Abstract
Background
Nicotine replacement therapy (NRT) has proven effect in assisting smoking cessation. However, its effectiveness varies across studies and population groups. This may be due to differences in the rate of adherence. Hence, this review aims to examine the level of adherence to NRT and to assess if the level of adherence to NRT affects success of smoking cessation.
Methods
A systematic review and meta-analysis was conducted using studies retrieved from five electronic databases (MEDLINE, Scopus, EMBASE, Web of science, and PsycINFO) and grey literature. Pooled analysis was conducted using Stata version 16 software. Methodological quality and risk of bias were assessed using the NIH Quality Assessment Tool. Analyses were done among those studies that used similar measurements to assess level of adherence and successful smoking cessation. Heterogeneity of studies was assessed using the Higgins’ I2 statistical test. Funnel plots and Egger’s regression asymmetry test were used to affirm presence of significant publication bias.
Results
A total of 7521 adult participants of 18 years old and above from 16 studies were included in the analysis. Level of adherence to NRT among participants of randomised controlled trials were found to be 61% (95% CI, 54–68%), p-value of < 0.001 and I2 = 85.5%. Whereas 26% of participants were adherent among participants of population-based studies with 95% CI, 20–32%, p-value of < 0.001 and I2 = 94.5%. Level of adherence was the lowest among pregnant women (22%) with 95% CI, 18–25%, p-value of 0.31 and I2 = 15.8%. Being adherent to NRT doubles the rate of successful quitting (OR = 2.17, 95% CI, 1.34–3.51), p-value of < 0.001 and I2 = 77.6%.
Conclusions
This review highlights a low level of adherence to NRT among participants of population-based studies and pregnant women as compared to clinical trials. Moreover, the review illustrated a strong association between adherence and successful smoking cessation. Hence, it is recommended to implement and assess large scale interventions to improve adherence. Health programs and policies are recommended to integrate the issue of adherence to NRT as a core component of smoking cessation interventions.
Trial registration
PROSPERO registration number: CRD42020176749. Registered on 28 April 2020.
Similar content being viewed by others
Background
Smoking remains the most common preventable cause of chronic diseases [1] and premature mortality [2]. Smoking cessation has shown a considerable effect in improving the health and survival of individuals [3]. Smoking cessation usually requires pharmacotherapy in addition to counseling from health care providers. Nicotine replacement therapy (NRT) is the most commonly utilised smoking cessation medication and it can be administered in the form of transdermal patches, gums, lozenges, sprays, or inhalators. NRT has been accepted as first-line pharmacotherapy for smoking cessation because of its safety and efficacy profile [4]..
Among participates who used NRT as a smoking cessation medication, relatively higher smoking cessation rates were reported in clinical trial participants as compared to participants of population-based studies. For instance, in a 2018 Cochrane review that includes only randomised controlled trials, NRT use increases the rate of successful smoking cessation by 50 to 60% [5]. Whereas, most population-based studies reported a 10 to 30% rise in the rate of successful smoking cessation among individuals who utilised NRT [3, 6, 7]. Variations in success rate may be due to underutilisation or non-proper use of prescribed smoking cessation medications illustrated by some studies conducted in USA and China especially in population-based studies, referred to studies that used data collected from diversified areas of daily life that are outside the scope of highly controlled randomised control trials [8,9,10]. In general, participants of randomised controlled trials were found to be more likely to take their medications as prescribed by the provider because of additional treatment-related counseling offered by the trial which may resolve their concerns and addressed safety issues. For instance, one study conducted among diabetic patients reported lower adherence rate and medication effectiveness among population-based settings than randomised control participants [11].
A comprehensive literature review on adherence to medications was conducted by the Medication and Compliance Special Interest Group of the International Society for Pharmacoeconomics and Outcomes Research. This review defined adherence as “the extent to which a patient takes treatment in accordance with the prescribed interval and dose of a dosing regimen” [12]. Similarly, the World Health Organisation (WHO) defines adherence as “the extent to which the patient follows medical instructions” [13]. The most important factor affecting the evaluation of adherence and success of quitting is likely to be resuming smoking. It can be controlled by establishing the sequence of non-adherence and relapse or assessing adherence during a pre-specified treatment period and determine abstinence only in those who had been continuously abstinent throughout this specified period [14]. There are inconsistencies across the literature in the definition of adherence to smoking cessation pharmacotherapies. Studies have reported that adherence to NRT is low both within and outside of the context of clinical trials [15].
