Abstract
Background
Evidence on the effects of bovine colostrum (BC) supplementation on gastrointestinal (GI) diseases is conflicting.
Objectives
This systematic review summarized the findings of clinical trials (CTs) on the effects of BC supplementation on GI diseases.
Methods
A systematic search was conducted in online databases, including PubMed, ISI Web of Science, and Scopus, until March 2021 and updated until December 2023. CTs investigated BC’s effect on any measurable symptomatic change in terms of GI health as the primary outcome variable or as one of the outcomes in any population eligible for this systematic review.
Results
Out of 6881 records, 22 CTs (uncontrolled = 4, cross-over = 1, and parallel = 17) with 1427 patients were enrolled in the systematic review. Diarrhea, the most frequently evaluated symptom (20 interventional arms), was decreased in frequency with BC supplementation in 15 of these arms. However, most studies reported no change in its duration. BC supplementation consistently reduced stool frequency across all seven studies. Abdominal pain relief was noted in four interventional arms but showed no improvement in five others. Assessment of other GI symptoms was limited, yielding inconclusive results.
Conclusions
There is limited evidence on the effects of BC on GI diseases, with mixed findings. More well-designed controlled clinical trials are required to explore its effects.
Similar content being viewed by others
Introduction
Gastrointestinal (GI) diseases, which significantly impact global health, affect the GI tract from the mouth to the anus [1]. GI diseases can be categorized as either functional, which are not accompanied by visible structural changes, or structural, such as inflammatory bowel disease (IBD), where both function and appearance of the GI tract are affected [2]. On the other hand, functional GI diseases are characterized by symptoms (including pain, constipation, nausea, bloating, and diarrhea) without any apparent structural changes to the GI tract [3]. GI diseases are among the most common reasons people seek medical care [4], and are typically caused by infections, unhealthy diet, stress, and medications’ side effects [2]. Given the varied causes of GI diseases, there is a growing interest in diverse treatment approaches, including dietary modifications [5, 6]. Dietary approaches are recognized as a novel alternative treatment option for managing of GI diseases [5, 6].
Recently, the therapeutic potential of colostrum in promoting gut health has garnered significant attention [7, 8]. Colostrum is the primary milk secretion of the mammary gland produced by mammals after parturition [9]. Its composition differs from the milk that is subsequently produced. Colostrum contains a higher concentration of fat, protein, peptides, immunoglobulins, vitamins, minerals, hormones, antimicrobial peptides (e.g., lactoferrin, lactoperoxidase), and growth factors, and lower concentration of lactose compared to mature milk [10]. Therefore, the additional benefit of colostrum in the prevention and treatment of GI diseases may be attributed to its higher concentration of immunoglobulins and antimicrobial factors than mature milk [8]. Similarly, bovine colostrum (BC) is a rich source of nutrients and immunological agents [9]. While BC is essential for the nutrition, growth, and development of newborn calves’ GI tract and immune system, its potential therapeutic applications in humans are also being explored [11, 12]. To date, BC has been investigated for several GI diseases [8, 13]. Preventing the effects of BC on intestinal permeability in healthy individuals and patients has been indicated in a recent systematic review [9]. Moreover, a recent meta-analysis demonstrated the effectiveness of BC in reducing the frequency and alleviating symptoms of childhood infectious diarrhea [14]. Despite multiple clinical trials (CTs) evaluating BC’s impact on GI diseases [15,16,17], there still needs to be a consensus on its efficacy [16, 18], while others reported no significant benefits [19, 20]. Recognizing this gap in the literature, our study aims to systematically review the current evidence on BC’s effects on GI diseases.
Methods
This systematic review was written with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [21].
Search strategy
The online databases including PubMed, ISI Web of Science and Scopus were searched systematically up to March 2021 to find relevant publications. We used combinations of the following search terms: (colostrum[all] OR colostrums[all] OR bovine[all] OR cow[all] OR cows[a ll] OR cattle[all]) AND ((Disease[all] AND Gastrointestinal[all]) OR (Diseases[all] AND Gastrointestinal[all]) OR “Gastrointestinal Disease*”[all] OR “Gastrointestinal Disorders”[all] OR “Gastrointestinal Disorder”[all] OR “Functional Gastrointestinal Disorders”[all] OR “Functional Gastrointestinal Disorder”[all] OR (“Gastrointestinal Disorder”[all] AND Functional[all]) OR (“Gastrointestinal Disorders”[all] AND Functional[all])). The complete search strategy is shown in Additional file 1. The reference lists of the retrieved articles were also hand-searched for additional relevant studies. No time or language limitation was applied, and the search was updated until December 2023 using PubMed’s e-mail alert service.
