Abstract
Background
We systematically reviewed and chronicled exposures and outcomes measured in the maternal and birth cohort studies in the Gulf Cooperation Council (GCC) countries and quantitatively summarized the weighted effect estimates between maternal obesity and (1) cesarean section (CS) and (2) fetal macrosomia.
Methods
We searched MEDLINE-PubMed, Embase, Cochrane Library, Scopus, and Web of Science electronic databases up to 30 June 2019. We considered all maternal and birth cohort studies conducted in the six GCC countries (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and United Arab Emirates (UAE)). We categorized cohort studies on the basis of the exposure(s) (anthropometric, environmental, medical, maternal/reproductive, perinatal, or socioeconomic) and outcome(s) (maternal or birth) being measured. Adjusted weighted effect estimates, in the form of relative risks, between maternal obesity and CS and fetal macrosomia were generated using a random-effects model.
Results
Of 3502 citations, 81 published cohort studies were included. One cohort study was in Bahrain, eight in Kuwait, seven in Qatar, six in Oman, 52 in Saudi Arabia, and seven in the UAE. Majority of the exposures studied were maternal/reproductive (65.2%) or medical (39.5%). Birth and maternal outcomes were reported in 82.7% and in 74.1% of the cohort studies, respectively. In Saudi Arabia, babies born to obese women were at a higher risk of macrosomia (adjusted relative risk (aRR), 1.15; 95% confidence interval (CI), 1.10–1.20; I2 = 50%) or cesarean section (aRR, 1.21; 95% CI, 1.15–1.26; I2 = 62.0%). Several cohort studies were only descriptive without reporting the magnitude of the effect estimate between the assessed exposures and outcomes.
Conclusions
Cohort studies in the GCC have predominantly focused on reproductive and medical exposures. Obese pregnant women are at an increased risk of undergoing CS delivery or macrosomic births. Longer-term studies that explore a wider range of environmental and biological exposures and outcomes relevant to the GCC region are needed.
Systematic review registration
PROSPERO CRD42017068910
Similar content being viewed by others
Background
A wide range of prenatal exposures including environmental, genetic, and socioeconomic factors can individually or jointly affect different maternal and birth health outcomes [1,2,3]. Such unfavorable health outcomes might manifest during the early or later stages of pregnancy or infancy leading to both short- and long-term consequences [1,2,3]. For instance, gestational diabetes mellitus (GDM) increases the risk of both the mother developing post-pregnancy type 2 diabetes mellitus (T2DM) [4] and macrosomia in the newborn [5]. Maternal obesity has also been associated with an increased risk of macrosomia in newborns [5]. Socioeconomic exposures including poverty and environmental factors, such as air pollution, have also been shown to be associated with various maternal and birth outcomes [6,7,8,9]. Pre-eclampsia is positively associated with a greater risk of developing cardiovascular diseases (CVD) or cardiac shock in the future [10,11,12], and it doubles the risk of stroke in the offspring [13].
High-quality and well-designed cohort studies provide robust data that can be used to explore associations between specific exposures and outcomes. Long-term birth cohort studies such as the Norwegian Mother and Child Study [14] and the Danish Birth Cohort Study [15] have revealed several important maternal and child factors operating in early life, fetal growth, and its determinants. However, the information obtained in these settings may not be easily generalized to different populations, such as the Gulf Cooperation Council (GCC) countries (i.e., Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates (UAE)), as they may have different individual, familial, lifestyle, environmental, and genetic exposures including, but not limited to consanguinity, physical inactivity, diet, and tobacco use.
In recent decades, there has been a dramatic rise in the prevalence of several adverse health outcomes in the GCC countries, in particular non-communicable diseases and their risk factors including obesity, T2DM, asthma, neurodevelopmental disorders, and CVD [16, 17]. Maternal and prenatal exposures and associated outcomes in these GCC countries have become of great interest due to changes in demographic dynamics, composition of the population, and lifestyle transition [16]. Among females, the prevalence of physical inactivity is very high (58.7–98.7%) and the proportion of women that report smoking cigarettes or water pipes varies considerably (0.5–20.7%) [18].
There are number of cohort studies that have been conducted in the GCC countries that pertain to specific exposures and outcomes affecting maternal and infant health [19,20,21,22]. These include anthropometric, environmental, socioeconomic, lifestyle, and medical physiological exposures that can bear consequences on the pregnancy condition, delivery process, neonatal status, perinatal growth, and possibly long-term health consequences for both the mother and offspring [23,24,25,26,27]. However, there has not been a synthesis and evaluation of the different cohort studies that have been conducted in the GCC countries on which to base more effective evidence-based public health policies. A comprehensive review of the maternal and birth cohort literature in the GCC will highlight research areas that have received considerable attention and identify knowledge gaps in the current body of scientific evidence. Highlighting understudied maternal and child health-related exposures and outcomes is important for grant funding bodies tasked with identifying priority areas and researchers planning future studies.
The objectives of this study are (i) to summarize and characterize the exposures and outcomes that have been examined and discussed in the maternal and birth cohort studies in the six GCC countries (qualitative synthesis) and (ii) to quantitatively generate weighted effect estimates on the association between maternal obesity and (a) cesarean section (CS) and (b) fetal macrosomia (quantitative synthesis).
Materials and methods
The protocol for this review has been published elsewhere [28] and is registered online on PROSPERO (registration number CRD42017068910). Minor necessary modifications not in line with the protocol were adapted in this review, whenever it was necessary. Our review was informed by the Cochrane Collaboration guidelines [29] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [30]. The PRISMA checklist can be found in (see Additional file 1: Table S1).
Data source and search strategy
We searched MEDLINE-PubMed, EMBASE, Scopus, Web of Science, and The Cochrane Library databases (up to 30 June 2019). We used comprehensive search criteria with no language restrictions. The literature search protocol is summarized in the (see Additional file 2: Box S1).
Study selection
Retrieved citations from the six databases were imported and compiled into EndNote reference manager [31], and duplicate records were removed. The remaining records were reviewed at the title/abstract level, and full texts of those records that were considered eligible or potentially eligible against our eligibility criteria were retrieved for full-text review. In this review, we use the term “cohort study” to refer to a full published research article containing a followed up maternal and/or birth cohort(s).
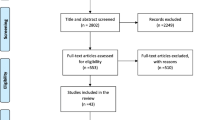
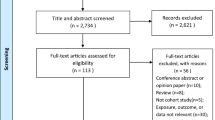
Two reviewers (NA and ETB) independently assessed retrieved citations for eligibility. Full-text articles deemed relevant or potentially relevant were retrieved and screened against specific inclusion and exclusion criteria. We also systematically screened the reference lists of all eligible cohort studies for further eligible publications (Fig. 1). Conflicts were resolved by discussion and consensus after consulting expert reviewers (RHA and LA).
Eligibility criteria
Inclusion criteria
-
Study design: prospective or retrospective cohort.
-
Study population: pregnant mothers and their offspring.
-
Geographical location: any of the six GCC countries, namely, Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, or the UAE.
-
Recruitment timing. Cohort studies should have recruited pregnant mothers and their newborns or recruited newborns immediately after delivery as long as relevant information on pregnancy was available.
-
Follow-up: no specific follow-up period. Cohort studies were required to have some prospective or retrospective data on exposure(s) and outcome(s) for mothers and/or their offspring.
-
Measurements: not specific. Cohort studies should have measured at least one maternal exposure and at least one maternal and/or newborn outcome.
Exclusion criteria
We excluded all other study designs including cross-sectional, case-control, randomized-controlled trials, reviews, qualitative studies, editorials, author commentaries, and case series studies regardless of the number of cases. Studies that were not conducted in GCC countries or were not attributed to any of the GCC countries were also excluded.
Data extraction and management
We extracted and summarized data following the PECO framework [32, 33]. The PECO stands for population, exposure, comparator, and outcome. Summarizing cohort studies using the PECO framework helps to identify the specific population, exposure(s), and outcomes(s) assessed in the cohort(s), being followed.
