of the briquettes will be reduced, but once they ignite, combustion will produce little or no smoke with a clean flame [50]. The results were similar to those obtained by Mopoung and Udeye [51], when using banana peels and banana bunch waste.
Ash content
The ash contents for uncarbonized and carbonized briquette samples are shown in Table 4. There was a significant difference (p ≤ 0.05) between different substrate combinations for both nominal variables. Water hyacinth briquette samples had the highest ash content, with 10.19 ± 0.161% and 36.11 ± 0.375% for uncarbonized and carbonized samples, respectively. A paired sample statistical t test had a significant difference (p = 0.001); carbonized briquettes of all samples had more ash content than the uncarbonized briquettes, revealing the effect of carbonization. Ash is incombustible; therefore, it does not provide useful energy in domestic cooking applications, rendering briquettes of minor quality [52]. Composite briquettes had higher ash content than single substrate briquettes. Lubwama et al. [25] reported similar results on rice husks, coffee husks and ground nut shells. The development of bio-composite briquettes had a net positive impact on ash content levels, which, consequently, lowers their calorific values.
Fixed carbon
Pineapple peels had a higher percentage of fixed carbon for both uncarbonized (17.12 ± 0.379%) and carbonized (58.89 ± 0.496%) briquette samples (Table 5). A paired sample statistical t test indicated that carbonized briquettes had a significantly higher (p = 0.001) fixed carbon content than uncarbonized briquettes. This is attributed to the carbonization process, which tends to reduce the moisture and volatile content, resulting in an increase in the fixed carbon [50, 51]. Higher values of fixed carbon represent higher values of calorific value for the developed bio-composite briquettes [52].
Thermogravimetric analysis
Thermogravimetric analysis (TGA) was used to evaluate the combustion properties of the developed briquettes. TGA plots (Figs. 3 and 4) for the developed briquettes illustrate the percentage weight loss as a function of temperature. The plots clearly show the difference in the thermograms for carbonized and uncarbonized briquettes, since the uncarbonized briquettes had to go through the whole process of decomposition and different biomass materials have their own degradation patterns.
The process occurred in three stages: dehydration process, devolatization and endothermic decomposition of lignin. For all the briquettes, TGA thermograms remain at plateau from combustion at room temperature to 104 °C, followed by undergoing a major weight loss at about 105 °C. This weight loss occurred due to the evaporation of moisture from the composite briquettes [25].
The second phase varies with different developed briquettes from about 230 to 330 °C. From the onset of devolatization to about 600 °C, the aliphatic side chains start splitting off from aromatic rings [53]. This was the reduction of the volatile matter content, including the hemicellulose, cellulose and part of the lignin fractions, and it was the cause of the drastic weight drop, as illustrated in the graphs, thus resulting in the least value in the differential thermal analysis (DTA) plot curves (Figs. 5 and 6) [54]. These variations in initial degradation temperatures of the briquettes are due to the differences in the elemental and chemical compositions of developed briquettes [54]. Between 600 and 900 °C, a stage governed by the thermal decomposition of inorganic minerals, such as carbonates and clay, the last decomposition phase was observed, owed to the degradation of lignin in the developed briquettes [55].
At approximately 900 °C, lignin in the developed bio-composite briquettes had decomposed off, implying that the remaining weight percentage was mainly composed of residues, including ash, tars and fixed carbon. Lignin contains both aliphatic and aromatic constituents and, thus, signifies the ability of the developed briquettes to resist hydrolysis. The lowest total percentage weight losses at the highest combustion temperature were 24.85% and 76.84%, obtained for water–hyacinth-carbonized and uncarbonized briquettes, respectively, while the highest percentage weight loss for carbonized briquettes was 29.73% for briquettes consisting of banana peels, pineapple peels and water hyacinth in the ratio of 1:2:2 and for uncarbonized briquettes, 83.2% for banana peels. Thermogravimetric analysis shows that the uncarbonized briquettes are thermally unstable.
