Abstract
Background
The acute and long-term benefits of exercise training on cardiovascular health have been well established. The systematic review and meta-analysis aimed to systematically assess the effectiveness of exercise training on arterial stiffness and blood pressure among postmenopausal women with elevated blood pressure.
Methods
A comprehensive search was conducted on PubMed, Embase, Web of Science, ProQuest, Cochrane Library, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov website from inception to September 30, 2023, to identify the randomized controlled trials (RCTs), which evaluated the effectiveness of exercise training on arterial stiffness and blood pressure in postmenopausal women. Standardized mean differences (SMD), weighted mean differences (WMD), and 95% confidence intervals (95% CIs) were calculated using random/fixed effects models. Quality assessment was performed using the modified Jadad scale and the Cochrane Risk of Bias Tool. Sensitivity analysis and subgroup analysis were conducted based on drug dosage, treatment duration, and age of administration to further explore potential heterogeneity. Funnel plots were performed to assess publication bias and Begg’s regression test was carried out for funnel plot asymmetry.
Results
Twenty-two RCTs involving 1978 participants were included in the quantitative analysis. The mean quality of eligible studies was 4.2 out of 7 based on the modified Jadad scale. The results indicated that exercise training had a significant effect on reducing brachial-ankle pulse wave velocity [MD = − 0.69, 95%CI (− 1.11, − 0.27), P = 0.001], decreasing augmentation index (AIx) [MD = − 6.00, 95%CI (− 6.39, − 5.61), P < 0.00001] and AIx normalized to a heart rate of 75 beats per minute (AIx@75%) [MD = − 7.01, 95%CI − 7.91 to − 6.12, P < 0.00001], lowering systolic blood pressure [MD = − 6.19, 95%CI − 9.24 to − 3.15, P < 0.0001], diastolic blood pressure [MD = − 3.57, 95%CI (− 6.10, − 1.03), P = 0.006) and pulse pressure [MD = − 8.52, 95%CI (− 16.27, − 0.76), P = 0.03]. Subgroup analysis revealed that baseline blood pressure levels had a large impact on the effect of exercise training.
Conclusions
The systematic review and meta-analysis suggested that exercise training may ameliorate arterial stiffness and reduce blood pressure in postmenopausal women with elevated blood pressure. However, the optimal mode of exercise training that improves arterial stiffness and blood pressure in this population requires further investigation.
Systematic review registration
PROSPERO CRD42021211268
Similar content being viewed by others
Introduction
The incidence of hypertension is significantly higher in postmenopausal women [1, 2]. Menopause is one of the risk factors for cardiovascular diseases [3], with sex hormone deficiency [4], endothelial dysfunction [5], and arterial stiffness [6]. It has been proved that estrogen plays an important role in inducing the mobilization of endothelial progenitor cells in the bone marrow to promote angiogenesis and repair endothelial damage [7]. However, estrogen deficiency reduces the repair capacity of endothelial cells, ultimately leading to arterial damage and endothelial dysfunction in older women [4, 8, 9]. Abnormal blood pressure may result from decreased arterial compliance associated with endothelial dysfunction [10, 11]. Arterial stiffness is widely regarded as an important predictor and potential therapeutic target for hypertensive patients, with prognostic value for cardiovascular disease [12,13,14,15]. Measurements of arterial stiffness have been recommended as a valuable method in the preventive management of cardiovascular disease [16]. Thereinto, the brachial-ankle pulse wave velocity (baPWV) and augmentation index (AIx) are two important indicators for evaluating arterial stiffness [17, 18].
Adverse reactions such as hypokalaemia, glucose intolerance, and dry cough may occur during the use of antihypertensive drugs, which may reduce patient compliance and decrease treatment effectiveness [16]. The risk of cardiovascular disease in hypertensive patients cannot be reduced to the same level as in healthy people even with rigorous blood pressure treatment [19, 20]. The study found an unsatisfactory effect of antihypertensive drugs on arterial stiffness and PWV [21]. Hormone replacement therapy is common in postmenopausal women, but the improvement in arterial stiffness with standard hormone replacement therapy has not been observed [22]. Lifestyle modifications including exercise training have been recommended by the International Society of Hypertension (ISH) guidelines as the preferred intervention before medications in hypertensive patients [23]. Substantial studies have shown that exercise can not only lower blood pressure but also reduce blood lipid levels [24] and enhance cardiac function [25]. Aerobic and resistance exercise training might be beneficial for the prevention and treatment of hypertension and arterial stiffness [26,27,28]. Data from a randomized control trial (RCT) revealed that stair climbing led to reductions in arterial stiffness, blood pressure, and increases in leg strength in stage 2 hypertensive postmenopausal women [29]. Moreover, exercise training for 12 weeks (180 min per week) improved arterial stiffness in elder women with hypertension [30]. Previous studies have found that aerobic and resistance exercise training improved arterial stiffness and lowered blood pressure in postmenopausal women with elevated blood pressure [29,30,31]. Nevertheless, similar effects were not observed in several other studies [32,33,34,35]. Thus, the potential effects of exercise training on arterial stiffness and blood pressure in postmenopausal women with elevated blood pressure need to be well understood.