Study selection
Relevant studies were identified based on our PICOS criteria (patients, intervention, comparator, outcome, and study design). Studies with the following criteria were selected: (1) randomized controlled trials (RCTs) or other CTs; (2) being conducted on any population (infants, pediatrics, adults aged, sick and healthy subjects); (3) considering BC or hyperimmune bovine colostrum (HBC) or mixed BC product as intervention; (4) considering no control group or any intervention as a control group; (5) measuring any type of symptomatic change in gastrointestinal health as the primary outcome variable or as one of the outcomes. Studies were excluded if they were non-original (commentaries, editorials, or reviews), animal or in vitro experimental studies, or used non-bovine colostrum (e.g., human colostrum). Unpublished studies or gray literature were also excluded from the current review. Moreover, when a study was performed on separate groups of participants, data of each group compared to the control group were considered an independent study. Two independent authors (PH, LH) screened the retrieved articles using our search strategy to identify potentially eligible studies. Titles and abstracts of articles were reviewed to decide which articles were relevant. Then, full texts of identified articles were reviewed to assess their eligibility based on predefined inclusion and exclusion criteria. Any disagreements were resolved in consultation with the principal investigator (PA).
Data extraction
Two reviewers (PH, LH) independently extracted the following information from each study: first author’s last name; publication date; trial design (single arm/parallel/cross-over); country of origin; mean age or age range of participants; sex of participants; sample size, number of individuals in intervention and control groups, duration of intervention, intervention and control diet, side effects, and outcomes assessed.
Quality assessment
At least two independent members (PH, FH) critically appraised each study. They assessed the risk of bias methodology using Cochrane Collaboration Risk of Bias Tool (ROB) [22] based on the following domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants, personnel (performance bias), and outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. According to the Cochrane Handbook, the judgment of each domain was done using the terms “Low,” “High,” or “Unclear” risk of bias. Low risk of bias interpreted as plausible bias unlikely to seriously alter the results. Unclear risk of bias interpreted as plausible bias that raises some doubt about the results. High risk of bias interpreted as plausible bias that seriously weakens confidence in the results. Any discrepancies were resolved by discussion.
Results
Search results and study selection
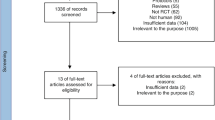
A total of 6881 records were identified through the initial search. After removing duplicates (n = 1940) and screening titles and abstracts, 33 articles remained for further evaluation. The full texts of these articles were read by two independent reviewers (PH and MR) to assess their eligibility, leading to the exclusion of 11 articles. These exclusions were due to the following reasons: non-relevant outcome (n = 6), in vitro studies (n = 2), and non-relevant intervention (n = 3) [23,24,25,26,27,28,29,30,31,32,33]. In order to avoid missing any relevant articles, the reference lists of identified eligible studies were assessed. Hand-searching these articles resulted in identifying n = 4 additional studies. Among these articles, 22 met our inclusion criteria and were enrolled [15,16,17,18,19,20, 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] (Fig. 1).
Study characteristics
Table 1 shows the characteristics of eligible studies for inclusion in the present systematic review. Two studies [29, 36] prescribed BC in intervention and control arms. In the study by Sanctuar et al. [36], participants in the intervention group consumed BC besides probiotics, while those in the control group consumed BC alone. In the other one, the effect of HBC with non-immunized BC was compared [34]. Of the 22 relevant articles (with a total of 31 effect sizes), four were uncontrolled [18, 25, 31, 32], one was cross-over [35], and the remaining 17 studies were parallel RCTs [15,16,17, 19, 20, 24, 26,27,28,29,30, 33,34,35, 37,38,39]. Seven studies (with ten effect sizes) were conducted in Asia [17, 18, 20, 26, 28, 29, 35], six studies (with seven effect sizes) in Europe [16, 27, 31, 32, 34], two studies (with five effect sizes) in Australia [15, 33], three studies in the US [30, 36, 39], and four studies in Africa [24, 25, 37, 38]. Two studies comprised only men [28, 31] and others, both men and women [15,16,17,18,19,20, 24,25,26,27, 29, 30, 32,33,34,35,36,37,38,39]. Of these 22 articles, 14 studies were conducted among infants and children [18,19,20, 26,27,28,29, 33,34,35,36,37,38,39], six studies among adults [15, 17, 24, 25, 30, 31], and two studies among both children and adults [16, 32]. Except for four studies on healthy subjects [15, 26, 30, 37], others were conducted on patients with diarrhea, AIDS, H. pylori, colitis, or hospitalized in intensive care unit (ICU) or deficient birth weight infants. The intervention duration ranged from 3 days [39, 40] to 3 months [41]. Patients in the intervention arm received either BC or HBC with a range of less than 1 [15, 36] to 100 g/d [42]. In all trials, oral BC was prescribed apart from one that used an enema supplement [16]. BC was prescribed solely in most of the studies, but in some studies, it was along with other interventions [24, 36,37,38,39]. In the control group, participants received conventional treatments or market milk or bovine serum albumin. The GI symptoms evaluated in these studies included diarrhea and bowel movement, the frequency and presence of bacterial pathogens in stool, H. pylori infection, abdominal pain, rectal bleeding, nausea, abdominal tenderness, abdominal distension, vomiting, pre-feed significant gastric residuals, constipation, gas frequency, and gut microbiota.