Data were extracted using a pre-piloted data extraction form. Data extraction was performed by two reviewers (NA and ETB). Random checks of at least 50% of the extracted studies were crosschecked by a third expert reviewer (RHA). Discrepancies in data extraction were resolved by agreement between data extractors and the third expert reviewer (RHA). The main features of the eligible cohort studies including author name(s), year of publication, and study design were extracted. In addition, we also extracted different characteristics of the studied population including recruited cohort population, country, size of the cohort, measured exposure(s) and outcome(s), and key findings of the cohort study, whenever reported and possible. If reported, we also extracted adjusted effect estimates of the association between maternal obesity and CS or macrosomia. In studies adjusting the effect estimate using different models, we extracted estimates from the model that we considered to adjust for the most appropriate confounders (e.g., age, parity, comorbidity). If the cohort study had discrepant effect estimates in the text and tables, we extracted estimates reported in text if the corresponding author(s) of these studies failed to respond to our email enquiries.
In this review, all factors or variables treated in the original cohorts as risk factors or independent/explanatory variables that could be determinant of health outcome(s) of interest were defined as exposures. According to the nature and source, we categorized the defined exposures into six exposure domains (anthropometric, environmental, medical/medical service, maternal/reproductive, perinatal/infant, and sociodemographic) and the measured outcomes into two outcome domains (birth and maternal outcomes). Hence, one cohort study could incorporate more than one exposure domain and/or more than one outcome domain. Indeed, one of the strengths of the cohort study design is the capability to assess multiple exposures and outcomes in the same cohort. Anthropometric domain included measures such as height, body mass, and body mass index (BMI) of the mother. Environmental domain included, for example, living conditions, nutritional exposures, and exposure to any type of smoke. Medical/medical service domain referred to pre-existing maternal health conditions that were not due to pregnancy including medical conditions such as DM or hypertension as well as a family history of diseases and/or medical services during pregnancy and delivery such as length of waiting time to receive healthcare, relationships with the healthcare professionals, consumption of medication, or depression. Maternal/reproductive domain referred to conditions that were specifically related to exposures experienced during present or previous pregnancies or post-delivery such as parity, GDM, or breastfeeding. Perinatal/infant domain included exposures, for example, birth weight, multiple birth, and mode of birth delivery. Sociodemographic domain included exposures such as age, education, or employment.
In this review, all variables or measures treated in the original cohorts as dependent variables that may stem from exposure to potential risk factor(s)/independent variable(s) were defined as outcomes. The defined outcomes were categorized into two domains (maternal or birth) indicating whether the mother or her newborn suffered from an outcome due to a specific exposure.
Risk of bias assessment
We evaluated the methodological quality and risk of bias (ROB) aspects for each cohort study using the National Heart, Lung, and Blood Institute (NIH) tool [34]. For each assessed criteria, each study has the potential to be categorized as “potentially of low ROB” if the answer was “yes” for that specific criteria, “potentially of high ROB” if the answer was “no” for that specific criteria, or “can’t determine, not applicable, or not reported” for that specific criteria. ROB was performed by at least two reviewers for each study.
Quantitative analysis
Meta-analysis
Meta-analyses of pre-calculated adjusted effect estimates were conducted, and the corresponding 95% confidence interval (CI) was estimated. We pooled adjusted estimates using a random-effects model [35]. We estimated the I-squared (I2) as a measure of heterogeneity [36, 37]. Meta-analyses were performed using the Review Manager (RevMan) version 5.3 [38]. In cohort studies reporting adjusted odds ratio (aOR) as a measure of effect estimate, we converted the aOR into adjusted relative risk (aRR) following a standard procedure [39]. Odds ratio (OR) are not well understood, and when the outcome is common, OR are always further away from 1 than relative risk (RR). Misinterpretation of the OR in cohort studies can potentially lead to serious overestimation of the effect estimate between an exposure and outcome being studied [39].
Ethics approval
In line with the United Arab Emirates University-Human Research Ethics Committee regulations, ethical approval or an exemption letter was not required for this study as it did not use any primary data.
Results
Scope of the review
We identified 3502 citations. Of which, 81 citations were found eligible as maternal and birth cohort studies for inclusion in the systematic review (Fig. 1).
Study characteristics
Table 1 summaries the 81 published cohort studies according to the measured six exposures and two outcomes domains in the six GCC countries. Additional file 3: Table S2 presents more information on the measured exposures and outcomes in addition to the summary of key findings of each of the reviewed 81 cohort studies in the GCC countries, stratified by the country.
The 81 cohort studies were published between 1990 in Saudi Arabia [40, 79] and 2019 in Kuwait [41], Qatar [42, 60, 61], and Saudi Arabia [80]. The size of the cohorts ranged from 23 pregnant women with a known diagnosis of idiopathic thrombocytopenic in Saudi Arabia [62] to 158,006 delivering mothers in Kuwait [81]. Majority (64.2%) of the cohort studies were in Saudi Arabia [25,26,27, 40, 43,44,45,46,47,48,49,50, 56, 57, 62,63,64,65,66,67,68,69,70,71,72, 79, 80, 82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98, 105,106,107,108,109,110,111,112], followed by eight (9.9%) in Kuwait [20, 21, 24, 41, 51, 73, 81, 99], seven (8.6%) in each of Qatar [42, 52, 53, 60, 61, 74, 113] and the UAE [58, 59, 75,76,77, 100, 101], six (7.4%) in Oman [54, 55, 78, 102,103,104], and one (1.2%) in Bahrain [22] (see Additional file 3: Table S2).
Thirty-four cohort studies (42.0%) were identified as a prospective design (19 in Saudi Arabia, seven in Kuwait, four in UAE, two in Qatar, one in Oman, and one in Bahrain) [20,21,22, 24, 40, 41, 45, 47, 50, 51, 53, 55, 57,58,59, 63,64,65, 73, 76, 79, 85, 92, 94, 97, 99, 100, 106,107,108,109, 111, 113] while 47 (58.0%) used a retrospective design [25,26,27, 42,43,44, 46, 48, 49, 52, 54, 56, 60,61,62, 66,67,68,69, 71, 72, 74, 75, 77, 78, 80,81,82,83,84, 86,87,88,89,90,91, 93, 95, 96, 98, 101,102,103,104,105, 110, 112] (33 in Saudi Arabia, five in Qatar, five in Oman, one in Kuwait [81], and three in the UAE). Fifty-two (64.2%) cohort studies enrolled pregnant mothers at varying stages of their pregnancy with different characteristics such as diabetic and non-diabetic mothers [26, 67], obesity [42, 47, 54], singleton [41, 48, 99] or triplet pregnancies [91], teenage women [112], multipara women [82, 86, 98], and women with systemic lupus erythematosus (SLE) [27]. Eight studies (9.9%) enrolled pregnant mothers at varying stages after delivery [24, 49, 58, 59, 81, 84, 88, 99]. Seventeen studies (21.0%) recruited newborns at varying stages after birth [20, 21, 45, 52, 56, 63, 65, 66, 70, 73, 75, 92, 101, 102, 105,106,107,108] such as preterm babies [20, 66, 73, 105, 108] (see Additional file 3: Table S2).
Studied exposures
Majority (65.2%) of the 81 cohort studies discussed maternal or reproductive exposures followed by medical/medical service exposures (39.5%) and sociodemographic exposures (30.9%) (Table 1).
Maternal or reproductive exposures
Maternal or reproductive exposures often measured were GDM and parity in 24.7% and 16.0% of the 81 cohort studies, respectively. GDM was investigated as an exposure for different maternal and birth outcomes including mode of birth delivery, birth weight, APGAR score, preterm delivery, intrauterine fetal death, and admission to neonatal intensive care unit (NICU) in four cohort studies [26, 48, 67, 88]. These cohorts consistently found that pregnant women with pre-GDM or GDM were at increased risk of various adverse maternal and birth outcomes including CS delivery, macrosomia, and preterm delivery [26, 48, 67, 88]. Pre-GDM was also independently associated with CS delivery (adjusted odds ratio “aOR,” 1.65), induction of labor (aOR, 1.67), macrosomia (aOR, 1.40), stillbirth (aOR, 3.66), and APGAR score < 7 at 5 min (aOR, 3.82) [67]. Various unfavorable health outcomes were more common in grand multipara compared to primigravida mothers [82, 114] (see Additional file 3: Table S2).
