Abstract
Background
Parkinson’s disease (PD) is a neurodegenerative disorder of the nervous system that affects movement. Individuals with PD commonly experience difficulty initiating movements, slowness of movements, decreased balance, and decreased standing ability. It has been shown that these motor symptoms adversely affect the independence of individuals with PD. Imagery is the cognitive process whereby a motor action is internally reproduced and repeated without overt physical movement. Recent studies support the use of imagery in improving rehabilitation outcomes in the PD population. However, these data have inconsistencies and have not yet been synthesised. The study will review the evidence on the use of imagery in individuals with PD and to determine its efficacy in improving rehabilitation outcomes.
Methods
Randomised controlled clinical trials comparing the effects of imagery and control on activities, body structure and function, and participation outcomes for people with PD will be included. A detailed computer-aided search of the literature will be performed from inception to June 2021 in the following databases: MEDLINE, EMBASE, CINAHL, PsycINFO, Cochrane Library, Web of Science, and Scopus. Two independent reviewers will screen articles for relevance and methodological validity. The Physiotherapy Evidence Database (PEDro) scale will be utilised to evaluate the risk of bias of selected studies. Data from included studies will be extracted by two independent reviewers through a customised, pre-set data extraction sheet. Studies using imagery with comparable outcome measures will be pooled for meta-analysis using the random effect model with 95% CI. If individual studies are heterogeneous, a descriptive review will analyse variance in interventions and outcomes. A narrative data analysis will be considered where there is insufficient data to perform a meta-analysis.
Discussion
Several studies investigating imagery in the PD population have drawn dissimilar conclusions regarding its effectiveness in rehabilitation outcomes and clinical applicability. Therefore, this systematic review will gather and critically appraise all relevant data, to generate a conclusion and recommendations to guide both clinical practice and future research on using imagery in the rehabilitation of people with PD.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Systematic review registration
PROSPERO registration number CRD42021230556.
Similar content being viewed by others
Background
Description of condition
Parkinson’s disease (PD) is a chronic, progressive, neuro-degenerative disease, characterised by a break-down of the cells of the substantia nigra—the area of the brain largely responsible for planning and controlling body movements [1, 2]. All individuals with PD experience different symptoms at different times with varying severity. Early-stage PD is characterised by unilateral and mild symptoms such as a slight tremor [3]. Due to its progressive nature, mid-stage PD typically presents with a significant slowing of body movements and slightly decreased balance, posture, and gait. Advanced-stage PD is distinguished by severe symptoms, limited or no walking and standing ability, rigidity, and bradykinesia (slowness of movements, difficulty initiating movements) [4].
These motor symptoms affect the three levels of functioning according to the International Classification of Functioning (ICF): body structure and function, activities, and participation [5,6,7]. They impact independence and an individual’s ability to perform their meaningful everyday activities [6, 8].
Description of intervention
Imagery may be a suitable non-pharmacologic treatment for such decline in individuals with PD. It can be classified into two categories, visual and kinesthetic imagery [9]. Both involve imagination or visualisation. In visual imagery, an individual will visualise moving a limb without the actual sensing of the muscles. During kinesthetic imagery, the person imagines the muscle movement for an action. In a therapeutic context, imagery is the cognitive process whereby a particular motor action or kinesthetic experience is internally reproduced (visualised or ‘imagined’) and repeated extensively without any physical movement [10]. Imagery can be used to promote one’s learning or enhancing of a motor skill or general wellbeing. During imagery, actions can be viewed internally (the individual views the task execution from a first-person perspective) or externally (the individual ‘views’ themselves executing task from a third-person perspective) [11]. Imagery can also be externally cued-guided by a therapist or other, or internally cued, by the individual themselves. For example, imagery may involve an individual dividing motor actions into single steps and imagining these single steps from their own perspective or through ‘viewing’ themselves completing the actions.
How the intervention might work
The theory behind imagery is that imagining an action shares the same neural mechanisms as unconscious motor preparation [10]. It has also been proven that imagined and executed actions share the same neural structures and recruit overlapping brain regions [12]. Thus, repeated practicing of imagined motor tasks is as effective as physical practice of motor tasks.