The small sample size of these studies may account for the observed differences. Meta-analysis plays a role in comprehensive evaluation by summarizing the results of multiple studies with small sample sizes and performing systematic analysis. The essence of this synthesis is equivalent to increasing the sample size to achieve the purpose of improving the estimation of the effect size. To our knowledge, no previous meta-analyses have been performed to examine the comprehensive effect of different exercise training on arterial stiffness and blood pressure in this population. Therefore, this study conducted a systematic review and meta-analysis to systematically assess the exercise effects on arterial stiffness and blood pressure in postmenopausal women with elevated blood pressure.
Methods
Protocol and registration
The systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Additional file 1) and the protocol has been recorded in PROSPERO (CRD42021211268).
Search strategy
A systematic search was conducted according to the PRISMA statement up to September 30, 2023, using PubMed, Embase, Web of Science, ProQuest, Cochrane Library, ClinicalTrials.gov website (https://clinicaltrials.gov/), and Cochrane Central Register of Controlled Trials (https://www.cochranelibrary.com) to identify eligible randomized trials. In addition, we obtained the references of published studies by manually retrieving, personal communication and other sources. The search terms included “postmenopausal”, “arterial stiffness”, “blood pressure”, “vascular stiffness”, “pulse wave velocity”, “augmentation index”, “pulse pressure” (Additional file 2).
Inclusion and exclusion criteria
We included the following studies: (1) postmenopausal women enrolled in the RCTs were diagnosed with elevated blood pressure based on the 2017 ACA/AHA Guidelines [36]. Postmenopausal women are defined as women with amenorrhea for at least 1 year and/or serum follicle stimulating hormone (FSH) concentration > 40 miU/ml, or women with a history of hysterectomy and bilateral oophorectomy [37]. (2) The intervention group underwent aerobic exercise training, resistance exercise training, or combined exercise training, and the participants were asked to complete the whole exercise course. Participants in the control group were instructed to maintain regular lifestyle habits, keep sedentary, and receive sham training or entertainment programs. Discontinued hormone replacement therapy or have been on stable hormone replacement therapy with or without hypotensive drugs for at least 1 year, and remain unchanged during exercise training. (3) Studies with at least one of the following outcomes: baPWV, systolic blood pressure (SBP), diastolic blood pressure (DBP), PP, AIx, and AIx normalized to a heart rate of 75 beats per minute (AIx@75%).
Excluded studies were as follows: (1) severe comorbidities that make exercise training intolerant during treatment. (2) Data were incomplete or inconsistent, or the full text of the literature could not be obtained. (3) Only one study with the most complete data was included for duplications.
Study selection
Data selection was independently performed by two researchers (DSY and SYT) using EndNote X9 reference management software. After eliminating repetitive literature, the titles and abstracts of all potentially relevant studies were independently examined and the full-text records were retrieved for eligibility, followed by a full-text review. Disagreement on inclusion was resolved by consensus and after discussion with the senior reviewer.
Data extraction
The data was independently extracted by DSY and SYT into an Excel table, including study information (first author, publication year, country, years of collection, registration number, sample size), patient demographics (age, gender), interventions, and the outcomes (baPWV, AIx, AIx@75%, SBP, DBP, PP, and adverse events). Finally, two researchers cross-checked the entered information, and disagreement on information was resolved by consensus after checking with the original studies.
Quality assessment
Quality assessment was conducted independently by two reviewers (DSY and SYT) using the modified Jadad scale. Any disagreement in opinion regarding quality was resolved by discussion consensus with a third investigator (MJS). The modified Jadad scale contains 5 items for RCTs, with a score ranging from 0 to 7: randomization, allocation concealment, blinding, and dropout/withdrawal. A score of 1 to 3 indicates low quality, whereas a score of 4 or more indicates high quality [38]. Cochrane Handbook 5.1.0 [39] was used for assessing the quality of RCTs whereby evaluated the random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. Each trial was ranked as having an unclear, high, or low risk of bias for each item.
Statistical analysis
The meta-analysis was performed using Review Manager (version 5.3.0, Cochrane Collaboration, The Nordic Cochrane Center, Copenhagen) and Stata 12.0. Meta-analysis was conducted if two or more studies provided the same effect concerning the outcomes. Adjusted mean difference (MD) along with their respective standard deviation (SD) were extracted from each of the studies, and each effect size was expressed in a 95% confidence interval (95% CI). Inter-study heterogeneity was evaluated using Cochran’s Q statistics and I2-test. Low heterogeneity was defined as an I2 value less than 25%, moderate heterogeneity as a value of 25 ~ 50%, and high heterogeneity as a value larger than 50%. Heterogeneity was considered significant as either P < 0.10 and I2 > 50%, prompting a random-effects modeling estimate. Otherwise, a fixed-effects approach was used. Sensitivity analysis was considered to examine the influence of each study on the stability of the meta-analysis results. Subgroup analyses were attempted to address potential sources of heterogeneity. Funnel plots were conducted to assess the publication bias of indicators with more than 10 included studies. Furthermore, we also performed Begg’s regression test for funnel plot asymmetry, to verify whether the association between effect sizes and the related standard error was statistically significant.
Results
Search results
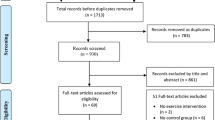
Nineteen thousand nine hundred fifty-five references were identified, including 19,582 trials from the database search, 328 studies from personal communication or hand-searching other review articles, and 45 from trial registries or other sources. Three hundred ninety-five potentially eligible articles were retrieved in full text, of which 22 parallel RCTs were included in the meta-analysis. Figure 1 illustrates the different phases of the search and selection processes.