Sociodemographic exposures
Maternal age was a common measured sociodemographic exposure studied in 18 cohort studies. Advanced maternal age was associated with GDM, CS, and preterm delivery [51, 53, 58, 92, 111, 113]. Primary education or less was independently associated with 69% lower likelihood of exclusive breastfeeding at 6 months (aOR, 0.31; 95% CI, 0.11–0.88) [58] (see Additional file 3: Table S2).
Medical or medical service exposures
Thirty-two (39.5%) cohort studies explored medical or medical services as exposures such as length of hospital stay [58] and other medical conditions such as SLE [27, 102] and diabetes [26, 60, 67, 68, 70, 74]. Pre–pregnancy T1DM or T2DM were independently associated with emergency (aOR, 2.67) or elective CS delivery (aOR, 6.73), macrosomia (aOR, 3.97), or preterm delivery at < 37 weeks (aOR, 2.24) in Saudi Arabia [26].
Other exposures
Seventeen (21.0%) cohort studies focused on perinatal exposures. These included factors such as birth weight [20, 21, 73, 106], head circumference [92], and birth multiplicity [93]. Environmental and anthropometric exposures were measured in only six (7.4%) and 16 (19.8%) of the 81 cohort studies, respectively. Environmental exposures included smoking and secondhand smoking [49, 56], and all six studies on anthropometric measures were on BMI (Table 1 and see Additional file 3: Table S2).
Studied outcomes
There were 21 cohort studies reporting only birth outcomes [20, 21, 27, 49, 51, 52, 57, 63, 65, 69, 70, 73, 75, 92, 101, 105,106,107,108,109,110], 14 cohort studies reporting only maternal outcomes [24, 41, 44, 53, 58, 59, 64, 76, 81, 83, 84, 99, 100, 104], and 46 cohort studies reporting both maternal and birth outcomes [22, 25, 26, 40, 42, 43, 45,46,47,48, 54,55,56, 60,61,62, 66, 68, 71, 72, 74, 77,78,79,80, 82, 85,86,87,88,89,90,91, 93,94,95,96,97,98, 101,102,103, 111,112,113] (Table 1 and, see Additional file 3: Table S2).
Maternal outcomes
Mode/type of birth delivery assessed in 15 cohort studies [14, 40, 47, 48, 50, 55, 62, 67, 68, 71, 72, 81, 86, 88, 111], followed by preeclampsia/eclampsia in 12 cohorts [47, 50, 53, 54, 68, 72, 93, 97, 102, 103, 111, 113], GDM in seven cohort studies [47, 50, 53, 54, 83, 111, 113], and maternal anemia in three cohort studies [50, 104, 113]. Postpartum depression was explored in only one prospective cohort study in the UAE [76]. Pregnancy anemia was examined in only one cohort in Oman [104]. In several cohort studies, obese pregnant women were at a higher risk of developing several unfavorable outcomes including GDM (aOR, 5.10 [47]; aOR, 6.60 [53]; RRs, 8.60 [50]), pregnancy hypertension (RRs, 6.10 [50]; RRs, 6.10 [50]; aOR, 2.23 [47]), pre-eclamptic toxemia (RRs, 5.90 [50]), CS delivery (aOR, 4.80 [47]; aOR, 2.16 [48]; RRs, 2.00 [50]), antepartum (aOR, 2.80) or postpartum hemorrhage (RRs, 2.50) [47], macrosomia (aOR, 6.80 [50], 9.18 [49], 3.90 [47]), 1 min APGAR score < 7 (RRs, 6.80) [50], postdate delivery (> 42 weeks) (RRs, 3.70) [50], and preterm birth (aOR, 2.20) [47]. Obese pregnant women with GDM (aOR, 3.45) or obese pregnant women with no GDM (aOR, 1.46) were more likely to deliver macrosomic babies compared to non-obese pregnant women with no GDM [48].
Birth outcomes
The most common measured birth outcome was birth weight in 33 cohort studies [22, 26, 40, 42, 47,48,49,50,51, 54,55,56, 67, 68, 70,71,72, 75, 78,79,80, 85, 86, 88,89,90, 96,97,98, 101,102,103, 111], followed by congenital malformations in nine cohort studies [22, 43, 54, 57, 68, 70, 78, 97, 113], preterm birth in 12 cohort studies [51, 67, 70, 71, 77, 79, 88, 90, 93, 97, 103, 111], and stillbirth in five cohort studies [22, 25, 51, 110, 111]. Retinopathy of prematurity was assessed in three preterm birth cohorts in Kuwait [20, 21, 73]. Early cognitive development of infants at different early life stages was explored in only one cohort [109], and mean umbilical cord blood lead level was also measured in one other cohort [92]. Maternal, fetal, or neonatal deaths were examined in 12 cohort studies [25, 26, 62, 63, 68, 82, 85, 87, 90, 93, 112]. Eczema in children at 2 years of age was assessed in only one cohort in Saudi Arabia that linked to the sub-optimal growth indexed by fetal abdominal circumference [69] (see Additional file 3: Table S2).
Weighted effect estimates
Obese pregnant women in Saudi Arabia were 15% more likely to give birth to a macrosomic baby compared to non-obese women (pooled aRRs, 1.15; 95% CI, 1.00–1.25; I2 = 50.0%) (Fig. 2) [47,48,49]. Following written communication with the study authors [48], we excluded two unverified point estimates due to the inaccuracy of the reported CI. Nonetheless, Saudi obese pregnant women remained at a higher risk of giving birth to a macrosomic baby compared to non-obese mothers (pooled aRR, 1.18; 95% CI, 1.14–1.22; I2 = 0.0%) (see Additional file 4: Figure S1). Obese pregnant Saudi women were also at a 21% increased risk of undergoing CS delivery compared to non-obese pregnant Saudi women (pooled aRRs, 1.21; 95% CI, 1.15–1.26; I2 = 62.0%) (Fig. 3). Excluding one estimate (aOR, 4.80; 95% CI, 1.50–6.40), due to the inability to verify the accuracy of the reported CI following written communication with the study authors [47], did not change the strength of this association (aRRs, 1.23; 95% CI, 1.19–1.28; I2, 15%) (see Additional file 5: Figure S2).
Pooled adjusted estimates of the association between maternal obesity and macrosomia. Note: Estimates from same author and year indicates to stratified estimates that were extracted from same study and included in the forest plot. Square indicates to the study-specific effect estimate. Size of the square is proportional to the precision (weight) of the study-specific effect estimates. Bars indicate the width of the corresponding 95% confidence interval (CI). The diamond centered on the summary effect estimate, and the width indicates the corresponding 95% CI
Pooled adjusted estimates of the association between maternal obesity and CS delivery. Note: Estimates from same author and year indicates to stratified estimates that were extracted from same study and included in the forest plot. Square indicates to the study-specific effect estimate. Size of the square is proportional to the precision (weight) of the study-specific effect estimates. Bars indicate the width of the corresponding 95% confidence interval (CI). The diamond centered on the summary effect estimate, and the width indicates the corresponding 95% CI
Quality assessment
Findings of our summarized and criteria-specific quality assessment of cohort studies can be found in the supplementary information. Briefly, all studies clearly stated the research question(s)/objective(s), clearly specified and defined the study population, and recruited subjects from the same or similar populations with stating the inclusion and exclusion criteria. Hence, all cohort studies were categorized as “potentially of low ROB” for these three assessment criteria. Over a half (57.0%) of the cohort studies either reported descriptive statistics for the burden of the exposure and outcomes or the association between the measured exposure(s) and outcome(s) was not adjusted for any potential confounding effect, and hence were classified as “potentially of high ROB”. Overall, cohort studies were of reasonable quality with “potentially of low ROB” in 9.8 and with “potentially of high ROB” in 1.6 of the 14 measured quality criteria (see Additional file 6: Figure S3, and, see Additional file 7: Table S3).