The way this happens is not fully understood. But it is hypothesised that both psychological and physiological mechanisms are involved [13]. The psychological mechanism is suggested to be improving the cognitive elements of skills, including breaking an action down into steps, attentional focus, and promoting learning of movement strategies by exploring different execution patterns. The theorised physiological mechanisms include neural changes in the central nervous system, like greater relaxation and altered programming of the motor system itself [14]. Therefore, individuals with PD who perform imagery in a therapeutic context may experience improvements in motor planning and motor action execution.
Importance of doing this review
Imagery has been proven effective in other neurological conditions including stroke [15,16,17,18,19]. There are a handful of studies showing that imagery can have positive effects in rehabilitation in PD [20, 21]. These changes include improved arm-hand ability, performance of activities of daily living, cognition, and motivation. New research has provided initial evidence on the positive use of imagery in PD rehabilitation when used in combination with other therapies, including action observation [22, 23].
Whilst there is a growing body of evidence highlighting the effectiveness of imagery in individuals with PD, there are conflicting data in current literature. Tremblay, Leonard [24] found that study participants with PD could not engage in observation and imagery as effectively as healthy controls. However, other studies indicate individuals with PD can successfully engage in imagery. One such study found no significant difference in completion times of imagined and physical tasks in participants with PD in an ‘ON’ medication state [25].
To date, there is no synthesised evaluation of the literature regarding the efficacy of imagery in PD rehabilitation. Da Silva, de Sales [26] offer comprehensive descriptions of imagery protocols in PD in their systematic review. Further research to determine the efficacy of these protocols is needed to bridge this gap in knowledge.
Objective
The objective of this study is to gather and synthesise current research on the use of imagery in individuals with PD and to determine its efficacy in improving rehabilitation outcomes as classified by the categories of the ICF [5].
Methods
The protocol was developed according to the Preferred Reporting Items of Systematic Reviews and Meta-Analyses Protocol (PRISMA-P) guidelines [27] and has been registered on PROSPERO database (Ref: CRD42021230556).
Criteria for selecting studies for this review
Type of studies
Randomised controlled clinical trials comparing one group undergoing treatment with imagery and a control group will be considered eligible for the present study.
Types of participants
Studies involving individuals with a diagnosis of PD (any sex, age, stage of disease progression) will be included. Studies that administer imagery to individuals with PD with dementia or other diseases, such as Alzheimer’s disease, stroke, or multiple sclerosis, will be excluded.
Types of interventions
Studies addressing the treatment of individuals with PD using imagery protocols with a focus on the outcomes of interest will be selected. Imagery was defined as the cognitive process of internally reproducing or ‘imagining’ a motor action repeatedly without any physical movement. Interventions requiring specialised equipment (including electro-myographic stimulation or virtual reality technologies) and those associated with medication beyond the participants’ habitual medications will not be included in the present systematic review. For the control groups, both active and passive control types will be considered.
Types of outcome measures
Studies that report results related to ‘body structure and function’ (i.e. muscle strength and cognition), ‘activities’ (i.e. activities of daily living, gait and mobility), and ‘participation’ (i.e. quality of life) using standardised or non-standardised assessments before and after the intervention or follow-up will be included. The primary outcome measures of interests will be activities of daily living performance under the ‘activities’ category. It includes self-care such as feeding and dressing and instrumental activities of daily living such as meal preparation and doing laundry. The secondary outcome measures include gait and walking in the ‘activities’ category such as functional mobility and measures capturing walking speed or cadence, motor and cognitive function in the ‘body structure and function’ category such as balance, attention and concentration, memory, visuospatial ability and executive function and quality of life measures in the ‘participation’ category. These categories have been selected as they are the three domains of functioning in the ICF [5] and thus have the capacity to represent a holistic perspective on rehabilitation.