Study characteristics
As shown in Table 1, among the 22 eligible studies, the efficacy and safety of three exercise types involving aerobic exercise (n = 18), resistance exercise (n = 2), and combined exercise (n = 2) on arterial stiffness and blood pressure in postmenopausal women with elevated blood pressure were examined. Duration of exercise training varied from 6 to 24 weeks. Trials were published from 2001 to 2020 and carried out in the USA, Korea, Canada, Egypt, Turkey, Japan, and Iran. A total of 1978 patients were retrieved in this study, of which 1390 participants were randomly assigned to an exercise training group and 588 were divided into the control group. The number of patients included in these studies ranged from 8 to 464. Taking into account different diseases and clinical conditions, was comprised of postmenopausal women with sedentary, untreated prehypertension, stage 1 hypertension, and stage 2 hypertension.
Arterial stiffness
baPWV
Six eligible RCTs with 243 participants [29, 30, 32, 35, 40, 41] examined the effect of exercise training on baPWV. Combining findings based on the random-effects model, we found that baPWV levels were significantly reduced in the exercise training group compared with the control group [MD = − 0.69, 95%CI (− 1.11, − 0.27), P = 0.001], with high heterogeneity among studies (I2 = 78%, P = 0.0004) (Fig. 2A-1). Sensitivity analysis was performed by excluding studies one by one, and heterogeneity decreased remarkably when the study of Mona Mohamed Taha et al. [41] was removed (Fig. 2A-2). Meanwhile, it was found that there were no significant differences in the sample size, duration of treatment, and other aspects of the six studies by tracing the original literature. The results suggested that exercise training may reduce baPWV and improve arterial stiffness in postmenopausal women with elevated blood pressure.
AIx and AIx@75%
Three trials involving 156 participants [32, 42, 43] reported AIx and AIx@75% as outcomes. Collectively, results from the fixed-effects model (I2 = 0%) indicated that both AIx [MD = − 6.00, 95%CI (− 6.39, − 5.61), P < 0.00001] (Fig. 2B) and AIx@75% [MD = − 7.01, 95%CI (− 7.91, − 6.12), P < 0.00001] (Fig. 2C) levels appeared substantially different in the exercise training group compared with the control group. The findings resulted in statistical significance for the pooled effects, indicating that exercise training can improve the elasticity of the arteries and arterial stiffness in postmenopausal women with elevated blood pressure.
Blood pressure
SBP
Twenty eligible RCTs involving 1934 participants [29,30,31,32,33,34, 40,41,42,43,44,45,46,47,48,49,50,51,52,53] examined the effect of exercise training on SBP. Combined results from the random-effects model (I2 = 94%, P < 0.00001) indicated that the changes in SBP levels were statistically significant before and after exercise training [MD = − 6.19, 95%CI (− 9.24, − 3.15), P < 0.0001] (Fig. 3A). No significant effect for sensitivity was observed. According to the baseline blood pressure levels, SBP between 120 and 129 mmHg was considered elevated SBP, SBP between 130 and 139 mmHg was considered stage 1 hypertension, and SBP ≥ 140 mmHg was defined as stage 2 hypertension [36]. Subgroup analysis showed that there was moderate or high heterogeneity in each subgroup (Fig. 3B). Combined results from the random-effects model (I2 = 57%, P = 0.03) showed that SBP levels changed after exercise training [MD = − 9.97, 95%CI (− 13.00, − 6.93), P < 0.0001] in the population with stage 1 hypertension, indicating a positive effect of exercise training on reducing blood pressure in postmenopausal women with stage 1 hypertension.
DBP
Nineteen eligible trials reported DBP as an outcome [29,30,31,32,33,34, 41,42,43,44,45,46,47,48,49,50, 52,53,54], and the combined results from the random-effects model showed that exercise training had a positive effect on the reduction of DBP [MD = − 3.57, 95%CI (− 6.10, − 1.03), P = 0.006). These studies included a total of 1893 participants, with high heterogeneity between studies (I2 = 96%, P < 0.00001) (Fig. 4A). No significant effect for sensitivity was observed. Meanwhile, we used baseline DBP as the criteria for subgroup analysis. DBP < 80 mmHg was considered as elevated blood pressure, DBP between 80 and 89 mmHg was considered stage 1 hypertension, and DBP ≥ 90 mmHg was defined as stage 2 hypertension, and individuals with SBP and DBP in 2 categories should be designated to the higher blood pressure category [36]. Subgroup analysis revealed that heterogeneity in each subgroup remained high (Fig. 4B). The results suggested that exercise training may reduce DBP, but the reduction value is smaller than that of SBP.
PP
Three eligible trials [35, 43, 49] examined the effect of exercise training on PP. Collectively, results from the fixed-effects model indicated that PP levels were reduced in the exercise training group compared with the control group [MD = − 8.52, 95%CI (− 16.27, − 0.76), P = 0.03), with low heterogeneity between studies (I2 = 0%, P = 0.72) (Fig. 5). The results suggested that exercise may reduce PP, indicating that exercise training can potentially improve the elastic function of arteries.
Adverse events
In all, 22 trials including 1978 patients provided no detailed data on adverse events except Khalid Turky et al. [47]. In this study, one participant had acute back pain at the end of training, which focused on the efficacy and safety of stretching and treadmill walking.