DTA curves illustrate the degradation of biomass feedstocks in relation to their lignocellulosic constituents, such as the cellulose, hemicellulose and lignin. The temperatures at which the maximum weight changes for all the briquettes ranged from 450 to 550 °C. The thermal stability of briquettes between 100 and 150 °C and the thermal degradation between 450 and 550 °C showed similarities with other plant biomasses. Therefore, briquettes produced from banana peels, pineapple peels and water hyacinth can go through slow pyrolysis.
Calorific value for the briquettes
The calorific values for both uncarbonized and carbonized briquette samples are presented in Fig. 7. The results show that briquette samples with high fixed carbon had higher calorific values, as was also reported by Deshannavar et al. [49] and Lubwama et al. [25]. The calorific values were significantly different (p ≤ 0.05) between the different substrate combinations, and a paired statistical sample t test between the uncarbonized and carbonized briquette samples was also significantly different (p = 0.001). Pineapple peels had the highest value for both carbonized (25.08 MJ/Kg) and uncarbonized briquettes (17.02 MJ/Kg). In general, single substrate briquettes had higher calorific values compared to the composite ones. However, compositing improved the heating values of water hyacinth, which were very low when used alone. The calorific values obtained in this study agree with those reported by Lubwama et al. [25] on bio-composite briquettes developed from rice husks, coffee husks and groundnut shells (16.6–22.0 MJ/Kg). Okia, Ndiema and Ahmed [54] also reported values between (16.22–21.59 MJ/Kg) for briquettes developed from water hyacinth, cow dung and charcoal dust.
Bulk density and compressive strength
The bulk density and compressive strength for the developed briquettes are illustrated in Figs. 8 and 9, respectively. Uncarbonized briquettes made from banana peels had the highest bulk density (1.089 g/cm3) compared to other briquettes. On the other hand, carbonized BP + PP had the highest compressive strength (53.22 N/mm2) among the developed briquettes. Bulk density expresses the amount of material per volume unit. Therefore, the higher the density, the more concentrated the energy in the fuel [56]. Compressive strength is the maximal load applied for a briquette to crack, and it estimates the weight a briquette can withstand during storage [57]. Thus, briquettes with a high compressive strength are desirable [55]. In addition, compressive strength could indicate the time that a briquette lasts burning, as well as the heat generated in the process. Briquettes with a low compressive strength burn for a short time and generate less heat in the process [30].
Water boiling test
Results for the time it takes to boil 2.5 L of water using 250 g of briquettes are shown in Fig. 10. In general, uncarbonized briquettes with low calorific values had longer boiling time (ranging between 27 and 36 min) than carbonized briquettes with higher calorific values (ranging between 26 and 41 min). Uncarbonized briquettes have a higher content of volatile matter, and during combustion the developed briquette ignites burned more readily and faster than briquettes with a lesser volatile matter content. The results obtained for banana peels agree with those found by Tumutegyereize et al. [39] who recorded times of between 31.5–52.5 min and boil 8–10 L of water in 2-L intervals. A relationship between calorific values and water boiling times for the developed composite briquettes is provided in Fig. 11. Calorific value is considered to influence the boiling time [25]. However, according to this study, calorific value alone does not affect the boiling time. The composite briquette of all the three substrates (banana peels, pineapple peels and water hyacinth) has a short boiling time, attributed to the combined volatile matter.
Conclusions
This study investigated the available agricultural waste materials (banana and pineapple peels) and water hyacinth, a water weed, to generate energy for usage in cooking, as a substitute for wood and charcoal. A manual pressing machine was used to produce carbonized and uncarbonized briquettes. Pineapple peel briquettes emerged with the highest calorific value (25.08 MJ/Kg), followed by a composite of banana peels and pineapple peels (22.77 MJ/Kg). When used individually, carbonized water hyacinth produced briquettes with low calorific values. However, the values significantly improved when composited with banana or pineapple peels. The results provide vital information on how water hyacinth, a most abundant substrate and water weed, can enhance energy provision when used for briquette production with banana and pineapple peels.
Availability of data and materials
Not applicable.