Discussions
Summary of major findings
The present systematic review summarizes the published evidence on the maternal and birth cohort studies that have been conducted in the six GCC countries. This is the first review to chronicle, synthesize, and appraise the maternal and birth cohort studies in the GCC countries. The review confirms, using peer-reviewed data, that pregnant women in the GCC countries have a high burden of various maternal and modifiable lifestyle and environmental exposures. These exposures were associated with a range of different unfavorable maternal and birth health-related outcomes. Saudi Arabia contributed the largest volume of literature to the review. The included cohort studies predominantly focused on maternal and reproductive exposures compared to other aspects of pregnancy such as the biological predisposition of the mother or the type of environment. Majority of the studies reported only descriptive estimates on the burden of exposures and/or outcomes or crude estimates on the association between exposure(s) and outcome(s). Our summary effect estimates strengthened the evidence base for a strong positive association between maternal obesity and macrosomia or CS delivery.
Implications for clinicians and policy makers
Globally, the prevalence of obesity has nearly tripled since 1975 [115]. The populations in the GCC countries have also been affected by this global trend in overweight and obesity. According to the World Health Organization (WHO) report in 2010, the prevalence of obesity in females in Kuwait was 48%, 44% in Saudi Arabia, 42% in the UAE, 38% in Bahrain, 32% in Qatar, and 17% in Oman [116]. A recent report issued by the WHO in 2017 documented that the age standardized prevalence of obesity in females in each of the six GCC countries exceeded 30% [117]. Our meta-analyses revealed that maternal obesity is independently positively associated with undergoing CS delivery or giving birth to a macrosomic baby. Our weighted estimate showed that obese women were 1.15-times more likely to have macrosomic babies (Fig. 3) which is similar to the pooled estimate from 16 case-control and cohort studies in Asia (India), Europe (Denmark, France, Germany, Italy, and the UK), the Middle East (Saudi Arabia), and North America (Canada, USA) (RRs, 1.20; 95% CI, 1.18–1.23) [118]. Also, maternal obesity increases the risk of GDM which is also a risk factor for different drivers of CS delivery including congenital disorders and anticipated low birth weight [70] and macrosomia and dystocia [67]. However, two of the three estimates included in our overall pooled estimate on the association between obesity and CS adjusted for GDM, maternal age, parity, gestational age, and exposure to environmental tobacco smoke [48]. Our weighted effect estimate on the maternal obesity and CS delivery (Fig. 3) is similar to previously published weighted estimates reported (1) in 2008 in 11 cohort studies in different European countries and the USA (RR, 1.21; 95% CI, 1.18–1.22) [119]; (2) in 2007 in 33 cohort studies in USA, Sweden, France, Denmark, Israel, Canada, the UK, Poland, and the United Arab Emirates (RR, 1.20; 95% CI, 1.18–1.22) [120]; and in 2015 in 22 cohort studies in low and middle-income countries in Southeast Asia, Middle East, and Central and South America (RR, 1.19; 95% CI, 1.10–1.26) [121]. Our weighted estimate was also similar to the previously reported weighted estimate (RR, 1.20; 95% CI, 1.16–1.23) on the association between severe maternal obesity and CS delivery reported in 33 cohort studies in 2007 [120]. This finding from studies in GCC countries strengthens the evidence base on the strong positive association between maternal obesity and undergoing CS delivery even after adjustment for maternal age and parity. Previous research has reported that if normal weight women have a 20% increased risk of CS delivery while obese women have a 40% increased risk of CS delivery, then every 1% decrease in the fraction of obese pregnant women would prevent 16,000 CS deliveries annually [120]. Hence, with our documented 21% increase risk of CS in obese women in the GCC countries, a substantial number of CS deliveries and related complications would be averted when reducing the prevalence of maternal obesity in these countries.
Gaps in evidence
Many of the cohort studies followed specific subpopulations rather than a representative population of the country. Nearly all cohorts were recruited through either convenience or consecutive sampling. The majority of studies only followed the cohorts for a short period of time (e.g., third trimester to birth). As such, larger population-based studies with longer follow-up periods are needed to further understand the long-term influence of early and late prenatal exposures on pregnancy outcomes as well as on health and developmental outcomes during infancy, early childhood, adolescence, and adulthood.
This review has shown that there is a lack of research exploring the relationship between environmental exposures and maternal and child health outcomes. This is problematic for several reasons. In the GCC, rapid modernization has occurred over the last 50 years which might have had detrimental effects on the environment [122]. Factors such as water pipe smoking and indoor incense use are prevalent and have been shown to increase the risk of several adverse maternal outcomes such as wheezing, asthma, and headache, which in turn may affect the developing fetus [123].
Furthermore, consanguinity is prevalent in the GCC region, which increases the risk of genetic-related health outcomes such as thalassemia [124] that can negatively impact pregnancy and child birth [125]. However, early intervention and management can minimize the negative consequences of these conditions on the delivery of the child and their prognosis.
The lack of the above exposures being studied leaves a gap in the literature on how a multitude of exposures which are not sociodemographic or reproductive in nature may affect the health and lives of the mother and offspring. Longitudinal prospective cohorts collecting a large and varied dataset are relevant and necessary to understand this knowledge gap.
9With respect to outcomes, longitudinal maternal and child cohort studies should endeavor to measure a broad range of health conditions. This has been discussed by Golding [126] who draws parallels between successful cohorts around the world such as the Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) cohort [126], the Danish National Birth Cohort (DNBC) [15], and the Norwegian Mother and Child Cohort Study (MoBa) [127]. Golding stipulates the studied outcomes should include parental, pregnancy-based, and baby- or child-based outcomes including anthropometric, signs and symptoms of illness during childhood, and behavioral and mental health measures in children and adolescents [126]. Such studies require a longer follow-up than the previously conducted mother and child cohort studies in GCC countries.
Implications for improved reporting and interpretation of future cohort studies
Cohort studies aim to identify risk factors leading to the development of unfavorable specific health outcomes. Designing and implementing cohort studies is a time- and effort-consuming process. Reporting results of cohort studies should follow a robust methodology and should be informative using appropriate scientific terms as well as appropriate bio-statistical analyses. In many of the reviewed cohort studies, there were weaknesses in appropriately reporting the correct study design, using the appropriate epidemiologic terms, or implementing the appropriate bio-statistical analyses. Cohort studies are not the same as case-control or cross-sectional studies [128]. “Incidence rate and incidence proportion” are different from “prevalence” [129]. Measuring strength and the magnitude of association between the measured exposure(s) and the outcome(s) after controlling for the influence of potential confounders is critical. Limiting the reporting to descriptive crude results in the form of proportions or correlations is not sufficient. What should we measure, OR or RRs? In medical research, there is a confusion on interpreting OR [39]. The OR is usually further away from 1 than the RR except in rare outcomes [39]. Misinterpretation of the OR can lead to serious overestimation of the benefits or risks in medical decision-making that may confuse healthcare professionals and policy makers, discussing treatment options or designing public health interventions [39]. Odds ratio is not well understood as a measure of effect size, and conversion to RRs by a simple calculation would improve understanding of findings [39]. When communicating results of medical research, it is important to be able to frame the statistics in a meaningful and easily understood metric [130]. Quantifying RR as a metric of the effect size is more appropriate and informative [39]. In the reviewed cohort studies, even in studies that went beyond descriptive analysis, researchers relied mainly on estimating the OR rather than the RR. Clinician researchers without a background in cohort methodology should involve epidemiologists in the design, conduct, analysis, and reporting of cohort studies as this would improve the quality and interpretation of the available evidence.
Strengths and limitations
To our knowledge, this is the first review to explore the types of exposures and outcomes being studied in maternal and birth cohorts in the GCC region. We implemented a comprehensive search strategy covering four electronic databases in addition to hand-searching of reference lists of included studies. We carefully screened studies and extracted data and critically assessed the ROB of the included cohort studies using the National Institute of Health scale (see Additional file 7: Table S3). Consequently, our paper represents a comprehensive review mapping gaps in evidence and provides critical recommendations to improve analyzing and reporting results of cohort studies.