Quality assessment
The qualities of included RCTs were evaluated by the Jadad scale, and the results were summarized in Fig. 6. A total of 12 eligible trials were found to be of high quality. The quality of results ranged from 2 to 7, with an average score of 4.2. All of the studies were randomized, and half of them reported the method used for randomization [29, 33, 40,41,42, 44, 45, 47, 49, 52, 53]. For blinding, seven articles blinded the observer during outcome assessment, but specific blinding methods were not available [40, 42, 44, 45, 47, 52, 53]. Furthermore, dropouts were listed and described in the one of articles [47].
Publication bias assessment
Funnel plots were conducted for SBP and DBP to analyze the potential publication bias of the included studies via Begg’s test (Fig. 7). The results revealed that most of the study sites in the funnel plot were located within 95%CI range, but the shape formed by the study sites was not completely asymmetric, suggesting a potential publication bias of these studies in SBP (P = 0.002) and DBP (P = 0.005), which may be related to the small sample size, different treatment courses, and low quality of included studies.
Discussion
This systematic review and meta-analysis presented a comprehensive overview of several exercise training on arterial stiffness and blood pressure in postmenopausal women with elevated blood pressure reported in 22 RCTs. Different types of aerobic exercise and/or resistance exercise training, such as walking, running, climbing stairs, swimming, resistance band exercises, whole-body vibration, rubber tube, dumbbells, isometric handgrip training, stretching exercises, bench step exercise, yoga, high-intensity interval training, and combined exercise training were included in our study. There was no restriction on the frequency and duration of training. Overall, current evidence may allow recommendations about exercise training for arterial stiffness and blood pressure in postmenopausal women with elevated blood pressure. However, the study findings should be interpreted cautiously given the substantial heterogeneity between available publications and the low quality of the majority of the included studies.
The baPWV is determined by the conduction time between the brachial artery and the ankle regional distance between these two segments. BaPWV reflects the structural and functional stiffness of the arterial wall and is often used to evaluate the level of arterial stiffness in clinical practice, with the characteristics of simple operation, repeatability, and non-invasiveness [55]. Studies showed that both aerobic and resistance exercise training may increase the diameter of the major arteries and help reduce peripheral arterial stiffness, especially the brachial or femoral arteries [56]. The beneficial effect of exercise training on the arteries may be related to the effect of increased blood flow on the endothelium, resulting in structural remodeling and the reduction of vascular smooth muscle tone [57]. The baPWV is primarily determined by central arterial stiffness and is generally higher than the PWV of the upper and lower limb arteries indicating that baPWV may be affected by the peripheral arterial stiffness [58,59,60]. However, the baPWV is an appropriate option for clinical applications. BaPWV reflects vascular stiffness, while AIx is a measure of wave reflection. The AIx is calculated from parameters measured by pulse waves and associated with arterial stiffness [61, 62], and AIx measurements are standardized to a heart rate of 75 bpm [63]. Higher AIx were associated with a higher risk of hypertension [64]. The presence of hypertension is one of the main determinants of the accelerated progression of aortic stiffness in treated hypertensive patients [65]. Arterial stiffness is related to the early return of reflex waves and the increase of amplitude, which is one of the main factors of abnormal blood pressure [66]. Previous studies showed that higher baPWV is closely related to the elderly [67, 68], higher mean arterial blood pressure [69], and hypertension [70]. Besides, elderly women often have a high wave reflection [71]. Arterial stiffness is an indicator of early adverse structural and functional changes in the arterial wall and has been proven as an independent predictor for cardiovascular morbidity and mortality [72,73,74]. Studies found that DBP levels were strongly correlated to AIx [75, 76]. Peripheral pulse pressure also provided a surrogate measure of arterial stiffness, which was considered as an independent predictor of cardiovascular outcomes in hypertensive patients [77].
The results of our systematic review and meta-analysis suggested that the effect of exercise training was superior to no exercise or sedentary in improving arterial stiffness and blood pressure in postmenopausal women with elevated blood pressure. Previous studies have investigated the exercise effects on arterial stiffness and blood pressure in different subjects such as healthy people [77], adults [78], overweight or obese populations [79], and chronic kidney disease [80]. We found that the baPWV of the intervention group was reduced by 0.79 m/s more than that of the control group. At present, there is no unified standard for the definition of minimum clinically important difference (MCID) of arterial stiffness. The findings should be considered meaningful compared with other results [35, 81, 82]. Moreover, heterogeneity decreased remarkably when the study of Mona Mohamed Taha et al. [41] was removed. The average age of participants in Mona Mohamed Taha et al. [41] was less than 50 years old, while patients were all over 50 years old in the remaining studies, suggesting that age might be the source of heterogeneity. Data from the Kailuan study cohort involving 940 participants [83] showed that aerobic exercise had an acute positive effect on arterial stiffness and provided evidence of a greater reduction in arterial stiffness in individuals without hypertension than in those with hypertension. While a meta-analysis [27] suggested that PWV decreased after aerobic exercise training. A review [26] of 10 RCTs indicated that resistance training stand-alone did not elicit changes in the prognosis of cardiovascular diseases in healthy subjects. Similarly, the same result was observed in another study [27]. In our systematic review and meta-analysis, the effect of resistance exercise training [30] and combined exercise training [35] on arterial stiffness was assessed in only one study, respectively, leading to a reduction in the reliability and generalizability of our findings. A meta-analysis involving 21 RCTS found that a combination of aerobic and resistance training interventions may reduce the beneficial effect on arterial stiffness, but did not appear to differ significantly with aerobic training alone [82]. We found that exercise training was beneficial for lowering blood pressure. Sensitivity analysis and subgroup analysis of SBP and DBP failed to reduce heterogeneity. Blood pressure was greatly affected by multiple factors such as small sample size, environment, measurement tools, measurement methods, different treatment courses, and low quality of the included studies, which may be the sources of high heterogeneity. Similar to our results, a systematic review found that exercise training was associated with a reduction in SBP and DBP in menopausal and postmenopausal women with elevated blood pressure [84]. Our findings indicated that exercise training could decrease SBP in postmenopausal women with elevated blood pressure, especially in those with stage 1 hypertension.