Side effects
Nausea (8.1%) and flatulence (10.8%) [36], skin rashes (5.6%), itching (0.6%) [18], and increased gassiness (25%), and stomachache (12.5%) [35] were the reported side effects by studies participants.
Risk of bias
The within-study risk of bias is summarized in Fig. 2. The Cochrane ROB tool for randomized control and cross-over trials was used to evaluate the risk of bias in the included studies [22]. For the selection bias domains (both randomization and allocation concealment), there were five studies with a high and nine studies with some concerns of bias, with the remaining studies (n = 8) with a low risk of bias. The inadequate generation of allocation sequences and allocation concealment are associated with biased intervention effects. In the domains of performance and detection biases, there was only one study with some concerns and six with a high risk of bias. The remaining 15 studies had low bias. Lack of blinding of participants or healthcare providers or outcome assessors could bias the results by affecting the actual outcomes of the individuals. Attrition bias was high in almost 50% of the included studies, while in the remaining ones, it was low. Differences between people lost to follow-up and those who continue can be the reason for any found effect and not the intervention itself.
All studies had a low risk of bias in the reporting domain. Five studies had a high risk of bias for the “other bias” domain, while the remainder had a low risk of bias (n = 17).
Effect of intervention on outcomes
Diarrhea
Diarrhea was the most evaluated symptom in the included studies (n = 14) [15, 17,18,19, 26, 28, 30,31,32, 35,36,37,38,39]. Out of 20 effect sizes (extracted from 14 studies) examining the frequency or incidence of diarrhea, BC supplementation decreased diarrhea frequency in 15 intervention groups [14, 15, 17, 26, 28, 30,31,32, 35, 36, 38]. In contrast, no beneficial effect was reported in five interventional groups [19, 26, 30, 37]. BC could not shorten diarrhea duration in most studies [26, 35, 39] except for two [26, 28].
Stool frequency/evacuations
Stool frequency/evacuations were also examined in seven trials (8 effect sizes), all consistently suggesting a reduction [24,25,26,27,28, 32, 34]. BC supplementation, either with probiotics or alone, also improved stool consistency [35]. BC did not affect diarrheal stool output in four intervention groups [15] conducted among healthy adults but reduced it in children with rotavirus diarrhea [45], without any significant improvement in virus shedding in the stool [39].
Abdominal pain
Bowel movements or abdominal pain associated with bowel movements and abdominal distention were also examined by six studies (9 interventional arms) [15,16,17, 19, 20, 35, 36]. BC could alleviate abdominal pain in five interventional groups [15, 16, 36] but not in the remaining four articles [17, 19, 20].
Others
Other symptoms were poorly studied. These limited findings demonstrated that BC supplementation could decrease the presence of pathogens in stools [33, 34], improve bowel symptoms score (defined as patients’ well-being, abdominal pain, rectal bleeding, anorexia/nausea, bowel frequency, stool consistency, abdominal tenderness, and the presence of extra-intestinal manifestations), and inflammation [16] and vomiting frequency [48]. No beneficial effect of BC on H. pylori infection [34], the occurrence and clinical signs of NEC including abdominal distension, vomiting, pre-feed significant gastric residuals, blood in stool and ileus [20], severity of intestinal mucositis [19], gut microbiota [35], constipation [17], gastric residuals [20], blood in stool [20], ileus [20], and β-diversity of fecal microbiota [41] was also reported (Table 2).