Some limitations should be considered when interpreting the findings of this review. First, the review was limited to the available adjusted effect estimates from a narrow range of specific exposure-outcome pairs from Saudi Arabia only. This has also limited our ability to quantify the sources of heterogeneity through meta-regression and subgroup analyses. Secondly, there were inherent differences in the designs of these cohorts and measurement methods of even similar exposures and outcomes which may account for some of the observed small-to-moderate heterogeneity (I2 = 15–62%) and could affect the strength of evidence from our meta-analyses. The results of our meta-analyses provide supporting evidence on the association between maternal obesity and fetal macrosomia or CS. However, careful consideration should be given when interpreting findings as some of the individual point estimates included in our meta-analyses might be biased. These individual point estimates were based on a varied cutoff point used to identify the exposed population. For example, the WHO defines obese people as those with a BMI ≥ 30 kg/m2 [131]; however, in one study which provided two adjusted estimates in the meta-analyses, obese women were defined as having a BMI ≥ 29.9 kg/m2 [47]. Using a slightly lower BMI cutoff to classify obesity may have overestimated the burden of the exposed population leading to misclassification bias. Residual confounding bias is also a potential limitation when interpreting any reported associations. Thirdly, as we did not search national databases, there is a limited possibility that we might have missed some unpublished maternal and birth cohort studies conducted in the GCC countries. However, this limited possibility is (i) supported by the robust searching and screening strategy we implemented and (ii) the lower likelihood that well-conducted cohort studies would not be published in indexed peer-reviewed journals.
Studies limited the recruited cohort to only citizens which minimizes the generalizability of the findings to the general population. For instance, our pooled estimates on CS and macrosomia were limited to the Saudi population which reduces the representativeness to the other five GCC countries or to other nationalities living in Saudi Arabia. Overall, this does not detract from the importance of our meta-analysis findings (Fig. 2, see Additional file 4: Figure S1, and Fig. 3, see Additional file 5: Figure S2) which are consistent with previously published findings from populations outside the GCC [118,119,120].
Despite these limitations, our review compiled and summarized important data and provided narrative information from a large number of maternal and birth cohort studies in the six GCC countries. Our review was also able to provide specific weighted estimates in Saudi Arabia, the largest of the six GCC countries in terms of population size and land mass.
Conclusions
The reviewed maternal and birth cohort studies in the GCC countries have focused on reproductive and sociodemographic exposures. Birth outcomes were studied more frequently than maternal outcomes. Obese pregnant women are at higher risk of undergoing CS delivery or giving birth to macrosomic babies. Designing future cohort studies should strive to explore a wide range of mother and child exposure outcomes that are relatively under-researched but prevalent in the GCC countries such as various forms of tobacco use and air quality, DM and GDM, and consanguinity. These future studies will provide informative data to fill gaps in the evidence. Such findings can be used by clinicians, researchers, and policy makers to address important maternal and child health issues.
Availability of data and materials
The datasets used and/or analyzed during the current study and its additional information files are available from the corresponding author on reasonable request.
Abbreviations
- aOR:
-
Adjusted odds ratio
- aRRs:
-
Adjusted relative risk
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular diseases
- DM:
-
Diabetes mellitus
- DNBC:
-
Danish National Birth Cohort
- GCC:
-
Gulf Cooperation Council
- GDM:
-
Gestational diabetes mellitus
- I 2 :
-
I-squared
- MoBa:
-
Norwegian Mother and Child Cohort Study
- NICU:
-
Neonatal intensive care unit
- NIH:
-
National Heart, Lung, and Blood Institute
- OR:
-
Odds ratio
- PECO:
-
Participants, exposure, comparator, and outcome
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- ROB:
-
Risk of bias
- RRs:
-
Relative risk
- SLE:
-
Systemic lupus erythematosus
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- UAE:
-
United Arab Emirates
- WHO:
-
World Health Organization
References
Rogers I, Emmett P, Ness A, Golding J. Maternal fish intake in late pregnancy and the frequency of low birth weight and intrauterine growth retardation in a cohort of British infants. J Epidemiol Community Health. 2004;58(6):486–92.
Kogan MD. Social causes of low birth weight. J R Soc Med. 1995;88(11):611–5.
Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environ Health Perspect. 2003;111(7):942–6.
Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9.
Alberico S, Montico M, Barresi V, Monasta L, Businelli C, Soini V, et al. The role of gestational diabetes, pre-pregnancy body mass index and gestational weight gain on the risk of newborn macrosomia: results from a prospective multicentre study. BMC Pregnancy Childbirth. 2014;14:23.
Gray SC, Edwards SE, Schultz BD, Miranda ML. Assessing the impact of race, social factors and air pollution on birth outcomes: a population-based study. Environ Health. 2014;13(1):4.
Weck RL, Paulose T, Flaws JA. Impact of environmental factors and poverty on pregnancy outcomes. Clin Obstet Gynecol. 2008;51(2):349–59.
Kent ST, McClure LA, Zaitchik BF, Gohlke JM. Area-level risk factors for adverse birth outcomes: trends in urban and rural settings. BMC Pregnancy Childbirth. 2013;13:129.
Genereux M, Auger N, Goneau M, Daniel M. Neighbourhood socioeconomic status, maternal education and adverse birth outcomes among mothers living near highways. J Epidemiol Community Health. 2008;62(8):695–700.
Lin YS, Tang CH, Yang CY, Wu LS, Hung ST, Hwa HL, et al. Effect of pre-eclampsia-eclampsia on major cardiovascular events among peripartum women in Taiwan. Am J Cardiol. 2011;107(2):325–30.
Kestenbaum B, Seliger SL, Easterling TR, Gillen DL, Critchlow CW, Stehman-Breen CO, et al. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am J Kidney Dis. 2003;42(5):982–9.
Stekkinger E, Zandstra M, Peeters LL, Spaanderman ME. Early-onset preeclampsia and the prevalence of postpartum metabolic syndrome. Obstet Gynecol. 2009;114(5):1076–84.
Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–80.
Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2006;35(5):1146–50.
Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, et al. The Danish National Birth Cohort--its background, structure and aim. Scand J Public Health. 2001;29(4):300–7.
Gulf Corporation Council Countries Statistics. https://www.gccstat.org/en/. Accessed 21 Mar 2019.
Rahim HF, Sibai A, Khader Y, Hwalla N, Fadhil I, Alsiyabi H, et al. Non-communicable diseases in the Arab world. Lancet. 2014;383(9914):356–67.
Alshaikh MK, Filippidis FT, Al-Omar HA, Rawaf S, Majeed A, Salmasi AM. The ticking time bomb in lifestyle-related diseases among women in the Gulf Cooperation Council countries; review of systematic reviews. BMC Public Health. 2017;17(1):536.
Al Juaid DA, Binns CW, Giglia RC. Breastfeeding in Saudi Arabia: a review. Int Breastfeed J. 2014;9(1):1.
Al-Essa M, Azad RV, Rashwan N. Threshold stage of retinopathy of prematurity: maternal and neonatal risk factors. Ann Saudi Med. 2000;20(2):129–31.
Al-Essa M, Rashwan N, Al-Ajmi M. Retinopathy of prematurity in infants with birth weight above 1500 grams. East Afr Med J. 2000;77(10):562–4.
Al Mahroos S, Nagalla DS, Yousif W, Sanad H. A population-based screening for gestational diabetes mellitus in non-diabetic women in Bahrain. Ann Saudi Med. 2005;25(2):129–33.
Al-Riyami IM, Al-Busaidy IQ, Al-Zakwani IS. Medication use during pregnancy in Omani women. Int J Clin Pharm. 2011;33(4):634–41.
Dashti M, Scott JA, Edwards CA, Al-Sughayer M. Predictors of breastfeeding duration among women in Kuwait: results of a prospective cohort study. Nutrients. 2014;6(2):711–28.
Al-Mulhim AA, Abu-Heija A, Al-Jamma F, El-Harith el HA. Pre-eclampsia: maternal risk factors and perinatal outcome. Fetal Diagn Ther. 2003;18(4):275–80.