Search strategy for identification of studies
Electronic searches
We will conduct electronic searches in the Medical Literature Analysis and Retrieval System Online (MEDLINE) (via OVID), Embase Biomedical Answers (Embase) (via OVID), Web of Science, The Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via Ebscohost), Scopus and PsycINFO (via Ebscohost). The search strategy was created considering terms related to the main outcomes of interest. A combination of search terms and Medical Subject Heading terms will be used (‘Parkinson disease’ or ‘parkinsonism’, ‘Parkinson’ or ‘hypokinesia’, ‘imagery’, ‘guided imagery’ or ‘imagination’, ‘motor imagery’, ‘mental imagery’, ‘simulated movement’ or ‘visuomotor imagery’, ‘rehabilitation’ (Table 1 in Appendix for a sample search). A systematic literature search strategy will be conducted from inception to June 2021.
Search of other sources
We will perform a hand search of the reference lists of the studies included in the review to identify any potentially relevant studies not retrieved during the electronic search. The grey literature will not be searched.
Data collection and analysis
Selection of studies
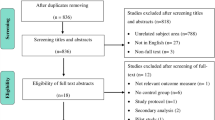
We follow the PRISMA guidelines in the study selection (Fig. 1). Databases will be searched by one reviewer (TS) to identify potential titles and abstracts. Two independent reviewers (KL and TS) will screen the titles and abstracts of the publications retrieved during the electronic search based on the eligibility criteria. Potentially relevant studies will then undergo full-text analysis. The entire selection process will be performed by consensus. If no consensus can be reached on a given study, a third reviewer will be consulted for the final decision. We will use the Covidence Review during the selection of the studies (www.covidence.org). Covidence is a systematic review software which assists in title and abstract screening, full-text screening and extracting study characteristics.
Data extraction
After the selection of the studies, the two reviewers (TS and KL) will work independently. A data extraction form will be developed by the research team and piloted independently by two reviewers on 10% of the identified studies and modified as required prior to use. The following information will be extracted from each study: country/setting, study design, sample size, sex of participants, mean stage of PD, mean disease duration characteristics of participants; intervention and control details, number of sessions and frequency of treatment, outcome measures and major findings. The intended outcomes will include activities of daily living performance, gait and walking, motor and cognitive function and quality of life measures. Disagreements concerning data extraction will be resolved through discussion. A third reviewer will be consulted where a consensus cannot be reached.
Assessment of risk of bias
Two independent reviewers (TS and KL) will utilise the Physiotherapy Evidence Database (PEDro) scale to assess the risk of bias [28]. The PEDro is scored out of 10, as item 1 is not included in score calculation as it represents external validity. Any disagreements between reviewers will be resolved through discussion. A third reviewer will be consulted where a consensus cannot be reached. We will acknowledge and report on concerns of bias that can influence the outcome of this review, in particular selection bias, performance bias and publication bias. Studies with a total score less than 50% of the maximum are considered to have low methodological quality [28].
Measures of treatment effect
For studies with comparable outcome measures, data will be pooled for meta-analysis. Continuous data will be presented as 95% confidence interval (CI) and mean difference (MD). For dichotomous data, the risk ratio (RR) and 95 % CI will be calculated. Furthermore, the number needed to treat (NNT) will be calculated.
Dealing with missing data
In case of missing data, authors will be contacted to provide further information. If authors fail to provide information within two months, intention-to-treat analysis will be used for the extrapolated data. The impact of this will be reported in the discussion section of the systematic review.
Assessment of heterogeneity
Heterogeneity will be addressed by pooling studies which investigate the same intervention and outcomes in individuals with PD. Statistical heterogeneity will be assessed through I2 statistics with a cut-off score of 50% using the ‘metafor’ package in R software.
Assessment of reporting biases
The methods section of the articles will be compared with the results section. If sufficient data is included, we will assess reporting bias by using a funnel plot.
Data synthesis
Data of the clinically important outcome measures from selected articles will be pooled to increase the overall sample size. This will produce a meta-analysis or descriptive review. If the studies are homogeneous and use the outcome measures reflecting activities of daily living performance, gait and walking, motor and cognitive function, or quality of life, variables will be statistically analysed. If outcome measures used in individual studies are heterogeneous and cannot be pooled for a meta-analysis, a descriptive review will analyse variance in interventions and outcomes.
If a meta-analysis is conducted, an analysis of the results on the outcome measures will be performed using post-intervention scores (means and SDs) to determine the overall effectiveness between the experimental and control interventions. An analysis of the long-term carryover effect post-intervention will also be conducted. The follow-up period is considered as the period following the initial post-intervention data collection. Corresponding authors will be contacted via email for original data where the published data was insufficient for data analysis.