Studies conducted on medical disorders have demonstrated a strong association between the level of adherence to medications and positive clinical outcomes [16]. Also, consumption of a higher number of gums, lozenges, and inhalers resulted in a better success rate for smoking cessation [9, 10, 17]. Most studies focused on smoking cessation outcomes, rather than on a thorough evaluation of the extent of adherence and its association with smoking cessation.
All in all, the rate of smoking cessation rate was reported to be substantially higher among participants of randomised controlled trials as compared to population-based studies. Most studies conducted in other medical conditions reported a significant association between adherence and treatment outcome. Hence, we hypothesised that this disparity in the success of quitting between study types and specific population groups like pregnant women, where there exists additional perceived concern about the fetal risk of NRT, may partially be explained by a difference in the level of adherence.
Our study aimed to examine the level of adherence to NRT among participants of population-based studies, randomised clinical trials, and during pregnancy. Disparity in the rate of adherence between population-based studies and randomised clinical trials was also evaluated in this review. Moreover, the impact of adherence on the rate of successful smoking cessation was also evaluated. Hence, the findings will inform policymakers and health care providers about the importance of addressing adherence to NRT to improve the rate of successful smoking cessation. These findings can be used to guide researchers to develop interventions that can enhance adherence to NRT.
Methods
Study design and search strategies
A systematic review and meta-analysis was conducted according to the PRISMA guidelines [18] and MOOSE for observational studies [19]. The protocol is registered in PROSPERO (registration number CRD42020176749), available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020176749. A detailed review protocol was developed before commencing the review. The review protocol was published and it is available from https://bmjopen.bmj.com/content/10/9/e039775. MEDLINE, Scopus, EMBASE, CINAHL, and PsycINFO databases were searched. The initial database search was conducted from the start of indexing to February 25, 2020. Citation alerts were created, and the most recent literature search was updated on July 20, 2020. The search strategy was developed with the assistance of a senior librarian. The free-text words (with truncation) and MeSH terms combined using Boolean logic operators: AND, OR, and NOT. A combination of keywords and phrases like: Smoking, “Smoking cessation”, Cessation, Smoke, Cigarette, Quitting, “Quitting Smoking”, “Medication Adherence”, Adherence, Discontinuation, Compliance, Non-Compliance, Non-adherence, “Treatment Compliance”, “Therapeutic Compliance”, “Nicotine replacement therapy”, NRT, “Nicotine patch”, Patch, “Nicotine gum”, “Nicotine inhaler”, Inhaler, Lozenge, “Nicotine spray”, Pharmacotherapies, “Drug therapies”, “Pharmacological therapy”, and “Medication treatment” were used to search articles in the databases [Supplementary material 1]. References from eligible studies were hand-searched for additional studies. Grey literature searches were conducted at the following websites and organisations: Centres for Disease Control and Prevention Smoking and Health Resource Library, National Institute for Health and Care Excellence, and the Ottawa Heart Institute’s Ottawa Model for Smoking Cessation. Citations were gathered using Endnote reference management software version 9 and exported to Covidence software for screening [20].
Eligibility criteria
Population
Studies that enrolled the adult population (18 years old and above) using NRT to quit smoking were included. Studies restricted to participants with mental illness and individuals with other substance use disorders were excluded from the review to maintain homogeneity among studies.
Intervention
The intervention included the use of different treatment durations and doses of NRT products taken in forms of gum, transdermal patch, nasal spray, lozenges, oral spray, or an oral inhalator. Studies using medications other than NRT were excluded from the review.
Comparator
In clinical trials, the control was either standard care or placebo, behavioural intervention, or no intervention. Studies that compare the effectiveness of NRT with other smoking cessation medications such as bupropion or varenicline were excluded.
Outcome
Studies that reported the level of adherence to NRT and/or impact of adherence on the rate of successful smoking cessation were included. We included studies if they reported both outcomes (level and impact of adherence) or one of the outcomes.
Study design
Studies that used quantitative methodology such as case-control, cohort, cross-sectional, longitudinal, randomised control trials without limitation to publication date, sample size, setting, language was included in this review. Commentaries, expert opinion, abstracts, conference presentations without complete results were excluded.