Moreover, two studies examining the effect of BC on deficient birth weight and ICU-hospitalized patients failed to find any beneficial effect of BC on mortality [17, 20]. Despite a reduction in hospital admission frequency in children with diarrhea [18] following BC consumption, no reduction in the mean length of hospital stay was observed in hospitalized infants [43].
Hyperimmune BC
Five studies (including six trials) evaluated the effect of HBC [9, 18, 29, 30, 35]. These studies were conducted among infants and children but not one [44]. These studies revealed controversial results for the effect of BC on GI symptoms.
While HBC could decrease diarrhea incidence in infants [39] and tended to decrease it in adults [44], no improvement in the diarrhea duration was observed [39]. In the two other trials that examined the effect of monovalent and polyvalent HBC, only polyvalent HBC decreased diarrhea incidence, whereas monovalent HBC showed no significant reduction [47].
HBC also had a beneficial effect on rotavirus [43] but not on H.pylori infection [34].
There was debate about the effect of BC on GI symptoms based on BC dosage and duration of consumption.
Discussion
Recently, the potential of BC as a therapeutic or nutraceutical product has been widely investigated. BC, also known as foremilk, has a nutrient profile and immunological composition, including nutritional factors, immunoglobulins, cytokines, growth factors, nucleosides, oligosaccharides, and antimicrobial agents [49]. Unlike human colostrum, which is enriched with IgA, BC predominantly contains IgG. To evaluate the efficacy of BC, it is essential to report the properties of its active components, such as the concentrations of IgG or antibody titers [50]. Supplementing BC may modulate immune responses, which improves symptoms of gastrointestinal tract disorders, especially inflammation, ulceration, and diarrhea. It seems that the anti-inflammatory effect of BC in GI epithelial cells is based on the suppression of nuclear factor-κB expression [51].
Although numerous BC products exist, details on their origin, extraction, manufacturing process, and standards must be better described, and analytical information is frequently missing from publications [52]. The information given in this review on the impacts of BC on GI diseases illustrates some of these problems.
In the current systematic review, 22 studies were identified which were heterogeneous in terms of their methodology, dosage, and preparation of BC, outcomes, and populations. Diarrhea is one of the GI diseases, which is among the leading causes of mortality and morbidity in the world and inflicts a tremendous health burden on children. Available evidence suggests the beneficial effects of BC supplementation on improving diarrhea in infants and patients [53].
Our systematic review showed that BC supplementation has the potential to improve diarrhea frequency, as well as stool evacuation/ consistency but not the duration of diarrhea [14, 15, 17, 26, 28, 30,31,32, 35, 36, 38]. Additionally, colostrum was found to be effective in reducing the frequency of hospital admissions due to diarrhea in children but not in reducing the length of hospital stay [18, 33]. Diarrhea can be caused by a combination of factors, including exposure to pathogens, the host’s immune system, and environment. Recent evidence indicates that BC supplementation may improve intestinal permeability and integrity, impacting diarrhea improvement [53]. The most common pathogens that cause diarrhea include rotavirus, coronavirus, enterotoxigenic Escherichia coli (E. coli), Cryptosporidium parvum (C. parvum), Salmonella spp., and Clostridium perfringens [54]. Our review showed that BC supplementation could improve bacterial (E. coli) and viral (rotavirus) infections related to diarrhea [33, 34]. Hence, BC may prevent or treat infectious diarrhea [55]. Since the gut is the epicenter of antibiotic resistance, this therapeutic approach is getting more attention from the research community to fight against bacterial infection of GIT than antibiotic-based medication.
Moreover, our systematic review has demonstrated that HBC has beneficial effects on diarrhea [9, 18, 34, 39, 44]. The immunization of cows produces HBC during pregnancy, which has a high level of antigen-specific IgG. HBC has been investigated to treat several enteric pathogens like E. coli, rotavirus, and H. pylori [51]. Two studies have investigated the quality parameters of HBC and found that only polyvalent HBC could reduce the incidence of diarrhea [47]. Polyvalent HBC may inhibit intestinal lipopolysaccharide (LPS) absorption. BC polyvalent immunoglobulins can also increase interleukin (IL)-10 and 13 and anti-inflammatory cytokine expression [56].