All analysis will be performed using the ‘metafor’ package in R software, where the fixed effect or the random effect model with 95% CI is applied. Random effects models will be used, if the estimated effects in the included studies are not identical. A funnel plot can be included to detect bias in the meta-analysis results or systematic heterogeneity in the selected studies [29].
Discussion
Given the impact of PD on individuals’ body structure and function, activities and participation, it is imperative to review the evidence regarding the effectiveness of imagery in the rehabilitation of people with PD. Therefore, the proposed systematic review will explore the efficacy of imagery as treatment in improving overall rehabilitation outcomes in individuals with PD. Imagery has a strong neuroscience base. It is a cost-effective and non-invasive treatment with limited adverse complications. As such, the outcomes of this review will guide clinical practice and inform future research in enhancing outcomes for people with PD. By conducting a systematic search and meta-analysis of available studies examining the effectiveness of imagery in populations with PD, this review will influence health care outcomes by providing best practice guidelines to promote and aid rehabilitation for people with PD.
This systematic review may be limited by a few factors. For example, most of the included studies may utilise imagery as part of the intervention. This may limit the examination of the effect of using imagery only on the reported outcomes. A report on the confidence in the cumulative evidence, such as using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, may be used. A structured approach for summarising the quality of evidence can be used in a clinical guideline.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CINAHL:
-
Cumulative Index to Nursing and Allied Health Literature
- Embase:
-
Embase Biomedical Answers
- ICF:
-
International Classification of Functioning
- MEDLINE:
-
Medical Literature Analysis and Retrieval System Online
- MD:
-
Mean difference
- NNT:
-
Number needed to treat
- PD:
-
Parkinson’s disease
- PEDro:
-
Physiotherapy Evidence Database
- PRISMA-P:
-
Preferred Reporting Items of Systematic Reviews and Meta-Analyses Protocol
- RR:
-
Risk ratio
References
Aragon A, Kings J. Occupational therapy for people with Parkinson’s. 2nd ed. London: Royal College of Occupational Therapists; 2018.
Pfeiffer R, Wszolek ZK, Ebadi MS, ebrary I. Parkinson’s disease. 2nd ed. Boca Raton: CRC Press; 2013.
Nieuwboer A, Rochester L, Müncks L, Swinnen SP. Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord. 2009;15:53–S8.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42.
World Health Organisation. International Classification of Functioning, Disability and Health. Geneva: ICF; 2001.
Parashos SA, Luo S, Biglan KM, Bodis-Wollner I, He B, Liang GS, et al. Measuring disease progression in early Parkinson disease: the National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) experience. JAMA Neurol. 2014;71(6):710–6.
Dixon L, Duncan D, Johnson P, et al. Occupational therapy for patients with Parkinson's disease. Cochrane Database Syst Rev. 2007;2007(3):CD002813. https://doi.org/10.1002/14651858.CD002813.pub2.
Barbieri FA, Rinaldi NM, Santos PCR, Lirani-Silva E, Vitório R, Teixeira-Arroyo C, et al. Functional capacity of Brazilian patients with Parkinson’s disease (PD): relationship between clinical characteristics and disease severity. Arch Gerontol Geriatr. 2012;54(2):83–8.
Mizuguchi N, Nakata H, Uchida Y, Kanosue K. Motor imagery and sport performance. J Phys Fitness Sports Med. 2012;1(1):103–11.
Abraham A, Duncan R, P., Earhart GM. The role of mental imagery in Parkinson’s disease rehabilitation. Brain Sci. 2021;11(2):185.
Nascimento IAPS, Santiago LMM, de Souza AA, Pegado CL, Ribeiro TS, Lindquist ARR. Effects of motor imagery training of Parkinson's disease: a protocol for a randomized clinical trial. Trials. 2019;20(1):1–8.
Abbruzzese G, Avanzino L, Marchese R, Pelosin E. Action observation and motor imagery: innovative cognitive tools in the rehabilitation of Parkinson's disease. Parkinsons Dis. 2015;2015:124214. https://doi.org/10.1155/2015/124214.