Screening and data extraction
Each citation was screened by two authors (AM, DT) by using Covidence [20]. Two authors (AM, DT) independently reviewed the full text. A data extraction template was developed and pretested by extracting data from three articles [8, 21, 22] and necessary modifications made before proceeding with the data extraction. The template had three main sections: study identification, methodological characteristics, main findings of the included studies.
Quality assessment
Two authors (AM, DT) independently assessed the quality of studies using the National Institutes of Health (NIH) quality assessment tool for observational and interventional studies [23]. The National Institutes of Health (NIH) quality assessment tool identifies the source of bias and study implementation errors through appraising each study against prespecified items including controlling for confounding factors, study power, the strength of causality in the association between interventions and outcomes. Disagreements were resolved by discussion and mutual agreement between the reviewers. [Supplementary material 2].
Statistical analysis
Meta-analyses were conducted using Stata software (V16, Stata Corp LP, College Station, TX) [24]. Heterogeneity was assessed using the Higgins’ I2 statistical analysis test. Heterogeneity was considered low, moderate, or high when the values were below 25%, between 25 and 75%, and above 75%, respectively [25]. Results were pooled using proportion and odds ratios, 95% confidence intervals calculated with p values for each outcome variable. When the level of heterogeneity was low, the Mantel-Haenszel fixed-effect model was applied to pool results. When the I2 test is above 75%, the DerSimonian-Laird (DL) random-effects model. Funnel plot test of asymmetry and Egger’s regression asymmetry test with p-value < 0.05 was used as a cut-off point to confirm a statistically significant publication bias [26].
To decrease heterogeneity between studies, we analysed randomised control trials (as defined by the Cochrane practice guide as the comparison groups generated by random allocation) [25] and population-based studies [27] separately. Pooled analyses were conducted among those studies that used similar measurements to assess the level of adherence and successful smoking cessation in a follow-up period between four and 10 weeks, as most of the published studies follow up period fell within this time frame. Studies that recruited pregnant women only were analysed alone as perinatal concerns may impose additional effects on NRT consumption and adherence [28].
Operational definitions
Adherence
Adherence is defined by The World Health Organisation (WHO) as “the extent to which the patient follows medical instructions” [13]. There exist inconsistencies in the definition of adherence to smoking cessation medications across studies. Hence, the definitions and measurements used to determine adherence to NRT in each study are presented in a summary table [Table 1].
Abstinence
Abstinence is defined as the proportion of participants who achieved point prevalence abstinence up to a given point of time. The assessment of abstinence is usually based on self-report or measuring a biomarker such as salivary cotinine or exhaled carbon monoxide [41]. The definition and measurements used to define successful smoking cessation are summarised in table one [Table 1].
Ethics consideration
As this is a literature review of the already published studies, it did not require ethical clearance to analyse published articles.
Results
Studies identified
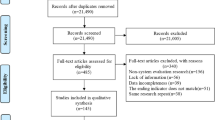
A total of 3404 articles were identified from five electronic databases and other sources such as grey literature. As illustrated in the flow chart, 16 studies with a total sample size of 7521 participants were included in the systematic review and meta-analysis [Fig. 1].
Description of the studies
Out of the included 16 studies, 11 studies were clinical trials [10, 21, 30,31,32, 34,35,36, 39, 42, 43] and five studies were population-based studies [8, 22, 29, 38, 40]. All of the population-based studies followed a cross-sectional study design [8, 22, 29, 38, 40]. Five studies were conducted in the USA [10, 31, 34, 37, 40]; two studies were conducted in Switzerland [22, 32]; two studies were from the UK [21, 33]; one study involved participants from four countries (Australia, USA, UK, and Canada) [29]. The remaining six studies were from Canada, Denmark, Australia, Syria, and China [8, 30, 35, 36, 38, 39]. The sample size of studies among studies that recruited general adult population [8, 21, 22, 29,30,31,32, 38, 40] ranged from a minimum of 82 individuals [22] to a maximum of 1605 participants [38]. All of the studies conducted among the general adult population evaluated adherence between four to 10 weeks [8, 21, 22, 29,30,31,32, 38, 40]. Most of the studies used Russell’s standard of smoking abstinence, the self-reported seven-day point prevalence of abstinence validated by expired-air carbon monoxide level [30], while a few studies used continuous abstinence for the follow-up period as a measure of successful smoking cessation. The specific assessment used to evaluate adherence and success of smoking cessation is illustrated in Table 1. Five studies enrolled only pregnant women and all of the five studies were clinical trials [33,34,35,36, 39]. The number of participants, in studies that recruited only pregnant women among those on the active nicotine group, was 20 participants in a study conducted in Australia [35] to a maximum of 521 in a study conducted in the UK [33]. All of the studies conducted among pregnant women enrolled those whose gestational age was in the second trimester and above [Table 1].