Wahabi HA, Esmaeil SA, Fayed A, Al-Shaikh G, Alzeidan RA. Pre-existing diabetes mellitus and adverse pregnancy outcomes. BMC Res Notes. 2012;5:496.
Al Arfaj AS, Khalil N. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus. 2010;19(14):1665–73.
Al-Rifai RH, Ali N, Barigye ET, Al Haddad AHI, Loney T, Al-Maskari F, et al. Maternal and birth cohort studies in the Gulf Cooperation Council countries: protocol for a systematic review and narrative evaluation. BMJ Open. 2018;8(1):e019843.
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41.
Reuters Thompson, Philadelphia, PA, USA EndNote X8 2017.
Woodruff TJ, Sutton P. The Navigation Guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ Health Perspect. 2014;122(10):1007–14.
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16.
National Heart, Lung, and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JAC. Metan: fixed- and randomeffects meta-analysis. Stata J. 2008;8(1):3–28.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009. xxviii,: 421 p.p. 2009
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014;348:f7450.
Meshari AA, De Silva S, Rahman I. Fetal macrosomia--maternal risks and fetal outcome. Int J Gynaecol Obstet. 1990;32(3):215–22.
Pampaka D, Papatheodorou SI, AlSeaidan M, Al Wotayan R, Wright RJ, Buring JE, et al. Postnatal depressive symptoms in women with and without antenatal depressive symptoms: results from a prospective cohort study. Arch Womens Ment Health. 2019;22(1):93–103.
Al-Obaidly S, Al-Ibrahim A, Saleh N, Al-Belushi M, Al-Mansouri Z, Khenyab N. Third trimester ultrasound accuracy and delivery outcome in obese and morbid obese pregnant women. J Matern Fetal Neonatal Med. 2019;32(8):1275–9.
Hijazi A, Althubaiti A, Al-Kadri H. Effect of antenatal care on fetal, neonatal and maternal outcomes: a retrospective cohort study. Internet J Gynecol Obstet. 2018;23(1):1–10.
Baradwan S, Shafi D, Baradwan A, Bashir MS, Al-Jaroudi D. The effect of endometrial thickness on pregnancy outcome in patients with Asherman’s syndrome post-hysteroscopic adhesiolysis. Int J Women's Health. 2018;10:77–82.
Al-Nemri AM, Alsohime F, Shaik AH, El-Hissi GA, Al-Agha MI, Al-Abdulkarim NF, et al. Perinatal and neonatal morbidity among infants of diabetic mothers at a university hospital in Central Saudi Arabia. Saudi Med J. 2018;39(6):592–7.
Al-Shaikh GK, Ibrahim GH, Fayed AA, Al-Mandeel H. Grand multiparity and the possible risk of adverse maternal and neonatal outcomes: a dilemma to be deciphered. BMC Pregnancy Childbirth. 2017;17(1):310.
Haseeb YA. Obstetric outcome in obese Saudi pregnant women: a cohort prospective study at a teaching hospital. Saudi J Med Med Sci. 2017;5(2):142–4.
Wahabi HA, Fayed AA, Alzeidan RA, Mandil AA. The independent effects of maternal obesity and gestational diabetes on the pregnancy outcomes. BMC Endocr Disord. 2014;14:47.
Wahabi HA, Mandil AA, Alzeidan RA, Bahnassy AA, Fayed AA. The independent effects of second hand smoke exposure and maternal body mass index on the anthropometric measurements of the newborn. BMC Public Health. 2013;13:1058.
El-Gilany AH, Hammad S. Body mass index and obstetric outcomes in pregnant in Saudi Arabia: a prospective cohort study. Ann Saudi Med. 2010;30(5):376–80.
AlSeaidan M, Al Wotayan R, Christophi CA, Al-Makhseed M, Abu Awad Y, Nassan F, et al. Birth outcomes in a prospective pregnancy-birth cohort study of environmental risk factors in Kuwait: the TRACER study. Paediatr Perinat Epidemiol. 2016;30(4):408–17.
Kunjachen Maducolil M, Abid H, Lobo RM, Chughtai AQ, Afzal AM, Saleh HAH, et al. Risk factors and classification of stillbirth in a Middle Eastern population: a retrospective study. J Perinat Med. 2018;46(9):1022–7.
Bener A, Saleh NM, Al-Hamaq A. Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast-developing community: global comparisons. Int J Women's Health. 2011;3:367–73.
Zutshi A, Santhosh J, Sheikh J, Naeem F, Al-Hamedi A, Khan S, et al. Implications of early pregnancy obesity on maternal, fetal and neonatal health: retrospective cohort study from Oman. Sultan Qaboos Univ Med J. 2018;18(1):e47–53.
Al-Hakmani FM, Al-Fadhil FA, Al-Balushi LH, Al-Harthy NA, Al-Bahri ZA, Al-Rawahi NA, et al. The effect of obesity on pregnancy and its outcome in the population of Oman, Seeb Province. Oman Med J. 2016;31(1):12–7.
Wahabi HA, Alzeidan RA, Fayed AA, Mandil A, Al-Shaikh G, Esmaeil SA. Effects of secondhand smoke on the birth weight of term infants and the demographic profile of Saudi exposed women. BMC Public Health. 2013;13:341.
Hammouda SA, Abd Al-Halim OA, Mohamadin AM. Serum levels of some micronutrients and congenital malformations: a prospective cohort study in healthy Saudi-Arabian first-trimester pregnant women. Int J Vitam Nutr Res. 2013;83(6):346–54.
Al Tajir GK, Sulieman H, Badrinath P. Intragroup differences in risk factors for breastfeeding outcomes in a multicultural community. J Hum Lact. 2006;22(1):39–47.
Sharief NM, Margolis S, Townsend T. Breastfeeding patterns in Fujairah, United Arab Emirates. J Trop Pediatr. 2001;47(5):304–6.
Bashir M, Dabbous Z, Baagar K, Elkhatib F, Ibrahim A, Brich SA, et al. Type 2 diabetes mellitus in pregnancy: the impact of maternal weight and early glycaemic control on outcomes. Eur J Obstet Gynecol Reprod Biol. 2019;233:53–7.
Bashir M, Baagar K, Naem E, Elkhatib F, Alshaybani N, Konje JC, et al. Pregnancy outcomes of early detected gestational diabetes: a retrospective comparison cohort study, Qatar. BMJ Open. 2019;9(2):e023612.
Al-Jama FE, Rahman J, Al-Suleiman SA, Rahman MS. Outcome of pregnancy in women with idiopathic thrombocytopenic purpura. Aust N Z J Obstet Gynaecol. 1998;38(4):410–3.
Eltawel M, AlHarbi T, AlJamaan K, Alsaif S, Ali Y, Salam M. A prospective study on the incidence and outcomes of neonatal thrombocytopenia at a tertiary care facility in central Saudi Arabia. Adv Neonatal Care. 2018;18(5):E3–E12.
Ellaithy M, Asiri M, Rateb A, Altraigey A, Abdallah K. Prediction of recurrent ectopic pregnancy: a five-year follow-up cohort study. Eur J Obstet Gynecol Reprod Biol. 2018;225:70–8.
Shalaby MA, Sawan ZA, Nawawi E, Alsaedi S, Al-Wassia H, Kari JA. Incidence, risk factors, and outcome of neonatal acute kidney injury: a prospective cohort study. Pediatr Nephrol. 2018;33(9):1617–24.
Al-Hathlol K. Relationship between in vitro fertilization and neonatal outcomes in very low birth weight preterm infants. Am J Perinatol. 2018;35(11):1113–8.
Wahabi H, Fayed A, Esmaeil S, Mamdouh H, Kotb R. Prevalence and complications of pregestational and gestational diabetes in Saudi women: analysis from Riyadh Mother and Baby cohort study (RAHMA). Biomed Res Int. 2017;2017:6878263.
