Abstract
Background
Patients with advanced critical illness often receive more intensive treatment than they would choose for themselves, which contributes to high health care costs near the end of life. The purpose of this study was to determine whether a family support intervention delivered by the interprofessional ICU team decreases hospitalization costs and hospital readmissions among critically ill patients at high risk of death or severe functional impairment.
Results
We examined index hospitalization costs as well as post-discharge utilization of acute care hospitals, rehabilitation and skilled nursing facilities, and hospice services for the PARTNER trial, a multicenter, stepped-wedge, cluster randomized trial of an interprofessional ICU family support intervention. We determined patients’ total controllable and direct variable costs using a computerized accounting system. We determined post-discharge resource utilization (as defined above) by structured telephone interview at 6-month follow-up. We used multiple variable regression modelling to compare outcomes between groups. Compared to usual care, the PARTNER intervention resulted in significantly lower total controllable costs (geometric mean: $26,529 vs $32,105; log-linear coefficient: − 0.30; 95% CI − 0.49, − 0.11) and direct variable costs ($3912 vs $6034; − 0.33; 95% CI − 0.56, − 0.10). A larger cost reduction occurred for decedents ($20,304 vs. $26,610; − 0.66; 95% CI − 1.01, − 0.31) compared to survivors ($31,353 vs. $35,015; − 0.15; 95% CI − 0.35,0.05). A lower proportion in the intervention arm were re-admitted to an acute care hospital (34.9% vs 45.1%; 0.66; 95% CI 0.56, 0.77) or skilled nursing facility (25.3% vs 31.6%; 0.63; 95% CI 0.47, 0.84).
Conclusions
A family support intervention delivered by the interprofessional ICU team significantly decreased index hospitalization costs and readmission rates over 6-month follow-up.
Trial registration Trial registration number: NCT01844492
Similar content being viewed by others
Introduction
Approximately 500,000 Americans die annually following admission to an intensive care unit (ICU) [1]. Because these patients typically lack decision-making capacity due to advanced illness or neuroactive medications, clinicians turn to patients’ surrogate decision-makers to assist with decisions regarding goals of care [2,3,4]. However, communication breakdowns are well documented [5,6,7,8,9,10,11,12,13,14,15] and surrogates report that the experience of authorizing withdrawal of life support is psychologically and emotionally difficult [16,17,18,19]. Consequently, patients near the end of life often receive more invasive, burdensome treatments than they would choose for themselves [20,21,22,23]. These problems threaten the ethical goal of patient-centered care and also contribute to the high costs of end-of-life care in the United States [24, 25]
We previously reported the results of a multicenter trial of a family support intervention delivered by the interprofessional ICU team [26]. The intervention improved surrogates’ ratings of the patient- and family-centeredness of care, decreased ICU and hospital length of stay, and did not impact surrogates’ psychological symptom burden at 6-month follow-up. The intervention was designed to be low-cost to deliver, to increase the role of nurses in supporting families, and to be feasible to deploy in most U.S. hospitals.
In our initial publication of the PARTNER trial, we did not report the effects of the intervention on costs of the index hospitalization or on hospital readmissions. Understanding whether the observed improvements in patient- and family-centeredness of care were accompanied by cost savings (or cost increases) is important for health systems and payers who may consider adopting the PARTNER intervention. Few studies of family support interventions have conducted a costing assessment, which we believe to be an important piece of establishing real-world benefit. In general, an intervention that simultaneously improves outcomes and decreases costs has high potential for broad adoption. If an intervention improves outcomes but increases costs, a detailed understanding of the magnitude of costs compared to benefits is often needed to inform funding decisions. We therefore determined the impact of the PARTNER intervention on costs during patients’ index hospitalization and on survivors’ utilization of acute care hospitals, skilled nursing and rehabilitation facilities, and hospice through 6-month follow-up.
Methods
Study design
From July 2012 to February 2016, we conducted a multicenter, stepped-wedge cluster randomized trial comparing a multicomponent family support intervention delivered by the interprofessional ICU team called PARTNER (PAiring Re-engineered ICU Teams with Nurse-driven Emotional Support and Relationship-building) to usual care. The study protocol and results of the primary analyses have been previously published [26]. The intervention was determined to be quality improvement because it was designed to improve the implementation of care practices recommended in professional society practice statements [27,28,29]. Surrogates provided informed consent for participation in long-term follow-up and were informed of the quality improvement project. The study was reviewed and approved by the University of Pittsburgh Institutional Review Board (PRO13020304), the UPMC Quality Improvement Committee, and the leadership of participating ICUs.