Our systematic review showed that HBC conferred a beneficial effect on acute rotavirus infection [33]. It is suggested that the vaccination of cows with uropathogenic Escherichia coli can stimulate a targeted immune response [57], which could be an interesting area for future studies. The specific type of HBC, with neutralizing titer activity against H. pylori, appears to have clinical utility in inhibiting the binding of H. pylori to lipid receptors [51]. In rodent models, HBC has been successfully effective in the reduction of H. pylori bacterial load [58]. However, our review did not find any beneficial effect of HBC on H. pylori infection [29], despite evidence showing that BC can inhibit the adhesion activity of H. pylori [29]. More studies are required to clarify whether HBC can be useful for eradicating H. pylori.
There is some evidence about BC’s anti-nociceptive activities [59]. The reduction in the frequency of bowel movement, abdominal distention, and pain associated with bowel movements following BC consumption was observed in three studies [15, 16, 35]. It has been demonstrated that colostrum contains several bioactive components that can influence the inflammatory process and antimicrobial activities and maintain intestinal immune balance [60]. The use of BC in treating IBD has been identified in just one study in which colostrum enemas ameliorated symptoms of left-side colitis [16]. Future trials should clarify the impact of oral consumption of colostrum in patients with IBD. Up to now, the therapeutic approaches for patients with IBD are still insufficient, and there is a need for alternative treatment options with novel mechanisms of action, like colostrum [61, 62]. Most benefits of BC in IBD seem to derive from its immune-modulating capabilities [24, 25].
Passive immunization is a logical alternative approach to protection from infectious diseases [63]. On a theoretical basis, it is expected that BC can balance and maintain intestinal microbiota. However, no beneficial effect of BC was reported on the β-diversity of fecal microbiota or intestinal microbiome [36, 37]. Additional research focused on the impact of BC on gut microbiota may be needed to confirm these findings.
Minor adverse reactions, such as nausea and flatulence [36], skin rashes/itching [14], and increased gassiness and stomachache [35], have been reported with the use of BC. Also, BC and HBC have been well tolerated. Therefore, BC is a valuable treatment for controlling gastrointestinal diseases with fewer side effects.
Strengths and limitations
Up to now, there is a limited number of RCTs with small sample sizes to evaluate the effects of BC on GI health or diseases. The major limitation of this systematic review is the need for studies on this subject and differences in BC doses, outcomes, and study populations, which did not allow us to perform a meta-analysis in this regard. The lack of unpublished evidence may also be another limitation of this systematic review. Furthermore, most of the included studies had a high risk of bias, which seriously weakens confidence in the results. This paper is a comprehensive systematic review evaluating various GI symptoms following BC consumption. In addition, no restriction on the language and date of publication was made.
Conclusion
This systematic review indicated that the nutraceutical approach of BC could improve the treatment of patients with diarrhea, inflammation, and bowel diseases.
Future lines of research
Further RCTs should be conducted to support the benefits or potential contraindications of BC application in different GI diseases and outcomes to facilitate critical appraisal and interpretation.
Since the quality of BC is affected by many different factors, such as the health status of the cow, mammary glands, the season of the birth, gestation cycle of the cow, health management of the dairy product, and quality of diet during the dry period before parturition in cows, future research should be conducted to shed light on the effects of these factors on gut health. Future research projects are warranted to focus on the BC optimum dose and duration of supplementation. It is also suggested to find possible mechanisms underlying the effects of BC on GI diseases.
References
Ananthakrishnan AN, Xavier RJ. Gastrointestinal diseases. Hunter’s tropical medicine and emerging infectious diseases. 2020. p. 16–26.
Knutsson A, Bøggild H. Gastrointestinal disorders among shift workers. Scand J Work Environ Health. 2010;36:85–95.
Vivier H, Ross EJ, Cassisi JE. Classification of gastrointestinal symptom patterns in young adults. BMC Gastroenterol. 2020;20:326.
Palka M, Krztoń-Królewiecka A, Tomasik T, Seifert B, Wójtowicz E, Windak A. Management of gastrointestinal disorders in Central and Eastern Europe: self-reported practice of primary care physicians. Slov J Public Health. 2014;53:294–303.
El-Salhy M. Nutritional management of gastrointestinal diseases and disorders. Nutrients. 2019;11:3013.
Corsello A, Pugliese D, Gasbarrini A, Armuzzi A. Diet and nutrients in gastrointestinal chronic diseases. Nutrients. 2020;12:1–19.
Menchetti L, Traina G, Tomasello G, Casagrande-Proietti P, Leonardi L, Barbato O, et al. Potential benefits of colostrum in gastrointestinal diseases. Front Biosci (Schol Ed). 2016;8:331–51.
Chandwe K, Kelly P. Colostrum therapy for human gastrointestinal health and disease. Nutrients. 2021;13:1956.