Serehi AA, Ahmed AM, Shakeel F, Alkhatani K, El-Bakri NK, Buhari BA, et al. A comparison on the prevalence and outcomes of gestational versus type 2 diabetes mellitus in 1718 Saudi pregnancies. Int J Clin Exp Med. 2015;8(7):11502–7.
AlMakoshi A, Ellahi A, Sallout B, Devereux G, Turner S. Fetal growth trajectory and risk for eczema in a Saudi population. Pediatr Allergy Immunol. 2015;26(8):811–6.
Lasheen AE, Abdelbasit OB, Seidahmed MZ, Hussein KA, Miqdad AM, Al Zahrani MH, et al. Infants of diabetic mothers. A cohort study. Saudi Med J. 2014;35(6):572–7.
Al-Qahtani MH. Infants of diabetic mothers: 4 years analysis of neonatal care unit in a teaching hospital, Saudi Arabia. Saudi J Med Med Sci. 2014;2(3):151–6.
El Mallah KO, Narchi H, Kulaylat NA, Shaban MS. Gestational and pre-gestational diabetes: comparison of maternal and fetal characteristics and outcome. Int J Gynaecol Obstet. 1997;58(2):203–9.
Al-Essa M, Azad RV, Rashwan N. Rate of and risk factors associated with retinopathy of prematurity: a prospective study from Kuwait. Med Princ Pract. 1999;8:115–8 Epub 118.
Bashir M, E Abdel-Rahman M, Aboulfotouh M, Eltaher F, Omar K, Babarinsa I, et al. Prevalence of newly detected diabetes in pregnancy in Qatar, using universal screening. PLoS One. 2018;13(8):e0201247.
Gardner H, Green K, Gardner AS, Geddes D. Observations on the health of infants at a time of rapid societal change: a longitudinal study from birth to fifteen months in Abu Dhabi. BMC Pediatr. 2018;18(1):32.
Hamdan A, Tamim H. Psychosocial risk and protective factors for postpartum depression in the United Arab Emirates. Arch Womens Ment Health. 2011;14(2):125–33.
Fareh OI, Rizk DE, Thomas L, Berg B. Obstetric impact of anaemia in pregnant women in United Arab Emirates. J Obstet Gynaecol. 2005;25(5):440–4.
Barakat MN, Youssef RM, Al-Lawati JA. Pregnancy outcomes of diabetic women: charting Oman’s progress towards the goals of the Saint Vincent Declaration. Ann Saudi Med. 2010;30(4):265–70.
Alfonso F, Macaya C, Iniguez A, Zarco P. Repeat coronary angioplasty during the same angiographic diagnosis of coronary restenosis. Am Heart J. 1990;119(2 Pt 1):237–41.
Magliah SF, Zarif HA, Almajnoni AO, Suwaidi AA, Aljohani NA, Kaneetah AH, et al. Assessment of maternal and neonatal outcomes of pregnant women with gestational diabetes mellitus at King Abdulaziz Medical City – Jeddah. Indo Am J Pharm Sci. 2019;6(1):2754–61.
Makhseed M, el-Tomi N, Moussa M. A retrospective analysis of pathological placental implantation--site and penetration. Int J Gynaecol Obstet. 1994;47(2):127–34.
Alhainiah MH, Abdulljabbar HSO, Bukhari YA. The prevalence, the fetal and maternal outcomes in grand multiparas women. Mater Sociomed. 2018;30(2):118–20.
Al-Ajlan A, Al-Musharaf S, Fouda MA, Krishnaswamy S, Wani K, Aljohani NJ, et al. Lower vitamin D levels in Saudi pregnant women are associated with higher risk of developing GDM. BMC Pregnancy Childbirth. 2018;18(1):86.
Mahzari MM, Alwadi FA, Alhussain BM, Alenzi TM, Omair AA, Al Dera HS. Development of type 2 diabetes mellitus after gestational diabetes in a cohort in KSA: prevalence and risk factors. J Taibah Univ Med Sci. 2018;13(6):582–6.
Alfadhli EM, Osman EN, Basri TH, Mansuri NS, Youssef MH, Assaaedi SA, et al. Gestational diabetes among Saudi women: prevalence, risk factors and pregnancy outcomes. Ann Saudi Med. 2015;35(3):222–30.
Alsammani MA, Ahmed SR. Grand multiparity: risk factors and outcome in a tertiary hospital: a comparative study. Mater Sociomed. 2015;27(4):244–7.
Al Rowaily MA, Alsalem FA, Abolfotouh MA. Cesarean section in a high-parity community in Saudi Arabia: clinical indications and obstetric outcomes. BMC Pregnancy Childbirth. 2014;14:92.
Wahabi HA, Esmaeil SA, Fayed A, Alzeidan RA. Gestational diabetes mellitus: maternal and perinatal outcomes in King Khalid University Hospital, Saudi Arabia. J Egypt Public Health Assoc. 2013;88(2):104–8.
Al-Khalifah R, Al-Subaihin A, Al-Kharfi T, Al-Alaiyan S, Alfaleh KM. Neonatal short-term outcomes of gestational diabetes mellitus in saudi mothers: a retrospective cohort study. J Clin Neonatol. 2012;1(1):29–33.
Gasim T. Gestational diabetes mellitus: maternal and perinatal outcomes in 220 Saudi women. Oman Med J. 2012;27(2):140–4.
Al-Sunaidi M, Al-Shahrani MS. Fetomaternal and neonatal outcome of triplet pregnancy. Promising results. Saudi Med J. 2011;32(7):685–8.
Al-Saleh I, Shinwari N, Nester M, Mashhour A, Moncari L, El Din Mohamed G, et al. Longitudinal study of prenatal and postnatal lead exposure and early cognitive development in Al-Kharj, Saudi Arabia: a preliminary results of cord blood lead levels. J Trop Pediatr. 2008;54(5):300–7.
Mansouri HA, Ghazawi AH. The maternal and neonatal outcome of high order gestation at King Abdulaziz University Hospital. Arch Gynecol Obstet. 2007;275(2):89–92.
Al-Hakeem MM. Pregnancy outcome of gestational diabetic mothers: experience in a tertiary center. J Fam Community Med. 2006;13(2):55–9.
Yamani Zamzami TY. Vaginal birth after cesarean section in grand multiparous women. Arch Gynecol Obstet. 2004;270(1):21–4.
Abdalrahman Almarzouki A. Maternal and neonatal outcome of controlled gestational diabetes mellitus versus high risk group without gestational diabetes mellitus: a comparative study. Med Glas (Zenica). 2013;10(1):70–4.
al-Dabbous IA, Owa JA, Nasserallah ZA, al-Qurash IS. Perinatal morbidity and mortality in offspring of diabetic mothers in Qatif, Saudi Arabia. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):165–9.
Fayed HM, Abid SF, Stevens B. Risk factors in extreme grand multiparity. Int J Gynaecol Obstet. 1993;41(1):17–22.
Scott JA, Dashti M, Al-Sughayer M, Edwards CA. Timing and determinants of the introduction of complementary foods in Kuwait: results of a prospective cohort study. J Hum Lact. 2015;31(3):467–73.
Gardner H, Green K, Gardner A. Infant feeding practices of Emirati women in the rapidly developing city of Abu Dhabi, United Arab Emirates. Int J Environ Res Public Health. 2015;12(9):10923–40.
Al-Ali FM, Hossain MM, Pugh RN. The associations between feeding modes and diarrhoea among urban children in a newly developed country. Public Health. 1997;111(4):239–43.
Abdwani R, Al Shaqsi L, Al-Zakwani I. Neonatal and obstetrical outcomes of pregnancies in systemic lupus erythematosus. Oman Med J. 2018;33(1):15–21.
Abu-Heija AT, Al-Bash M, Mathew M. Gestational and pregestational diabetes mellitus in Omani women: comparison of obstetric and perinatal outcomes. Sultan Qaboos Univ Med J. 2015;15(4):e496–500.
Al-Farsi YM, Brooks DR, Werler MM, Cabral HJ, Al-Shafei MA, Wallenburg HC. Effect of high parity on occurrence of anemia in pregnancy: a cohort study. BMC Pregnancy Childbirth. 2011;11:7.