Setting and eligibility criteria
Patients were recruited from five ICUs from five hospitals within the University of Pittsburgh Medical Center (UPMC) Health System: a neurological ICU, a surgical transplant ICU, two medical surgical ICUs, and a medical ICU. Details about physician and nurse staffing models were previously described.
Patient inclusion criteria included: (1) age greater than or equal to 18 years; (2) lack of decision-making capacity based on the clinical examination of the patient’s attending physician; and (3) at least one of the following clinical characteristics: (a) receipt of mechanical ventilation for ≥ 4 consecutive days; (b) an estimated ≥ 40% chance of hospital mortality as judged by the patient’s attending physician; or (c) an estimated ≥ 40% chance of severe long term functional impairment as judged by the patient’s attending physician. Patients were excluded if they lacked a surrogate decision-maker or were receiving only comfort-focused treatment at the time of screening. We enrolled one surrogate decision-maker per patient, based on the family’s judgement of who was acting as the main surrogate for the patient.
Randomization and study intervention
We used a computer-generated randomization scheme to determine the order in which sites transitioned from control phase (usual care) to intervention phase, in 6-month intervals. A detailed description of the PARTNER intervention has been previously published [26]. Briefly, the intervention is grounded in the Cognitive-Emotional Decision Making framework [30]. This framework conceptualizes the challenges of medical decision making as not only cognitive in nature, but also related to the affective and psychological difficulty of making high-stakes health decisions for a critically ill loved one [31]. The PARTNER intervention therefore was designed to attend to the cognitive, affective, and psychological challenges that surrogates face, through delivering guideline-recommended emotional support and ensuring frequent clinician-family communication [27,28,29]. The intervention was deployed by members of the existing ICU interprofessional team; it was overseen by 4–6 nurses in each ICU, called the PARTNER nurses, who were nominated by their ICU director and who received additional training, as described below.
There were three main components to the PARTNER intervention. First, the PARTNER nurses in each ICU received advanced communication skills training to provide intensive support to surrogates. This training was delivered through a 12-h course that employed a series didactic teaching, modelling of the communication skills by an expert instructor, extensive skills practice with trained medical actors, and personalized feedback provided by the instructor. Second, each ICU revised their care processes to deploy a structured family support pathway in which the PARTNER nurses met with families daily according to a standardized protocol and arranged interdisciplinary clinician-family meetings within 48 h of enrollment and every 5–7 days thereafter. Third, a quality improvement specialist from the UPMC health system provided each ICU with intensive implementation support to assist them in adopting the family support pathway in their ICU.
Data collection
We used the UPMC health system electronic health record to obtain the following information: patient demographics, primary diagnosis, admission source, severity of illness on ICU admission as determined by a modified Simplified Acute Physiology Score (SAPS) III [32], comorbidities measured with the Elixhauser score [33], and disposition upon hospital discharge. We determined hospitalization costs using the UPMC health system computerized cost accounting, described below. Research staff masked to treatment group conducted telephone interviews with surrogate decision-makers 6 months after the patient’s hospital discharge, during which they ascertained surrogates’ demographic information and information about patients’ post-discharge use of acute inpatient care, skilled nursing and rehabilitation facilities, and hospice services.
Outcomes
We quantified hospitalization costs by determining both the total controllable hospitalization costs and the direct variable costs of each admission. To calculate total controllable hospitalization costs, the UPMC computerized cost accounting system assigns specific costs to each service based on hospital expenses. UPMC developed this activity-based costing (ABC) system to align costs with patients based on actual utilization of resources. Direct expenses, such as blood products, drugs, and supplies, are allocated to individual patients based on usage. Departmental labor, including clinician services, and other fixed clinically oriented expenses such as utilities and facility maintenance are calculated as global costs and allocated to patients using specific cost drivers, such minutes on a nursing unit or time in an OR. This costing method excludes fully indirect expenses such as organizational business and administrative activities (i.e., marketing, legal, finance, human resources, talent acquisition) and information technology support. Because this costing method excludes these categories of fixed costs, which account for approximately 25% of total hospitalization costs, the label “total controllable hospitalization costs” is more accurate than total hospitalization costs.