Guberti M, Botti S, Capuzzo MT, Nardozi S, Fusco A, Cera A, et al. Bovine colostrum applications in sick and healthy people: a systematic review. Nutrients. 2021;13:2194.
Glówka N, Durkalec-Michalski K, Woźniewicz M. Immunological outcomes of bovine colostrum supplementation in trained and physically active people: a systematic review and meta-analysis. Nutrients. 2020;12:1023.
Bagwe-Parab S, Yadav P, Kaur G, Tuli HS, Buttar HS. Therapeutic applications of human and bovine colostrum in the treatment of gastrointestinal diseases and distinctive cancer types: the current evidence. Front Pharmacol. 2020;11:01100.
Blair M, Kellow NJ, Dordevic AL, Evans S, Caissutti J, McCaffrey TA. Health benefits of whey or colostrum supplementation in adults ≥35 years; a systematic review. Nutrients. 2020;12:299.
Playford RJ, Macdonald CE, Johnson WS. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am J Clin Nutr. 2000;72:5–14.
Li J, Xu YW, Jiang JJ, Song QK. Bovine colostrum and product intervention associated with relief of childhood infectious diarrhea. Sci Rep. 2019;9:3093.
Otto W, Najnigier B, Stelmasiak T, Robins-Browne RM. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scand J Gastroenterol. 2011;46:862–8.
Khan Z, Macdonald C, Wicks AC, Holt MP, Floyd D, Ghosh S, et al. Use of the “nutriceutical”, bovine colostrum, for the treatment of distal colitis: results from an initial study. Aliment Pharmacol Ther. 2002;16:1917–22.
Eslamian G, Ardehali SH, Baghestani AR, Vahdat SZ. Effects of early enteral bovine colostrum supplementation on intestinal permeability in critically ill patients: a randomized, double-blind, placebo-controlled study. Nutrition. 2019;60:106–11.
Saad K, Abo-Elela MGM, El-Baseer KAA, Ahmed AE, Ahmad FA, Tawfeek MSK, et al. Effects of bovine colostrum on recurrent respiratory tract infections and diarrhea in children. Medicine (Baltimore). 2016;95:e4560.
Rathe M, De Pietri S, Wehner PS, Frandsen TL, Grell K, Schmiegelow K, et al. Bovine colostrum against chemotherapy-induced gastrointestinal toxicity in children with acute lymphoblastic leukemia: a randomized, double-blind, placebo-controlled trial. JPEN J Parenter Enteral Nutr. 2020;44:337–47.
Balachandran B, Dutta S, Singh R, Prasad R, Kumar P. Bovine colostrum in prevention of necrotizing enterocolitis and sepsis in very low birth weight neonates: a randomized, double-blind, placebo-controlled pilot trial. J Trop Pediatr. 2017;63:10–7.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;021(372):n71.
Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training; Available from: https://training.cochrane.org/handbook. [Cited 2022 Feb 24]
Sharma D, Kaur A, Farahbakhsh N, Agarwal S. Role of oropharyngeal administration of colostrum in very low birth weight infants for reducing necrotizing enterocolitis: a randomized controlled trial. Am J Perinatol. 2020;37:716–21.
Playford RJ, Choudhry N, Kelly P, Marchbank T. Effects of bovine colostrum with or without egg on in vitro bacterial-induced intestinal damage with relevance for SIBO and infectious diarrhea. Nutrients. 2021;13:1024.
Inagaki M, Yamamoto M, Jier X, Zhuoma C, Uchida K, Yamaguchi H, et al. In vitro and in vivo evaluation of the efficacy of bovine colostrum against human rotavirus infection. Biosci Biotechnol Biochem. 2010;74:680–2.
Lund P, Sangild PT, Aunsholt L, Hartmann B, Holst JJ, Mortensen J, et al. Randomised controlled trial of colostrum to improve intestinal function in patients with short bowel syndrome. Eur J Clin Nutr. 2012;66:1059–65.
Aunsholt L, Jeppesen PB, Lund P, Sangild PT, Ifaoui IBR, Qvist N, et al. Bovine colostrum to children with short bowel syndrome: a randomized, double-blind, crossover pilot study. JPEN J Parenter Enteral Nutr. 2014;38(1):99–106.
Lodinová-Zádníková R, Korych B, Bartáková Z. Treatment of gastrointestinal infections in infants by oral administration of colostral antibodies. Nahrung. 1987;31:465–7.