References
GIZ. Sector Brief Uganda: Renewable Energy. 2022. https://www.giz.de/en/downloads/giz2022-en-sectorbrief-uganda-renewable-energy.pdf. Accessed on 2 Feb 2022
Fashina A, Mundu M, Akiyode O, Abdullah L, Sanni D, Ounyesiga L (2018) The drivers and barriers of renewable energy applications and development in Uganda: a review. J Clean Technol 1(1):9–39. https://doi.org/10.3390/cleantechnol1010003
Kabyanga M, Balana BB, Mugisha J, Walekhwa PN, Smith J, Glenk K (2018) Economic potential of flexible balloon biogas digester among smallholder farmers: a case study from Uganda. Renew Energy J 120:392–400. https://doi.org/10.1016/j.renene.2017.12.103
MEMD (Ministry of energy and mineral development). (2016) National charcoal survey for Uganda. https://pfccparliament.go.ug/wp-content/uploads/2019/04/NationalCharcoalSurvey_FINAL.pdf. Accessed on 12 Nov 2022
MWE (Ministry of water and environment) (2016) State of Uganda’s Forestry.. https://www.mwe.go.ug/sites/default/files/library/2.3%20The%20status%20of%20the%20national%20tree%20cover%20in%20Uganda.pdf. Accessed on 12 Aug 2021
Bamwesigye D, Kupec P, Chekuimo G, Pavlis J, Asamoah O, Darkwah SA, Hlaváčková P (2020) Charcoal and wood biomass utilization in Uganda: the socioeconomic and environmental dynamics and implications. J Sustain 12(20):1–18. https://doi.org/10.3390/su12208337
Abigaba G, Niyibizi G, Turinayo Y, Nansereko S (2017) Implications of fuel wood scarcity on livelihoods of rural communities of Nyarubuye Sub-County in Kisoro District, South western Uganda. Uganda J Agric Sci 17(1):43. https://doi.org/10.4314/ujas.v17i1.5
UBOS (Uganda bureau of statistics) (2022) Population clock. https://www.ubos.org/. Accessed on 30 Mar 2022
Menno JVV (2020) An overview of recent developments and the current state of the Ugandan energy sector (Uganda’s energy sector: A fiscal risk No. E-20046-UGA-1). London. https://www.theigc.org/wp-content/uploads/2020/12/UGA-20046-Paper-1_IGC-project_Uganda-energy-sector_Fiscal-risk_state-of-the-energy-sector_25062020.pdf. Accessed on 30 Mar 2022
Nsubuga D, Banadda N, Kiggundu N (2019) Innovations in value-addition of agricultural by-products in Uganda. J Environ Prot 10:1493–1506. https://doi.org/10.4236/jep.2019.1011089
Baidhe E, Kigozi J, Mukisa I, Muyanja C, Namubiru L, Kitarikawe B (2021) Unearthing the potential of solid waste generated along the pineapple drying process line in Uganda: a review. Environ Chall. https://doi.org/10.1016/j.envc.2020.100012
Kabasiita JK, Malinga GM, Odongo JCW, Opolot E (2021) Factors influencing utilization of municipal solid waste compost among urban farmers in western Uganda. CABI Agric Biosci 2(1):1–10. https://doi.org/10.1186/s43170-021-00067-2
Kamoga G, Ssekyewa C (2021) Waste management systems among smallholder farmers in Masaka and Lyantonde Districts, Central Uganda. J Agric Chem Environ 10(03):314–326. https://doi.org/10.4236/jacen.2021.103020
UBOS (Uganda bureau of statistics). Uganda bureau of statistics 2020 Statistical abstract. https://www.ubos.org/wp-content/uploads/publications/11_2020STATISTICAL__ABSTRACT_2020.pdf. Accessed on 30 Aug 2021
NAADS (National agricultural advisory services) (2021). Kayunga pineapple factory pre-tested, ready for operation. https://naads.or.ug/kayunga-pineapple-factory-pre-tested-ready-for-operation/. Accessed 15 April 2022
Komakech AJ, Banadda NE, Kinobe JR, Kasisira L, Sundberg C, Gebresenbet G, Vinnerås B (2014) Characterization of municipal waste in Kampala, Uganda. J Air Waste Manag Assoc 64(3):340–348. https://doi.org/10.1080/10962247.2013.861373