Al-Mouqdad MM, Aljobair F, Alaklobi FA, Taha MY, Abdelrahim A, Asfour SS. The consequences of prolonged duration of antibiotics in premature infants with suspected sepsis in a large tertiary referral hospital: a retrospective cohort study. Int J Pediatr Adolesc Med. 2018;5(3):110–5.
Sobaih BH. Long-term cognitive outcome of very low birth-weight Saudi preterm infants at the corrected age of 24-36 months. Saudi Med J. 2018;39(4):368–72.
Al-Qashar F, Sobaih B, Shajira E, Saif SA, Ahmed IA, Al-Shehri H, et al. Impact of intrauterine growth restriction and birth weight on infant's early childhood neurodevelopment outcome. J Clin Neonatol. 2018;7:1–6.
Waheeb S, Alshehri K. Incidence of retinopathy of prematurity at two tertiary centers in Jeddah, Saudi Arabia. Saudi J Ophthalmol. 2016;30(2):109–12.
Al-Saleh I, Nester M, Mashhour A, Moncari L, Shinwari N, Mohamed Gel D, et al. Prenatal and postnatal lead exposure and early cognitive development: longitudinal study in Saudi Arabia. J Environ Pathol Toxicol Oncol. 2009;28(4):283–302.
Archibong EI, Sobande AA, Asindi AA. Antenatal intrauterine fetal death: a prospective study in a tertiary hospital in south-western Saudi Arabia. J Obstet Gynaecol. 2003;23(2):170–3.
Fayed AA, Wahabi H, Mamdouh H, Kotb R, Esmaeil S. Demographic profile and pregnancy outcomes of adolescents and older mothers in Saudi Arabia: analysis from Riyadh Mother (RAHMA) and Baby cohort study. BMJ Open. 2017;7(9):e016501.
Shawky S, Milaat W. Early teenage marriage and subsequent pregnancy outcome. East Mediterr Health J. 2000;6(1):46–54.
Bener A, Al-Nufal M, Vachhani PJ, Ali AI, Samson N, Saleh NM. Maternal complications and neonatal outcome in Arab women of a fast developing country. J Fam Community Med. 2013;20(1):27–34.
Al-Farsi YM, Brooks DR, Werler MM, Cabral HJ, Al-Shafei MA, Wallenburg HC. Effect of high parity on the occurrence of prediabetes: a cohort study. Acta Obstet Gynecol Scand. 2010;89(9):1182–6.
World Health Organization. Obesity and overweight. 2017. Available:http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
S AL. Obesity in gulf countries. Int J Health Sci (Qassim). 2014;8(1):79–83.
World Health Organization. Prevalence of obesity, ages 18+, age standardized: female, 2016. Global Map. Available: http://gamapserver.who.int/mapLibrary/app/searchResults.aspx.
Gaudet L, Ferraro ZM, Wen SW, Walker M. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. Biomed Res Int. 2014;2014:640291.
Poobalan AS, Aucott LS, Gurung T, Smith WC, Bhattacharya S. Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women--systematic review and meta-analysis of cohort studies. Obes Rev. 2009;10(1):28–35.
Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007;8(5):385–94.
Rahman MM, Abe SK, Kanda M, Narita S, Rahman MS, Bilano V, et al. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: a systematic review and meta-analysis. Obes Rev. 2015;16(9):758–70.
Funk WE, Pleil JD, Pedit JA, Boundy MG, Yeatts KB, Nash DG, et al. Indoor air quality in the United Arab Emirates. J Environ Prot. 2014;11(5):709–22.
Yeatts KB, El-Sadig M, Leith D, Kalsbeek W, Al-Maskari F, Couper D, et al. Indoor air pollutants and health in the United Arab Emirates. Environ Health Perspect. 2012;120(5):687–94.
Kim S, Tridane A. Thalassemia in the United Arab Emirates: why it can be prevented but not eradicated. PLoS One. 2017;12(1):e0170485.
Abdulle AM, Nagelkerke NJ, Abouchacra S, Pathan JY, Adem A, Obineche EN. Under- treatment and under diagnosis of hypertension: a serious problem in the United Arab Emirates. BMC Cardiovasc Disord. 2006;6:24.
Golding J. Measuring outcomes in a longitudinal birth cohort. Paediatr Perinat Epidemiol. 2009;23(Suppl 1):185–200.
Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, et al. Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016;45(2):382–8.
Noordzij M, Dekker FW, Zoccali C, Jager KJ. Study designs in clinical research. Nephron Clin Pract. 2009;113(3):c218–21.
Greenberg RS, Daniels SR, Flanders WD, Eley JW, Boring JR. Chapter 2. Epidemiologic measures. In: Greenberg RS, Daniels SR, Flanders WD, Eley JW, Boring JR, editors. Medical epidemiology. 4th ed. New York: McGraw-Hill; 2005.
Ahmed H, Naik G, Willoughby H, Edwards AG. Communicating risk. BMJ. 2012;344:e3996.
World Health Organization. Health topics: Obesity. http://www.who.int/topics/obesity/en/. Accessed 19 Nov 2018.
Acknowledgements
The authors are greatly thankful for the infrastructure provided by the Institute of Public Health at the College of Medicine and Health Sciences, United Arab Emirates University.
Funding
This systematic review was supported by Zayed Center for Health Sciences (Grant Number 31R076) and the United Arab Emirates University Program for Advanced Research (UPAR Grant Number 31 M364).
Author information
Authors and Affiliations
Contributions
RHA, NA, ETB, AHA, TL, FAM, and LAA conceptualized and designed the review. NA and ETB searched the literature and retrieved the eligible cohort studies. RHA, NA, and ETB extracted the data. RHA conducted the meta-analysis and interpreted the findings. RHA, NA, ETB, TL, and LAA drafted the manuscript. RHA, NA, ETB, AHA, TL, FAM, and LAA critically reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
There is no need for any ethical approval or an exemption letter according to the United Arab Emirates University-Human Research Ethics Committee.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2009 checklist [30].
Additional file 2: Box S1
. Data sources and search criteria for systematically reviewing literature reporting on maternal and birth cohort studies in the GCC Countries.
Additional file 3: Table S2
. Summary characteristics and key findings of the 81 maternal and child published cohort studies conducted in the GCC countries, stratified by country.
Additional file 4: Figure S1
. Modified Fig. 2 showing the pooled adjusted estimates on the association between maternal obesity and macrosomia after excluding two adjusted odds ratio estimates (1.53 and 9.18) that are converted to relative risk in Fig. 2 (1.19 and 1.12 respectively) reported by Wahabi HA et al. 2013 [49]. Note: Square indicates to the study-specific effect estimate. Size of the square is proportional to the precision (weight) of the study-specific effect estimate in the pooled estimate. Bars indicate the width of the 95% confidence interval (CI). The diamond centered on the summary effect estimate, and the width indicates the corresponding 95% CI of the pooled estimate.
Additional file 5: Figure S2.
Modified Fig. 3 showing pooled adjusted estimates on the association between maternal obesity and CS delivery after excluding one estimate (4.80, 95% CI: 1.50–6.40) that is converted to relative risk in Fig. 3 (1.16) reported by Hassib YA., 2017 [47]. Note: Square indicates to the study-specific effect estimate. Size of the square is proportional to the precision (weight) of the study-specific effect estimate in the pooled estimate. Bars indicate the width of the 95% confidence interval (CI). The diamond centered on the summary effect estimate, and the width indicates the corresponding 95% CI of the pooled estimate.
Additional file 6: Figure S3.
Summary of risk of bias (ROB) assessment of the 81 cohort studies using the NIH quality assessment tool for the cohort and cross-sectional studies.
Additional file 7: Table S3.
Risk of bias (ROB) assessment of the 81 cohort studies using the NIH quality assessment tool for the cohort studies.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Al-Rifai, R.H., Ali, N., Barigye, E.T. et al. Maternal and birth cohort studies in the Gulf Cooperation Council countries: a systematic review and meta-analysis. Syst Rev 9, 14 (2020). https://doi.org/10.1186/s13643-020-1277-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-020-1277-0