Risk of bias
Generally, almost all of the included studies were assessed to have good quality by both reviewers. More detailed assessments of study qualities are illustrated in Supplementary Table 1. Clinical trials scored from a minimum of 11 [21, 34,35,36] to a maximum of 14 [32, 42] out of a score of 14 on the NIH quality assessment tool for randomised controlled trials. Whereas, population-based studies scored from a minimum of 9 [22] to a maximum of 13 [8] out of a score of 14 on the NIH Quality Assessment Tool for observational studies. [Supplementary material 2].
Level of adherence to NRT in clinical trials
A pooled analysis using random effects model was conducted among clinical trials that assessed level of adherence to NRT for a period of four to ten weeks [21, 30,31,32] found that 61% (95% CI, 54–68%) of adults met study adherence criteria with a p-value of < 0.001 and Higgins’ I2 = 85.5% [Fig. 2]. Test of publication bias was examined by using a funnel plot that illustrated the relatively symmetrical distribution of the studies. Furthermore, Egger’s test was conducted and there was no evidence of significant publication bias (p-value = 0.093). [Table 2].
Among the clinical trials the highest level of adherence of 68% (95% CI, 62–73%) was reported in a study conducted in Syria which assessed adherence by asking participants whether they had followed treatment instructions to use one patch every day over the past week and adherence to patch use was defined as responding “yes” to this question during at least 5 of the 6 weeks (> 80%) [30]. Whereas, the lowest adherence level was from a study conducted in the UK 55% (95% CI, 52–59%) that defined adherence as consumption of at least 80% of the prescribed NRT averaged over 4 weeks treatment period [44] [Table 1].
Level of adherence to NRT in population-based studies
A meta-analysis of five studies [8, 22, 29, 38, 40] that assessed adherence to NRT in population-based studies for a follow-up period between four and 10 weeks among adults was conducted. Using the DerSimonian-Laird random effect model a quarter of participants were found to met the study adherence criteria 26% (95% CI, 20–32%) with a p-value of < 0.001 and Higgins’ I2 = 94.5%. [Fig. 3]. Funnel plot symmetry test was conducted to evaluate the presence of publication bias and all studies were found to be symmetrical. Egger’s test was also performed, and it demonstrates the absence of significant publication bias (p-value = 0.439) [Table 2].
In population-based studies, the extent of adherence ranges from as low as 16% (95% CI, 14–18%) in a study conducted in China [8], that used self-reported daily use of NRT for at least 4 weeks period during 3 months of treatment as a measure of adherence, to a high of 35% (95% CI, 29–42%) in a study from the USA that defined adherence as a self-reported number of days, out of 28 days, that the nicotine patch was worn during the quit attempt. Participants were considered adherent if the patch was worn during all 28 days and non-adherent if the nicotine patch was worn for less than 28 days [Table 1].
Level of adherence among pregnant women
A meta-analysis of five studies [35, 37, 38, 40, 45] assessed pregnant women’s adherence to NRT by using the Mantel-Haenszel fixed-effect model. This illustrated that 22% of pregnant women met the definition of adherence used by the study (95% CI, 18–25%) with a p-value of < 0.31 and Higgins’ I2 = 15.8% [Fig. 4]. Funnel plot asymmetry test was conducted to evaluate the presence of publication bias and all studies were found to be symmetrical (p-value = 0.453) [Table 2]. The rate of adherence was found to be as low as 17% in a study conducted in Denmark [15] to a high of 29% in a study conducted in the USA that used total days of NRT use at delivery to measure the level of adherence [11] [Table 1].
Association between adherence and successful quitting
A pooled analysis was done using random effects model among studies that controlled for reverse causality, a potential confounder in assessing adherence and successful smoking cessation [8, 10, 37], illustrated that being adherent to NRT doubled the rate of successful quitting (OR = 2.17, 95% CI 1.34–3.51) with a p-value of < 0.001 and Higgins’ I2 = 77.6% [Fig. 5]. Test for small-study effect was examined by using Egger regression-based test and there was no evidence for small study effect in the pooled result. Both visual inspection and formal test for funnel plot asymmetry indicated symmetrical distribution (Egger’s test P-value = 0.091) [Table 2].