Okello C, Pindozzi S, Faugno S, Boccia L (2013) Bioenergy potential of agricultural and forest residues in Uganda. Biomass Bioenerg 56:515–525. https://doi.org/10.1016/j.biombioe.2013.06.003
Röder M, Chong K, Thornley P (2022) The future of residue-based bioenergy for industrial use in Sub-Saharan Africa. Biomass Bioenergy. https://doi.org/10.1016/j.biombioe.2022.106385
Bot B, Tamba J, Sosso O (2022) Assessment of biomass briquette energy potential from agricultural residues in Cameroon. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02388-2
Gabisa EW, Gheewala SH (2018) Potential of bio-energy production in Ethiopia based on available biomass residues. Biomass Bioenerg 111:77–87. https://doi.org/10.1016/j.biombioe.2018.02.009
Batidzirai B, Valk M, Wicke B, Junginger M, Daioglou V, Euler W, Faaij APC (2016) Current and future technical, economic and environmental feasibility of maize and wheat residues supply for biomass energy application: Illustrated for South Africa. Biomass Bioenerg 92:106–129. https://doi.org/10.1016/j.biombioe.2016.06.010
Katimbo A, Kiggundu N, Kizito S, Kivumbi HB, Tumutegyereize P (2014) Potential of densification of mango waste and effect of binders on produced briquettes. Agric Eng Int CIGR J 16(4):146–155
Lubwama M, Yiga VA (2017) Development of groundnut shells and bagasse briquettes as sustainable fuel sources for domestic cooking applications in Uganda. Renew Energy 111:532–542. https://doi.org/10.1016/j.renene.2017.04.041
Lubwama M, Yiga VA (2018) Characteristics of briquettes developed from rice and coffee husks for domestic cooking applications in Uganda. Renew Energy 118:43–55. https://doi.org/10.1016/j.renene.2017.11.003
Lubwama M, Yiga VA, Muhairwe F, Kihedu J (2020) Physical and combustion properties of agricultural residue bio-char bio-composite briquettes as sustainable domestic energy sources. Renewable Energy 148:1002–1016. https://doi.org/10.1016/j.renene.2019.10.085
Balirwa J, Wanda F (2005) Impacts of water quality change on beneficial uses of Lake Victoria, Uganda. https://www.mwe.go.ug/sites/default/files/library/Chapter%2012%20Impacts%20on%20beneficial%20uses.pdf. Accessed on 30 Mar 2022
Wanda FM, Namukose M, Matuha M (2015) Water hyacinth hotspots in the Ugandan waters of Lake Victoria in 1994–2012: implications for management. Afr J Aquat Sci 40(1):101–106. https://doi.org/10.2989/16085914.2014.997181
Twongo T, Bugenyi FWB, Wanda FM (1991) The potential for further proliferation of water hyacinth in Lakes Victoria, Kyoga and Kwania, and some urgent aspects for research. Afr J Trop Hydrobiol Fish 6:1–10
Rezania S, Fadhil M, Fatimah S (2016) Evaluation of water hyacinth (Eichhornia crassipes) as a potential raw material source for briquette production. Energy 111:768–773. https://doi.org/10.1016/j.energy.2016.06.026
Carnaje NP, Talagon RB, Peralta JP, Shah K, Paz-Ferreiro J (2018) Development and characterisation of charcoal briquettes from water hyacinth (Eichhornia crassipes)-molasses blend. PLoS ONE 13(11):1–14. https://doi.org/10.1371/journal.pone.0207135
Shanab SMM, Hanafy EA, Shalab EA (2018) Water hyacinth as non-edible source for biofuel production. Waste Biomass Valoriz 9(2):255–264. https://doi.org/10.1007/s12649-016-9816-6
Barua VB, Rathore V, Kalamdhad AS (2019) Anaerobic co-digestion of water hyacinth and banana peels with and without thermal pretreatment. Renew Energy 134:103–112. https://doi.org/10.1016/j.renene.2018.11.018