Oxidative stress and inflammation are the main mechanisms that cause arterial stiffness [85, 86]. The mechanisms associated with hypertension in postmenopausal women are initiated by the loss of endogenous estradiol and changes in other reproductive hormones. Endothelial dysfunction is an important precursor of cardiovascular disease. Importantly, the decline in endothelial function is independent of age but may be associated with the deficiencies of estrogen and L-arginine in postmenopausal women [87]. Postmenopausal women suffer from a series of changes that include the deficiency of estrogen, increment in proinflammatory cytokines production, strengthening of the oxidative stress response, deduction on L-arginine production, reduction of NO bioavailability, and inferior arterial response to acetylcholine, which are considered to be major causes of vasodilation impairment and endothelial dysfunction [88,89,90]. Potential therapeutic targets include enhancing L-arginine bioavailability and estrogen receptor activation to prevent endothelial dysfunction in postmenopausal women [91]. Aerobic exercise training is beneficial to lowering blood pressure, with a focus on improvements in cardiovascular autonomic control [92] and baroreflex sensitivity [93]. High-intensity resistance training may strongly stimulate the activity of the sympathetic nervous system, leading to increased blood pressure and aggravation of arterial stiffness [94]. Therefore, the long-term benefits of resistance training for postmenopausal women are still worth exploring.
The underlying mechanism of exercise training on arterial stiffness and blood pressure involves multiple pathways. Exercise training plays an active role in increasing the shear stress in the artery wall, enhancing endothelial cell integrity through remodeling, and improving NO bioavailability [95]. Moreover, exercise training is also associated with increased endothelin-1 and NO, enhanced endothelial function, reduced peripheral vascular resistance, and improved arterial stiffness [35]. Other potential mechanisms include improved autonomic nerve function and baroreflex sensitivity, reduced oxidative stress, and lipid deposition [96].
To appropriately interpret our results, several limitations need to be understood. Firstly, studies on aerobic training accounted for the majority of the obtained RCTs, possibly masking the effect of resistance training and combined training on the results. Secondly, the interventions in our study determined that exercise training was difficult to perform using blinding, thus affecting the methodological quality of this review and potential publication bias. However, due to the quality assessment and publication bias evaluation, the impact of the aforementioned potential conflicts on the procedures or results of this systematic review and meta-analysis may be reduced. Thirdly, heterogeneity in some outcomes remained undiminished even after differences in the patient’s characteristics, exercise type, and duration had been considered. The applicability of this systematic review and meta-analysis to the broader patient population may be limited given that most studies involved were conducted in specific countries. Therefore, further studies are required to confirm our results and determine the mechanisms behind the connection between exercise training and arterial stiffness and blood pressure in postmenopausal women with elevated blood pressure.
Conclusions
To conclude, this systematic review and meta-analysis determined a positive association between exercise training and arterial stiffness and blood pressure in postmenopausal women with elevated blood pressure. Existing reviews did not provide more granular evidence in terms of different exercise patterns (e.g., type, quantity, and intensity), and therefore this should be a priority for future studies. Methodologically robust RCTs are required to determine causal links between exercise training and arterial stiffness and blood pressure, and whether this should be by aerobic training or resistance training or a mixture of the two.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. Other data supporting the results of this study are available from the corresponding author upon reasonable request.
References
Rahman M, Williams G, Al MA. Gender differences in hypertension awareness, antihypertensive use and blood pressure control in Bangladeshi adults: findings from a national cross-sectional survey. J Health Popul Nutr. 2017;36(1):23.
Mohanty P, Patnaik L, Nayak G, Dutta A. Gender difference in prevalence of hypertension among Indians across various age-groups: a report from multiple nationally representative samples. BMC Public Health. 2022;22(1):1524.
Shin J, Han K, Jung JH, et al. Age at menopause and risk of heart failure and atrial fibrillation: a nationwide cohort study. Eur Heart J. 2022;43(40):4148–57.
Fu L, Adu-Amankwaah J, Sang L, et al. Gender differences in GRK2 in cardiovascular diseases and its interactions with estrogen. Am J Physiol Cell Physiol. 2023;324(2):C505–16.
Moreau KL, Hildreth KL, Klawitter J, Blatchford P, Kohrt WM. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. Geroscience. 2020;42(6):1699–714.