The strongest association between adherence and successful smoking cessation was observed in a study conducted in the USA which concluded that 21 days of adherence to nicotine patch increases the odds of successful quitting by four-fold [OR = 4.20; 95% CI, 1.51–11.72; P = 0.006)] with a significant P-value of 0.006 [37] [Table 1].
Discussion
Overall, the rate of adherence to NRT was found to be more than two folds higher in participants of clinical trials compared to participants of population-based studies. Moreover, only one in five pregnant women were found to be adherent to NRT among clinical trials that enrolled only pregnant women. This review demonstrated that being adherent to NRT doubles the rate of successful smoking cessation among adult daily smokers.
Participants of clinical trials are more motivated to quit smoking and have a better opportunity of obtaining adequate information regarding the safety and efficacy of NRT which could have contributed to the disparity in the level of adherence between participants of trials and population-based studies, as misinformation is a common reason for non-adherence [45, 46]. In a study conducted in the US, misinformation about the safety and efficacy of NRT was linked to a low compliance rate during their smoking cessation attempt. Delivering corrective suggestions was also found to increase awareness and intention to utilise smoking cessation medication s[46]. Randomised controlled trials that incorporate interventions such as counseling with specific emphasis on adherence to NRT and giving information about overcoming challenges to continuing medication use have improved adherence to NRT as well as smoking cessation rates [47, 48]. Compared to non-trials, in clinical trials more attention is given to accomplishing greater medication adherence to NRT [49, 50]. Moreover, participants enrolled in clinical trials may also be more motivated to improve their health, leading to better medication adherence and may differ from the broader population in ways that influence adherence.
The level of adherence was found to be less than a quarter (22%) among pregnant women. This could be due to health professionals’ and women’s concern about the efficacy and safety associated with NRT consumption [51]. Most pregnant women reported safety issues as a cause of non-adherence to NRT, even if evidence showed NRT does not increase the risk of miscarriage, stillbirth, premature birth, low birth weight, admissions to neonatal intensive care, cesarean section, congenital abnormalities or neonatal death [52, 53]. Although NRT is recommended for pregnant women in clinical guidelines in most countries [44, 54, 55], clinicians still hesitate in prescribing NRT during pregnancy [56]. A recent meta-analysis reported a low level of NRT prescription rates for pregnant smokers. Only 25.4% of health care providers reported prescribing NRT for pregnant smokers ever and even very few percentages (6.2%) reported prescribing NRT to pregnant smokers all the time [57]. This hesitancy affects physician counselling concerning adherence and affects clinician-client trust, which has a significant role in medication adherence [58]. Moreover, these reservations regarding the safety of NRT may hamper clinicians to prescribe adequate doses of NRT, as pregnancy is a high metabolic state that requires a relatively higher dose of nicotine to alleviate withdrawal symptoms that may lead to non-adherence [59, 60]. Similarly, a recent review showed the effect of health professionals’ view on the utilisation of NRT. Women feel more confident to use as instructed when the clinician tells NRT is safer than smoking and vice-versa [61].
In this review, adherence to NRT increased the rate of successful smoking cessation by more than two-fold (OR = 2.17, 95% CI 1.34–3.51). This finding is in line with a systematic review conducted in 2013 using clinical trials, which also reported a positive relationship between adherence to NRT and smoking cessation even if it did not compute the magnitude of the impact [62]. Another study assessed the efficacy of nicotine patches and found that consistent use of medications for 3 weeks tripled quitting at the 6 weeks follow up as compared to non-consistent users [43]. Furthermore, interventions aimed at improving adherence to smoking cessation medications raised the rate of short-term and long-term successful smoking cessation [63]. This finding can be explained by the fact that NRT reduces withdrawal symptoms such as craving, depression, restlessness, and irritation by replacing nicotine levels in the bloodstream [30, 64]. Hence, NRT reduces the occurrence of both frequency and strength of urges to smoke [65]. In those participants, who are not taking the medication as prescribed, the extent of withdrawal symptoms will be higher leading to resumed smoking or relapse [65].