BaruaVB KAS (2019) Biogas production from water hyacinth in a novel anaerobic digester: a continuous study. Process Saf Environ Prot 127:82–89. https://doi.org/10.1016/j.psep.2019.05.007
Sarto S, Hildayati R, Syaichurrozi I (2019) Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics. Renew Energy. 132:335–350. https://doi.org/10.1016/j.renene.2018.07.121
Unpaprom Y, Pimpimol T, Whangchai K, Ramaraj R (2021) Sustainability assessment of water hyacinth with swine dung for biogas production, methane enhancement, and biofertilizer. Biomass Convers Biorefinery 11(3):849–860. https://doi.org/10.1007/s13399-020-00850-7
Suthar S, Sharma B, Kumar K, Rajesh JB, Tyagi VK (2022) Enhanced biogas production in dilute acid-thermal pretreatment and cattle dung biochar mediated biomethanation of water hyacinth. Fuel. https://doi.org/10.1016/j.fuel.2021.121897
Kyayesimira J, Muheirwe F (2021) Health concerns and use of biomass energy in households: voices of women from rural communities in Western Uganda. Energy Sustain Soc 11(42):1–13. https://doi.org/10.1186/s13705-021-00316-2
Mahto P, Dubey V, Panhotra J (2015) Indoor air pollution: health hazards and techniques to reduce the hazardous effects. Int J Res 3(9SE):1–5. https://doi.org/10.29121/granthaalayah.v3.i9se.2015.3155
Tumutegyereize P, Mugenyi R, Ketlogetswe C, Gandure J (2016) A comparative performance analysis of carbonized briquettes and charcoal fuels in Kampala-urban, Uganda. Energy Sustain Dev 31:91–96. https://doi.org/10.1016/j.esd.2016.01.001
Tembe ET, Ekhuemelo DO, Asue MW (2018) Physical and combustion properties of briquettes produced from composite materials of Daniela Oliveri, Gmelina Arborea and Bambara nut shells in Benue State, Nigeria. Int J Pure Agric Adv 2(1):1–6
Kizito S, Jjagwe J, Ssewaya B, Nekesa P, Tumutegyereize T, Zziwa A, Komakech AJ (2022) Biofuel characteristics of non-charred briquettes from dried fecal sludge blended with food market waste: Suggesting a waste-to-biofuel enterprise as a win–win strategy to solve energy and sanitation problems in slums settlements. Waste Manag 140:173–182. https://doi.org/10.1016/j.wasman.2021.11.029
FAO (1983) Simple technologies for charcoal making, vol 41. FAO, Rome
Bot BV, Sosso OT, Tamba JG, Lekane E, Bikai J, Ndame MK (2021) Preparation and characterization of biomass briquettes made from banana peels, sugarcane bagasse, coconut shells and rattan waste. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01762-w
Vale AT, Gentil LV (2014) Energy balance and efficiency in wood sawdust briquettes production. Floresta 45(2):281–288. https://doi.org/10.5380/rf.v45i2.36954
Eltra Elemental Analyzers (2021) TGA ELTRA Thermostep thermogravimetric analyzer: Instruction manual. https://www.eltra.com/dltmp/www/53e4b56a-4790-4469-9a6a-636500000000-4eddac3ac644/brochure_TGA_en.pdf. Accessed 9 Nov 2021
LECO corporation. 2010. Moisture, volatile matter, ash, and fixed carbon determination-solid fuel characterization measurements in coke, organic application note, form 203-821-381, LECO Corporation, St. Joseph, Mich, USA. http://www.leco.co.za/wp-content/uploads/2012/02/TGA701-COKE-203-821-381.pdf
Liu Z, Jiang Z, Cai Z, Fei B, YanYu LX (2013) Effects of carbonization conditions on properties of bamboo pellets. Renew Energy J 51:1–6. https://doi.org/10.1016/j.renene.2012.07.034
Fernández RG, García CP, Lavín AG, de Las Heras JL, Pis JJ (2013) Influence of physical properties of solid biomass fuels on the design and cost of storage installations. Waste Manag J 33(5):1151–1157. https://doi.org/10.1016/j.wasman.2013.01.033