Silva DM, Coriolano MGWS, Macêdo JGF, Silva LP, Lins OG. Practice of mental protocols used in rehabilitation of patients with Parkinson’s disease: a systematic review. Acta Fisiátrica. 2016;23(3):155–60.
Ponde PDS, Neto WK, Rodrigues DN, Cristina L, Bastos MF, Sanches IC, et al. Chronic responses of physical and imagery training on Parkinson’s disease. Rev Bras De Med Do Esporte. 2019;25(6):503–8.
Bovend'Eerdt TJ, Dawes H, Sackley C, Izadi H, Wade DT. An integrated motor imagery program to improve functional task performance in neurorehabilitation: a single-blind randomized controlled trial. Arch Phys Med Rehabil. 2010;91(6):939–46.
Bovend’Eerdt TJH, Dawes H, Sackley C, Wade DT. Practical research-based guidance for motor imagery practice in neurorehabilitation. Disabil Rehabil. 2012;34(25):2192–200.
Liu KPY, Chan CCH. Metacognitive mental imagery strategies for training of daily living skills for people with brain damage: the self-regulation and mental imagery program. In: Söderback I, editor. International Handbook of Occupational Therapy Interventions: New York, Springer New York; 2009. p. 233–9.
Liu KP, Chan CC, Lee TM, Hui-Chan CW. Mental imagery for promoting relearning for people after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85(9):1403–8.
Liu KPY. Use of mental imagery to improve task generalisation after a stroke. Hong Kong Med J. 2009;15(3 SUPP4):37–41.
Braun S, Kleynen M, van Heel T, Kruithof N, Wade D, Beurskens A. The effects of mental practice in neurological rehabilitation; a systematic review and meta-analysis. Front Hum Neurosci. 2013;7:390. https://doi.org/10.3389/fnhum.2013.00390.
Mahmoud LSE-D, Shady NAERA, Hafez ES. Motor imagery training with augmented cues of motor learning on cognitive functions in patients with Parkinsonism. Int J Ther Rehabil. 2018;25(1):13–9.
Sarasso E, Agosta F, Piramide N, Gardoni A, Canu E, Leocadi M, et al. Action observation and motor imagery improve dual task in Parkinson's disease: a clinical/fMRI study. Mov Disord. 2021;36(11):2569–82.
Bek J, Gowen E, Vogt S, Crawford TJ, Poliakoff E. Combined action observation and motor imagery influences hand movement amplitude in Parkinson's disease. Parkinsonism Relat Disord. 2019;61:126–31.
Tremblay F, Leonard G, Tremblay L. Corticomotor facilitation associated with observation and imagery of hand actions is impaired in Parkinson's disease. Exp Brain Res. 2008;185(2):249–57.
Maillet A, Thobois S, Fraix V, Redoute J, Le Bars D, Lavenne F, et al. Neural substrates of levodopa-responsive gait disorders and freezing in advanced Parkinson’s disease: a kinesthetic imagery approach. Hum Brain Mapp. 2015;36(3):959–80.
Da Silva DM, de Sales MGW, de Macêdo JGF, da Silva LP, Lins OG. Practice of mental protocols used in rehabilitation of patients with Parkinson’s disease: a systematic review. Acta Fisiátrica. 2016;23(3):155–60.
Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation [published correction appears in BMJ. BMJ. 2015;350:g7647. https://doi.org/10.1136/bmj.g7647.
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–21.
Sedgwick P. Meta analysis: how to read a funnel plot. BMJ. 2013;2013(346):f1342.
Acknowledgements
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
TS and KL were involved in the study design and the development of the search strategies. TS and KL drafted the manuscript of the protocol. TS, KL and PF revised the manuscript. TS and KL will screen the potential studies, extract the data and assess quality. Any discrepancies identified will be cross-checked by PF. TS and PF performed the meta-analysis. KL is the guarantor of the review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Singer, T., Fahey, P. & Liu, K.P.Y. The efficacy of imagery in the rehabilitation of people with Parkinson’s disease: protocol for a systematic review and meta-analysis. Syst Rev 11, 158 (2022). https://doi.org/10.1186/s13643-022-02041-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-022-02041-z