Strength and limitations of the study
This is the first systematic review and meta-analysis to assess the level of adherence to NRT and its impact on the success of smoking cessation. Although pooled analyses were conducted among studies that used relatively similar definitions of adherence, studies that recruited the general adult population, and studies with relatively similar follow up periods, the level of heterogeneity was found to be high. This could be due to a lack of uniform strategies to define and measure adherence to NRT and smoking cessation across the literature. Additional limitation could be excluding studies that compared NRT with other active medications such as Varenicline and Bupropion were not included in the analysis. Hence, caution should be taken in interpreting the findings of this review. The limited number of studies evaluating outcomes and the higher level of heterogeneity among the included studies make it challenging to generate strong conclusions, which should be taken into consideration while using the results of the review.
Conclusions and recommendations
This review demonstrated the existing low levels of adherence to NRT among adult participants of population-based studies as compared to clinical trial participants. The level of adherence was found to be the lowest among pregnant women enrolled in clinical trials which could be attributed to additional fetal safety concerns. Moreover, this review demonstrated a strong association between the level of adherence to NRT and the success of a smoking cessation attempt.
This review found that smokers participating in clinical trials are more than two times adherent to NRT than participants in population-based studies, which may explain the gap in the effectiveness of the smoking cessation medications between population-based studies and clinical trials highlighted in the literature. This signifies a need for addressing adherence among individuals on smoking cessation medications. Based on the above-mentioned results, it is recommended to improve attention to adherence as a way to potentially improve smoking cessation success. Furthermore, advocating policies and strategies that improve adherence may potentially improve the quality of care an individual receives during his/her quit attempt. Policies and strategies that may enhance the health professionals’ capacity in providing smoking cessation care and adherence to NRT should be advocated. It is also recommended that clinicians support smokers by enhancing their understanding of NRT and supporting them to address their uncertainties about the safety and efficacy of NRT. As pregnancy is found to have a motivational effect to quit smoking, health professionals and researchers are recommended to support and proactively address women’s concerns about nicotine’s fetal and neonatal effects and discuss the relative benefits and harms of continuing to smoke versus a clean safer source of NRT for cessation [28]. Health programs, policies, and activities should incorporate adherence to NRT as a core component of the intervention. Finally, as the area of adherence to NRT is under investigated, future research is recommended to focus on improving adherence at a broader community level as compliance predicts the success of smoking cessation.
Availability of data and materials
All relevant materials and data supporting the findings of this review are included within the manuscript.
Abbreviations
- PRISMA :
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- MOOSE:
-
Meta-analyses of observational studies in epidemiology
- NRT:
-
Nicotine replacement therapy
References
GBD 2016 Risk Factors Collaborators, Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345-422.
GBD 2015 Tobacco Collaborators, Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389(10082):1885-906.
2008 PHS Guideline Update Panel, Liaisons, and Staff, Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53(9):1217-22.
West R. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol Health. 2017;32(8):1018–36.
Dobrzanski T, Bizon E. Growth hormone (HGH), IRI and total IRI responses to glucose load in selected groups of mental patients. II. Studies on the so-called chlorthalidone-diabetes in chronic schizophrenics. Endokrynol Polska. 1974;25(6):455–60.
Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38.
Yilmazel Ucar E, et al. Effectiveness of pharmacologic therapies on smoking cessation success: three years results of a smoking cessation clinic. Multidiscip Resp Med. 2014;9(1):9–9.
Lam TH, et al. Adherence to nicotine replacement therapy versus quitting smoking among Chinese smokers: a preliminary investigation. Psychopharmacology. 2005;177(4):400–8.
Shiffman S, et al. Real-world efficacy of prescription and over-the-counter nicotine replacement therapy. Addiction. 2002;97(5):505–16.
Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction. 2007;102(5):809–14.
Carls GS, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diab Care. 2017;40(11):1469.
Leslie S, et al. Calculating medication compliance, adherence and persistence in administrative pharmacy claims databases. Pharmaceut Program. 2008;1:13–9.
Comandini A, et al. Markers of anti-oxidant response in tobacco smoke exposed subjects: a data-mining review. Pulm Pharmacol Ther. 2010;23(6):482–92.
Etter JF, Schneider NG. An internet survey of use, opinions and preferences for smoking cessation medications: nicotine, varenicline, and bupropion. Nicotine Tob Res. 2013;15(1):59–68.