Deshannavar UB, Hegde PG, Dhalayat Z, Patil V, Gavas S (2018) Production and characterization of agro-based briquettes and estimation of calorific value by regression analysis: an energy application. Mater Sci Energy. https://doi.org/10.1016/j.mset.2018.07.003
Choudhury HA, Chakma S, Moholkara VS (2015) Biomass gasification integrated Fischer-Tropsch synthesis: perspectives, opportunities and challenges. In: Pandey A, Stöcker M, Bhaskar T, Sukumaran RK (eds) Recent advances in thermo-chemical conversion of biomass. Elsevier, Amsterdam, pp 383–435. https://doi.org/10.1016/B978-0-444-63289-0.00014-4
Mopoung S, Udeye V (2017) Characterization and evaluation of charcoal briquettes using banana peel and banana bunch waste for household heating. Am J Eng Appl Sci 10(2):353–365. https://doi.org/10.3844/ajeassp.2017.353.365
Mythili R, Venkatachalam P (2015) Product yield and characteristics of char. Energy Sourc. https://doi.org/10.1080/15567036.2012.721862
Bartocci P, Bidini G, Asdrubali F, Beatrice C, Frusteri F, Fantozzi F (2018) Batch pyrolysis of pellet made of biomass and crude glycerol: mass and energy balances. Renew Energy 124:172–179. https://doi.org/10.1016/j.renene.2017.06.049
Okia DO, Ndiema CK, Ahmed MS (2016) Physical and chemical properties of water hyacinth based composite briquettes. World. 4:28–36
Bai F, Sun Y, Liu Y, Li Q, Guo M (2015) Thermal and kinetic characteristics of pyrolysis and combustion of three oil shales. Energy Convers Manage 97:374–381. https://doi.org/10.1016/j.enconman.2015.03.007
Amarasekara A, Tanzim FS, Asmatulu E (2017) Briquetting and carbonization of naturally grown algae biomass for low-cost fuel and activated carbon production. Fuel 208:612–617. https://doi.org/10.1016/j.fuel.2017.07.034
Kabenge I, Omulo G, Banadda N, Seay J, Zziwa A, Kiggundu N (2018) Characterization of banana peels wastes as potential slow pyrolysis feedstock. J Sustain Dev 11(2):14–24. https://doi.org/10.5539/jsd.v11n2p14
Acknowledgements
The Federal Ministry of Food and Agriculture (BMEL), based on a decision of the Parliament of the Federal Republic of Germany, via the Federal Office for Agriculture and Food (BLE), is acknowledged for funding this work through Makerere University, Kampala (Uganda). We are also grateful to Deo Kawalya of the School of Languages, Literature and Communication, Makerere University, for proofreading the manuscript.
Funding
The study was funded by the Federal Ministry of Food and Agriculture (BMEL) of the Federal Republic of Germany, through Makerere University, Kampala (Uganda).
Author information
Authors and Affiliations
Contributions
TM conceptualized the study, carried out the experiments, collected and analyzed the data and wrote the major parts of the paper. DN contributed to the methodological design, supervised and critiqued data collection and analysis. IK and KDW contributed to the conceptualization of the study, revised the manuscript, and made suggestions for improvement. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors agreed to publish the article.
Competing interests
The authors declare that there is no competing interests regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mibulo, T., Nsubuga, D., Kabenge, I. et al. Characterization of briquettes developed from banana peels, pineapple peels and water hyacinth. Energ Sustain Soc 13, 36 (2023). https://doi.org/10.1186/s13705-023-00414-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13705-023-00414-3