Bölke E, Jehle PM, Hausmann F, Däubler A, Wiedeck H, Steinbach G, et al. Preoperative oral application of immunoglobulin-enriched colostrum milk and mediator response during abdominal surgery. Shock. 2002;17:9–12.
Malin M, Verronen P, Korhonen H, Syvaoja EL, Salminen S, Mykkanen H, et al. Dietary therapy with Lactobacillus GG, bovine colostrum or bovine immune colostrum in patients with juvenile chronic arthritis: evaluation of effect on gut defence mechanisms. Inflammopharmacology. 1997;5:219–36.
Morrison SA, Cheung SS, Cotter JD. Bovine colostrum, training status, and gastrointestinal permeability during exercise in the heat: a placebo-controlled double-blind study. Appl Physiol Nutr Metab. 2014;39:1070–82.
Davison G, Marchbank T, March DS, Thatcher R, Playford RJ. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am J Clin Nutr. 2016;104:526–36.
Marchbank T, Davison G, Oakes JR, Ghatei MA, Patterson M, Moyer MP, et al. The nutriceutical bovine colostrum truncates the increase in gut permeability caused by heavy exercise in athletes. Am J Physiol Gastrointest Liver Physiol. 2011;300:477–84.
Casswall TH, Sarker SA, Albert MJ, Fuchs GJ, Bergström M, Björck L, et al. Treatment of Helicobacter pylori infection in infants in rural Bangladesh with oral immunoglobulins from hyperimmune bovine colostrum. Aliment Pharmacol Ther. 1998;12:563–8.
Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS ONE. 2019;14:e0210064.
Rump JA, Arndt R, Arnold A, Bendick C, Dichtelmüller H, Franke M, et al. Treatment of diarrhoea in human immunodeficiency virus-infected patients with immunoglobulins from bovine colostrum. Clin Investig. 1992;70:588–94.
Plettenberg A, Stoehr A, Stellbrink HJ, Albrecht H, Meigel W. A preparation from bovine colostrum in the treatment of HIV-positive patients with chronic diarrhea. Clin Investig. 1993;71:42–5.
Florén CH, Chinenye S, Elfstrand L, Hagman C, Ihse I. ColoPlus, a new product based on bovine colostrum, alleviates HIV-associated diarrhoea. Scand J Gastroenterol. 2006;41:682–6.
Ebina T, Sato A, Umezu K, Ishida N, Ohyama S, Oizumi A, et al. Prevention of rotavirus infection by oral administration of cow colostrum containing antihumanrotavirus antibody. Med Microbiol Immunol. 1985;174:177–85.
Gaensbauer JT, Melgar MA, Calvimontes DM, Lamb MM, Asturias EJ, Contreras-Roldan IL, et al. Efficacy of a bovine colostrum and egg-based intervention in acute childhood diarrhoea in Guatemala: a randomised, double-blind, placebo-controlled trial. BMJ Glob Health. 2017;2:e000452.
Bierut T, Duckworth L, Grabowsky M, Ordiz MI, Laury ML, Callaghan-Gillespie M, et al. The effect of bovine colostrum/egg supplementation compared with corn/soy flour in young Malawian children: a randomized, controlled clinical trial. Am J Clin Nutr. 2021;113:420–7.
Kaducu FO, Okia SA, Upenytho G, Elfstrand L, Florén CH. Effect of bovine colostrum-based food supplement in the treatment of HIV-associated diarrhea in Northern Uganda: a randomized controlled trial. Indian J Gastroenterol. 2011;30:270–6.
Davidson GP, Daniels E, Nunan H, Moore AG, Whyte PBD, Franklin K, et al. Passive immunisation of children with bovine colostrum containing antibodies to human rotavirus. Lancet. 1989;2:709–12.
Okhuysen PC, Chappell CL, Crabb J, Valdez LM, Douglass ET, DuPont HL. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin Infect Dis. 1998;26:1324–9.
Sarker SA, Casswall TH, Mahalanabis D, Alam NH, Albert MJ, Brüssow H, et al. Successful treatment of rotavirus diarrhea in children with immunoglobulin from immunized bovine colostrum. Pediatr Infect Dis J. 1998;17:1149–54.
Huppertz HI, Rutkowski S, Busch DH, Eisebit R, Lissner R, Karch H. Bovine colostrum ameliorates diarrhea in infection with diarrheagenic Escherichia coli, Shiga toxin-producing E. Coli, and E. coli expressing intimin and hemolysin. J Pediatr Gastroenterol Nutr. 1999;29:452–6.