Tsai SS, Lin YS, Hwang JS, Chu PH. Vital roles of age and metabolic syndrome-associated risk factors in sex-specific arterial stiffness across nearly lifelong ages: possible implication of menopause and andropause. Atherosclerosis. 2017;258:26–33.
Masuda H, Kalka C, Takahashi T, Yoshida M, Wada M, Kobori M, et al. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res. 2007;101(6):598–606.
Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97(12):4692–700.
Gersh F, O'Keefe JH, Elagizi A, Lavie CJ, Laukkanen JA. Estrogen and cardiovascular disease. Prog Cardiovasc Dis. 2024;84:60–7.
Kostov K. The causal relationship between endothelin-1 and hypertension: focusing on endothelial dysfunction, arterial stiffness, vascular remodeling, and blood pressure regulation. Life (Basel). 2021;11(9):986.
Dong L, Liu J, Qin Y, Yang WJ, Nie L, Liu HN, et al. Relationship between ambulatory arterial stiffness index and the severity of angiographic atherosclerosis in patients with H-type hypertension and coronary artery disease. Clin Exp Hypertens. 2023;45(1):2228517.
Wu S, Tian X, Chen S, Zhang Y, Zhang X, Xu Q, et al. Arterial stiffness and blood pressure in treated hypertension: a longitudinal study. J Hypertens. 2023;41(5):768–74.
Tan L, Liu Y, Liu J, Zhang G, Liu Z, Shi R. Association between insulin resistance and uncontrolled hypertension and arterial stiffness among US adults: a population-based study. Cardiovasc Diabetol. 2023;22(1):311.
Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–45.
McEniery CM, Yasmin, Maki-Petaja KM, McDonnell BJ, Munnery M, Hickson SS, et al. The impact of cardiovascular risk factors on aortic stiffness and wave reflections depends on age: the Anglo-Cardiff Collaborative Trial (ACCT III). Hypertension. 2010;56(4):591–7.
Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, et al. 1999 World Health Organization-International Society of Hypertension. Guidelines for the management of hypertension Guidelines sub-committee of the World Health Organization. Clin Exp Hypertens. 1999;21(5–6):1009–60.
Yue X, Chen L, Shi Y, Suo Y, Liao S, Cheang I, et al. Comparison of arterial stiffness indices measured by pulse wave velocity and pulse wave analysis for predicting cardiovascular and all-cause mortality in a Chinese population. Hypertens Res. 2024;47(3):767–77.
Matsui Y, Ishikawa J, Shibasaki S, Shimada K, Kario K. Association between home arterial stiffness index and target organ damage in hypertension: comparison with pulse wave velocity and augmentation index. Atherosclerosis. 2011;219(2):637–42.
Mitchell GF, Conlin PR, Dunlap ME, Lacourcière Y, Arnold JM, Ogilvie RI, et al. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension. 2008;51(1):105–11.
Satoh H, Saijo Y, Kishi R, Tsutsui H. Brachial-ankle pulse wave velocity is an independent predictor of incident hypertension in Japanese normotensive male subjects. Environ Health Prev Med. 2011;16(4):217–23.
Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension. 2010;55(1):9–14.
Langrish JP, Mills NL, Bath LE, Warner P, Webb DJ, Kelnar CJ, et al. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension. 2009;53(5):805–11.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982–1004.
Fletcher B, Berra K, Ades P, Braun LT, Burke LE, Durstine JL, et al. Managing abnormal blood lipids: a collaborative approach. Circulation. 2005;112(20):3184–209.
Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol. 2009;587(Pt 23):5551–8.
Ceciliato J, Costa EC, Azevêdo L, Sousa JC, Fecchio RY, Brito LC. Effect of resistance training on arterial stiffness in healthy subjects: a systematic review and meta-analysis. Curr Hypertens Rep. 2020;22(8):51.
Evans W, Willey Q, Hanson ED, Stoner L. Effects of resistance training on arterial stiffness in persons at risk for cardiovascular disease: a meta-analysis. Sports Med. 2018;48(12):2785–95.
Costa EC, Hay JL, Kehler DS, Boreskie KF, Arora RC, Umpierre D, et al. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with pre- to established hypertension: a systematic review and meta-analysis of randomized trials. Sports Med. 2018;48(9):2127–42.
Wong A, Figueroa A, Son WM, Chernykh O, Park SY. The effects of stair climbing on arterial stiffness, blood pressure, and leg strength in postmenopausal women with stage 2 hypertension. Menopause. 2018;25(7):731–7.
Miura H, Takahashi Y, Maki Y, Sugino M. Effects of exercise training on arterial stiffness in older hypertensive females. Eur J Appl Physiol. 2015;115(9):1847–54.
Staffileno BA, Braun LT, Rosenson RS. The accumulative effects of physical activity in hypertensive post-menopausal women. J Cardiovasc Risk. 2001;8(5):283–90.
Wong A, Figueroa A. Eight weeks of stretching training reduces aortic wave reflection magnitude and blood pressure in obese postmenopausal women. J Hum Hypertens. 2014;28(4):246–50.
Arsenault BJ, Côté M, Cartier A, Lemieux I, Després JP, Ross R, et al. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207(2):530–3.
Swift DL, Earnest CP, Blair SN, Church TS. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. Br J Sports Med. 2012;46(10):753–8.