Coleman T, et al. Efficacy and safety of nicotine replacement therapy for smoking cessation in pregnancy: systematic review and meta-analysis. Addiction. 2011;106(1):52–61.
DiMatteo MR, et al. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811.
Tonnesen P, et al. A double-blind trial of a nicotine inhaler for smoking cessation. JAMA. 1993;269(10):1268–71.
Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Stroup DF, et al. Meta-analysis of observational studies in EpidemiologyA proposal for reporting. JAMA. 2000;283(15):2008–12.
Dobrzanski T. In vitro study on utilization of glucose in adipose tissue of patients with diabetes mellitus and schizophrenia. [polish]. Endokrynol Polska. 1970;21(1):65–74.
Hollands GJ, et al. Adherence to and consumption of nicotine replacement therapy and the relationship with abstinence within a smoking cessation trial in primary care. Nicotine Tob Res. 2013;15(9):1537–44.
Schneider MP, et al. Electronic monitoring of long-term use of the nicotine nasal spray and predictors of success in a smoking cessation program. Nicotine Tob Res. 2003;5(5):719–27.
Gallagher JE, et al. Public health aspects of tobacco control revisited. Int Dent J. 2010;60(1):31–49.
Heron KE, Smyth JM. Ecological momentary interventions: incorporating mobile technology into psychosocial and health behaviour treatments. Br J Health Psychol. 2010;15(Pt 1):1–39.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from https://www.training.cochrane.org/handbook.
Huedo-Medina TB, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206.
Garrison LP Jr, et al. Using real-world data for coverage and payment decisions: the ISPOR real-world data task force report. Value Health. 2007;10(5):326–35.
Bar-Zeev Y, et al. Nicotine replacement therapy for smoking cessation during pregnancy. Med J Aust. 2018;208(1):46–51.
Balmford J, et al. Adherence to and reasons for premature discontinuation from stop-smoking medications: data from the ITC four-country survey. Nicotine Tobacco Res. 2011;13(2):94–102.
Ben Taleb Z, et al. Predictors of adherence to pharmacological and behavioral treatment in a cessation trial among smokers in Aleppo, Syria. Drug Alcohol Depend. 2015;153:167–72.
Berg CJ, Ahluwalia JS, Cropsey K. Predictors of adherence to behavioral counseling and medication among female prisoners enrolled in a smoking cessation trial. J Correct Health Care. 2013;19(4):236–47.
Bolliger CT, et al. Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety. BMJ. 2000;321(7257):329–33.
Coleman T, et al. A randomized trial of nicotine-replacement therapy patches in pregnancy. Obstet Gynecol Surv. 2012;67(7):387–8.
Fish LJ, et al. Adherence to nicotine replacement therapy among pregnant smokers. Nicotine Tob Res. 2009;11(5):514–8.
Hotham ED, Gilbert AL, Atkinson ER. A randomised-controlled pilot study using nicotine patches with pregnant women. Addict Behav. 2006;31(4):641–8.
Kapur B, et al. Randomized, double-blind, placebo-controlled trial of nicotine replacement therapy in pregnancy. Curr Ther Res. 2001;62(4):274–8.
Shiffman S, et al. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10 week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008;30(10):1852–8.
Voci SC, et al. Association between adherence to free nicotine replacement therapy and successful quitting. Addict Behav. 2016;61:25–31.
Wisborg K, et al. Nicotine patches for pregnant smokers: a randomized controlled study. Obstet Gynecol. 2000;96(6):967–71.
Yingst JM, et al. Reasons for non-adherence to nicotine patch therapy during the first month of a quit attempt. Int J Clin Pract. 2015;69(8):883–8.
West R, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100(3):299–303.
Coleman T, et al. A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med. 2012;366(9):808–18.
Shiffman S, et al. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008;30(10):1852–8.
Canadian Action Network for the Advancement. Dissemination and adoption of practice-informed tobacco treatment. Toronto: Canadian smoking cessation clinical practice guideline; 2011.
Shiffman S, et al. Perceived safety and efficacy of nicotine replacement therapies among US smokers and ex-smokers: relationship with use and compliance. Addiction. 2008;103(8):1371–8.
Ferguson SG, et al. Providing accurate safety information may increase a smoker's willingness to use nicotine replacement therapy as part of a quit attempt. Addict Behav. 2011;36(7):713–6.