Tawfeek HI, Najim NH, Al-Mashikhi S. Efficacy of an infant formula containing anti-Escherichia coli colostral antibodies from hyperimmunized cows in preventing diarrhea in infants and children: a field trial. Int J Infect Dis. 2003;7:120–8.
Barakat SH, Meheissen MA, Omar OM, Elbana DA. Bovine colostrum in the treatment of acute diarrhea in children: a double-blinded randomized controlled trial. J Trop Pediatr. 2020;66:46–55.
Uruakpa FO, Ismond MAH, Akobundu ENT. Colostrum and its benefits: a review. Nutr Res. 2002;22:755–67.
Spalinger MR, Atrott K, Baebler K, Schwarzfischer M, Melhem H, Peres DR, et al. Administration of the hyper-immune bovine colostrum extract IMM-124E ameliorates experimental murine colitis. J Crohns Colitis. 2019;13:785–97.
Gomes RDS, Anaya K, Galdino ABS, Oliveira JPF, Gama MAS, Medeiros CACX, et al. Bovine colostrum: a source of bioactive compounds for prevention and treatment of gastrointestinal disorders. NFS Journal. 2021;1:1–11.
Struff WG, Sprotte G. Bovine colostrum as a biologic in clinical medicine: a review. Part I: biotechnological standards, pharmacodynamic and pharmacokinetic characteristics and principles of treatment. Int J Clin Pharmacol Ther. 2007;45:193–202.
Ali M, Abbas F, Shah AA. Factors associated with prevalence of diarrhea among children under five years of age in Pakistan. Child Youth Serv Rev. 2022;132:106303.
Bartels CJM, Holzhauer M, Jorritsma R, Swart WAJM, Lam TJGM. Prevalence, prediction and risk factors of enteropathogens in normal and non-normal faeces of young Dutch dairy calves. Prev Vet Med. 2010;93:162–9.
Choudhry N, Scott F, Edgar M, Sanger GJ, Kelly P. Reversal of pathogen-induced barrier defects in intestinal epithelial cells by contra-pathogenicity agents. Dig Dis Sci. 2021;66:88–104.
Ghosh S, Iacucci M. Diverse immune effects of bovine colostrum and benefits in human health and disease. Nutrients. 2021;13:3798.
Larcombe S, Hutton ML, Lyras D. Hyperimmune bovine colostrum reduces gastrointestinal carriage of uropathogenic Escherichia coli. Hum Vaccin Immunother. 2019;15:508–13.
Casswall TH, Nilsson HO, Björck L, Sjöstedt S, Xu L, Nord CE, et al. Bovine anti-Helicobacter pylori antibodies for oral immunotherapy. Scand J Gastroenterol. 2002;37:1380–5.
Ceniti C, Costanzo N, Morittu VM, Tilocca B, Roncada P, Britti D. Review: colostrum as an emerging food: nutraceutical properties and food supplement. 2022. p. 1–29.
Sienkiewicz M, Szymańska P, Fichna J. Supplementation of bovine colostrum in inflammatory bowel disease: benefits and contraindications. Adv Nutr. 2021;12:533–45.
Bernstein CN, Eliakim A, Fedail S, Fried M, Gearry R, Goh KL, et al. World Gastroenterology Organisation global guidelines inflammatory bowel disease: update August 2015. J Clin Gastroenterol. 2016;50:813–8.
Bagwe S, Tharappel LJP, Kaur G, Buttar HS. Bovine colostrum: an emerging nutraceutical. J Complement Integr Med. 2015;12:175–85.
Godhia ML, Patel N. Colostrum - its composition, benefits as a nutraceutical : a review. Curr Res Nutr Food Sci. 2013;1:37–47.
Acknowledgements
The authors would like to thank the research chancellor of Isfahan University of Medical Sciences in support of the conduct of this research.
Funding
There was no financial support for this research.
Author information
Authors and Affiliations
Contributions
PA, PH, and NK contributed to the design of review protocol, define the research theme, and screen the eligible studies. MRK, FH, and LH contributed to screen the eligible studies, conduct data extraction, and revise the article. PH, FH, and NK contributed to make the tables, and write the article. BT and HN contributed to interpret the results and give expert advice on the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hajihashemi, P., Haghighatdoost, F., Kassaian, N. et al. Therapeutics effects of bovine colostrum applications on gastrointestinal diseases: a systematic review. Syst Rev 13, 76 (2024). https://doi.org/10.1186/s13643-024-02489-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-024-02489-1