Son WM, Sung KD, Cho JM, Park SY. Combined exercise reduces arterial stiffness, blood pressure, and blood markers for cardiovascular risk in postmenopausal women with hypertension. Menopause. 2017;24(3):262–8.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e426–83.
Sarri G, Davies M, Lumsden MA, Guideline Development Group. Diagnosis and management of menopause: summary of NICE guidance. BMJ. 2015;351:h5746.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:15928.
Figueroa A, Kalfon R, Wong A. Whole-body vibration training decreases ankle systolic blood pressure and leg arterial stiffness in obese postmenopausal women with high blood pressure. Menopause. 2015;22(4):423–7.
Taha MM, Mohamed MAE, Hasanin ME. Effect of high intensity interval training on endothelial function in postmenopausal hypertensive patients: randomized controlled trial. Int J Physiother. 2016;3(1):39–44.
Wong A, Kwak YS, Scott SD, Pekas EJ, Son WM, Kim JS, et al. The effects of swimming training on arterial function, muscular strength, and cardiorespiratory capacity in postmenopausal women with stage 2 hypertension. Menopause. 2018;26(6):653–8.
Figueroa A, Kalfon R, Madzima TA, Wong A. Effects of whole-body vibration exercise training on aortic wave reflection and muscle strength in postmenopausal women with prehypertension and hypertension. J Hum Hypertens. 2014;28(2):118–22.
Wong A, Alvarez-Alvarado S, Kinsey AW, Figueroa A. Whole-body vibration exercise therapy improves cardiac autonomic function and blood pressure in obese pre- and stage 1 hypertensive postmenopausal women. J Altern Complement Med. 2016;22(12):970–6.
Swift DL, Earnest CP, Katzmarzyk PT, Rankinen T, Blair SN, Church TS. The effect of different doses of aerobic exercise training on exercise blood pressure in overweight and obese postmenopausal women. Menopause. 2012;19(5):503–9.
Lee JA, Kim JW, Kim DY. Effects of yoga exercise on serum adiponectin and metabolic syndrome factors in obese postmenopausal women. Menopause. 2012;19(3):296–301.
Khalid T, Nesreen E, Ramadhan O. Effects of exercise training on postmenopausal hypertension: implications on nitric oxide levels. Med J Malaysia. 2013;68(6):459–64.
Ohta M, Hirao N, Mori Y, Takigami C, Eguchi M, Tanaka H, et al. Effects of bench step exercise on arterial stiffness in post-menopausal women: contribution of IGF-1 bioactivity and nitric oxide production. Growth Horm IGF Res. 2012;22(1):36–41.
Gregory M. The effects of isometric handgrip training on carotid arterial compliance and resting blood pressure in postmenopausal women. Electronic Theses and Dissertations. 2012;4808. https://scholar.uwindsor.ca/etd/4808
Azadpour N, Tartibian B, Koşar ŞN. Effects of aerobic exercise training on ACE and ADRB2 gene expression, plasma angiotensin II level, and flow-mediated dilation: a study on obese postmenopausal women with prehypertension. Menopause. 2017;24(3):269–77.
Nuri R, Kordi MR, Moghaddasi M, Rahnama N, Damirchi A, Rahmani-Nia F, et al. Effect of combination exercise training on metabolic syndrome parameters in postmenopausal women with breast cancer. J Cancer Res Ther. 2012;8(2):238–42.
Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297(19):2081–91.
Son WM, Pekas EJ, Park SY. Twelve weeks of resistance band exercise training improves age-associated hormonal decline, blood pressure, and body composition in postmenopausal women with stage 1 hypertension: a randomized clinical trial. Menopause. 2020;27(2):199–207.
Moreau KL, Degarmo R, Langley J, McMahon C, Howley ET, Bassett DR Jr, et al. Increasing daily walking lowers blood pressure in postmenopausal women. Med Sci Sports Exerc. 2001;33(11):1825–31.
Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. 2017;69(6):1045–52.
da Silva RSN, da Silva DS, Waclawovsky G, Schaun MI. Effects of aerobic, resistance, and combined training on endothelial function and arterial stiffness in older adults: study protocol for a systematic review and meta-analysis. Syst Rev. 2022;11(1):171.
Niebauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. J Am Coll Cardiol. 1996;28(7):1652–60.
Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27(10):2022–7.
O’Rourke MF, O’Rourke JG. Biomarkers: anatomical and physiological. Am J Hypertens. 2007;20(5):467–8.
Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension. 2012;60(2):556–62.
Wilkinson IB, Cockcroft JR, Webb DJ. Pulse wave analysis and arterial stiffness. J Cardiovasc Pharmacol. 1998;32:S33–7.
Kelly R, Hayward C, Avolio A, O’Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80(6):1652–9.
Wilkinson IB, Mohammad NH, Tyrrell S, Hall IR, Webb DJ, Paul VE, et al. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15:24–30.
Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308(9):875–81.
Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105(10):1202–7.
Lyle AN, Raaz U. Killing me unsoftly: causes and mechanisms of arterial stiffness. Arterioscler Thromb Vasc Biol. 2017;37(2):e1–11.
Lehmann ED, Riley WA, Clarkson P, Gosling RG. Non-invasive assessment of cardiovascular disease in diabetes mellitus. Lancet. 1997;350:SI14–9.
Joo HJ, Cho SA, Cho JY, Lee S, Park JH, Hwang SH, et al. Brachial-ankle pulse wave velocity is associated with composite carotid and coronary atherosclerosis in a middle-aged asymptomatic population. J Atheroscler Thromb. 2016;23(9):1033–46.