Schmitz JM, et al. Medication compliance during a smoking cessation clinical trial: a brief intervention using MEMS feedback. J Behav Med. 2005;28(2):139–47.
Mooney M, et al. Interventions to increase use of nicotine gum: a randomized, controlled, single-blind trial. Nicotine Tobacco Res. 2005;7(4):565–80.
Baker TB, et al. Effects of nicotine patch vs Varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical trial. JAMA. 2016;315(4):371–9.
Hartmann-Boyce J, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018:5.
Bowker K, et al. Understanding pregnant smokers' adherence to nicotine replacement therapy during a quit attempt: a qualitative study. Nicotine Tobacco Res. 2016;18(5):906–12.
Coleman T, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2015;12.
Taylor L, Claire R, Campbell K, Coleman‐Haynes T, Leonardi‐Bee J, Chamberlain C, Berlin I, Davey MA, Cooper S, Coleman T. Fetal safety of nicotine replacement therapy in pregnancy: systematic review and meta‐analysis. Addiction. 2021;116:239–77. https://doi.org/10.1111/add.15185.
National Institute for Health and Care Excellence. Smoking: stopping in pregnancy and after childbirth. London: NICE; 2010.
Ministry of Health. Background and recommendations of the New Zealand guidelines for helping people to stop smoking. Wellington: Ministry of Health; 2014.
Bar-Zeev Y, et al. Clinician factors associated with prescribing nicotine replacement therapy in pregnancy: a cross-sectional survey of Australian obstetricians and general practitioners. Aust N Z J Obstet Gynaecol. 2018;58(3):366–70.
Gould GS, et al. What components of smoking cessation care during pregnancy are implemented by health providers? A systematic review and meta-analysis. BMJ Open. 2019;9(8):e026037.
Jin J, et al. Factors affecting therapeutic compliance: a review from the patient's perspective. Ther Clin Risk Manag. 2008;4(1):269–86.
Taghavi T, et al. Longitudinal influence of pregnancy on nicotine metabolic pathways. J Pharmacol Exp Ther. 2018;364(2):238–45.
Bowker K, et al. Changes in the rate of nicotine metabolism across pregnancy: a longitudinal study. Addiction (Abingdon, England). 2015;110(11):1827–32.
Campbell K, et al. Factors influencing the uptake and use of nicotine replacement therapy and e-cigarettes in pregnant women who smoke: a qualitative evidence synthesis. Cochrane Database Syst Rev. 2020:5.
Raupach T, et al. A systematic review of studies assessing the association between adherence to smoking cessation medication and treatment success. Addiction. 2014;109(1):35–43.
Hollands GJ, et al. Interventions to increase adherence to medications for tobacco dependence. Cochrane Database Syst Rev. 2019;8:Cd009164.
Wiggers LC, et al. Adherence to nicotine replacement patch therapy in cardiovascular patients. Int J Behav Med. 2006;13(1):79–88.
Lindson N, Aveyard P. An updated meta-analysis of nicotine preloading for smoking cessation: investigating mediators of the effect. Psychopharmacology. 2011;214(3):579–92.
Acknowledgments
The authors of this review would like to thank Ms. Debbie Booth for her support during the development of search strategies and Mr. Dinberu Shibeshi for his support during data analysis.
Funding
AM is supported by the University of Newcastle Vice-Chancellor’s Higher Degree by Research Training Scholarship. GG is supported by a National Health and Medical Research Council Translating Research into Practice Fellowship. MB is supported by the National Health and Medical Research Council Early Career Research Fellowship. No other financial support was gained to conduct this review.
Author information
Authors and Affiliations
Contributions
The study was conceptualised by AM, PE, MB, and GG, and overseen by GG. The manuscript was drafted by AM, and revised by GG, PE, and MB. The search strategies developed by AM, and PE. AM and DT involved in citation screening, data extraction, and assessing the quality of the included studies. AM performed the statistical analysis. All authors approved the final draft of the review. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Competing interests
None declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Draft Medline search – Ovid interface.
Additional file 2:
Quality Assessment Tool for observational studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mersha, A.G., Eftekhari, P., Bovill, M. et al. Evaluating level of adherence to nicotine replacement therapy and its impact on smoking cessation: a systematic review and meta-analysis. Arch Public Health 79, 26 (2021). https://doi.org/10.1186/s13690-021-00550-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13690-021-00550-2