Zeki Al Hazzouri A, Newman AB, Simonsick E, Sink KM, Sutton Tyrrell K, Watson N, et al. Pulse wave velocity and cognitive decline in elders: the Health, Aging, and Body Composition study. Stroke. 2013;44(2):388–93.
Chou YT, Chen HY, Shen WC, Wu IH, Su FL, Lee WH, et al. Blood pressure levels within normotensive range are independently associated with increased risk of arterial stiffness in adults without hypertension or prehypertension. Nutr Metab Cardiovasc Dis. 2023;33(12):2363–71.
Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, et al. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60(2):362–8.
Tian X, Chen S, Xu Q, Zhang Y, Xia X, Wang P, et al. Temporal relationship between arterial stiffness and blood pressure variability and joint effect on cardiovascular disease. Hypertens Res. 2024;47(5):1133–43.
Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–63.
Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62(6):1105–10.
Nürnberger J, Dammer S, Opazo Saez A, Philipp T, Schäfers RF. Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J Hum Hypertens. 2003;17(3):153–8.
Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92(10):595–600.
Zhou Z, Hou L, Cui M, Mourot L, Zhu W. Acute effects of low-volume intermittent versus higher-volume continuous exercise on arterial stiffness in healthy young men. Sci Rep. 2022;12(1):1749.
Zhang Y, Zhang YJ, Ye W, Korivi M. Low-to-moderate-intensity resistance exercise effectively improves arterial stiffness in adults: evidence from systematic review, meta-analysis, and meta-regression analysis. Front Cardiovasc Med. 2021;8:738489.
Gong L, Liu Y. Effect of exercise training on arterial stiffness in overweight or obese populations. Int J Sports Med. 2022;43(12):996–1012.
Mustata S, Groeneveld S, Davidson W, Ford G, Kiland K, Manns B. Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol. 2011;43(4):1133–41.
Lopes S, Afreixo V, Teixeira M, Garcia C, Leitão C, Gouveia M, et al. Exercise training reduces arterial stiffness in adults with hypertension: a systematic review and meta-analysis. J Hypertens. 2021;39(2):214–22.
Montero D, Vinet A, Roberts CK. Effect of combined aerobic and resistance training versus aerobic training on arterial stiffness. Int J Cardiol. 2015;178:69–76.
Zang Y, Ding X, Zhao MX, Zhang X, Zhang L, Wu S, et al. Arterial stiffness acute changes following aerobic exercise in males with and without hypertension. J Clin Hypertens (Greenwich). 2022;24(4):430–7.
Loaiza-Betancur AF, Chulvi-Medrano I, Díaz-López VA, Gómez-Tomás C. The effect of exercise training on blood pressure in menopause and postmenopausal women: a systematic review of randomized controlled trials. Maturitas. 2021;149:40–55.
Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258–61.
Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, et al. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218(1):90–5.
Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–73.
Hildreth KL, Kohrt WM, Moreau KL. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause. 2014;21(6):624–32.
Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda). 2014;29(4):250–64.
Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol. 2006;41(5):501–7.
Somani YB, Pawelczyk JA, De Souza MJ, Kris-Etherton PM, Proctor DN. Aging women and their endothelium: probing the relative role of estrogen on vasodilator function. Am J Physiol Heart Circ Physiol. 2019;317(2):H395–404.
Sanches IC, Sartori M, Jorge L, Irigoyen MC, De Angelis K. Tonic and reflex cardiovascular autonomic control in trained-female rats. Braz J Med Biol Res. 2009;42(10):942–8.
Bertagnolli M, Campos C, Schenkel PC, de Oliveira VL, De Angelis K, Belló-Klein A, et al. Baroreflex sensitivity improvement is associated with decreased oxidative stress in trained spontaneously hypertensive rat. J Hypertens. 2006;24(12):2437–43.
Raastad T, Glomsheller T, Bjøro T, Hallén J. Changes in human skeletal muscle contractility and hormone status during 2 weeks of heavy strength training. Eur J Appl Physiol. 2001;84(1–2):54–63.
Park SY, Rossman MJ, Gifford JR, Bharath LP, Bauersachs J, Richardson RS, et al. Exercise training improves vascular mitochondrial function. Am J Physiol Heart Circ Physiol. 2016;310(7):H821–9.
Lin YY, Lee SD. Cardiovascular benefits of exercise training in postmenopausal hypertension. Int J Mol Sci. 2018;19(9):2523.
Acknowledgements
All authors are grateful to reviewers and editors for their help and suggestions.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81703894) and the Elite Medical Professionals Project of China-Japan Friendship Hospital (Grant NO. ZRJY2021-GG10).
Author information
Authors and Affiliations
Contributions
DY and ST contributed equally to this study. DY, ST, and MS were responsible for designing the study. DY and ST contributed to the literature searching, data collection, data processing, and manuscript drafting. LH, XX, and JZ contributed to the literature searching and data checking. RY and ZS contributed to the data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, D., Tao, S., Shao, M. et al. Effectiveness of exercise training on arterial stiffness and blood pressure among postmenopausal women: a systematic review and meta-analysis. Syst Rev 13, 169 (2024). https://doi.org/10.1186/s13643-024-02589-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-024-02589-y