To calculate direct-variable costs, we removed the fixed costs of overhead that are not related to patient throughput, determined through individual departmental usage patterns [34] and aggregated each patient’s total service specific costs. The categories included in direct variable costs consist of blood products (the cost of all blood products and related services), drugs (the costs of all drugs directly attributable to that encounter), and supplies (the costs of all supplies directly attributable to that patient). Further details of the activity-based costing system can be found at: https://www.healthcatalyst.com/success_stories/activity-based-costing-in-healthcare-service-lines-upmc.
To determine patients’ post-discharge inpatient and hospice care utilization, trained research staff used an established interview guide, which they administered to surrogates by telephone 6 months after hospital discharge. Interviewers determined whether the patient was subsequently admitted to an acute care hospital, a long-term acute care hospital, a skilled nursing facility, or a rehabilitation facility. Interviewers also determined whether patients utilized hospice services during the 6-month follow-up period. Emergency department visits, outpatient visits, and non-hospice homecare use were excluded.
As previously reported [26], we calculated the cost per patient to deploy the intervention by summing the overall costs to deploy the intervention and dividing this by the number of patients in the intervention arm. Cost data was analyzed according to 2012–2016 prices, unadjusted for inflation. Time was included in the multiple variable model to account for the potential for confounding due to cost increases over the study period.
Statistical analysis
All analyses were performed on an intention-to-treat basis using Stata 15 software [35]. The individual ICU was the unit of randomization and the individual patient was the unit of analysis. Because of the nonnegative and skewed nature of cost data, we used log-linear mixed modeling with log-transformed costs to examine the intervention’s impact on total controllable and direct-variable hospitalization costs. We incorporated an ICU-level random effect to address clustering by ICU and random slopes of time to account for temporal effects, as delineated by Hussey and Hughes [36, 37]. We prespecified that all analyses would be adjusted for patient age, sex, race, modified Simplified Acute Physiology Score (SAPS) III, Elixhauser index, mechanical ventilation usage, admission source, and primary diagnosis. We also prespecified that we would conduct stratified analyses by whether the patient survived to hospital discharge.
To determine whether the intervention affected utilization of acute care, skilled nursing, and rehabilitation facilities, and hospice after the index hospitalization, we used multivariable logistic regression with robust standard errors for site clustering. We selected this modelling approach rather than mixed effects modelling because the time effect was not statistically significant and because the site effect led to model instability. We adjusted for the same covariates noted above. We assessed the stability of final models using routine model diagnostics to identify potential outliers and/or influential observations.
Results
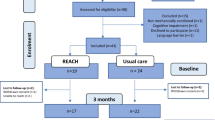
As previously described, 1420 patients met entry criteria and were included in the trial. Table 1 shows the demographic characteristics of the patients. There were baseline differences between treatment groups, including higher age, acute severity of illness, and number of chronic comorbidities in the intervention arm. Surrogates for 1106 patients agreed to be contacted for long-term follow-up and 809 of these (73%) completed long-term follow up; Table 2 summarizes their demographic characteristics. The overall 6-month mortality rate among patients in the trial was 56.7% (805/1420).
Table 3 summarizes the main cost analyses of the trial. Because the UPMC activity-based costing system was put into place after the trial commenced, cost data were available for 1012 of 1420 patients (71.3%; Supplemental Table 1). The intervention resulted in significantly lower total controllable hospitalization costs ($26,529 vs $32,105; unadjusted p-value 0.005; adjusted log-linear model coefficient: − 0.30; 95% CI − 0.49 to − 0.11; p = 0.002). A larger reduction in total controllable hospitalization costs occurred among patients who died during index hospitalization ($20,304 vs. $26,610; unadjusted p-value 0.028; log-linear model coefficient: − 0.66; 95% CI − 1.01 to − 0.31; p < 0.001) compared to those who survived to hospital discharge ($31,353 vs. $35,015; unadjusted p-value 0.173; adjusted log-linear model coefficient: − 0.15; 95% CI − 0.35 to 0.05; p = 0.135). The intervention also significantly decreased direct variable costs, both for decedents and survivors, as summarized in Table 3.
Table 4 summarizes the post-discharge utilization of inpatient and hospice services for patients who survived the index hospitalization, stratified by treatment group. Among the 809 patients whose surrogate completed the 6-month follow-up call (Supplemental Table 2), 241 died during the index hospitalization and therefore had no post-discharge health care utilization. Among the 568 survivors of the index hospitalization, a lower proportion of patients in the intervention arm were subsequently re-admitted to an acute care hospital (34.9% vs 45.1%; adjusted OR: 0.66; 95% CI 0.56 to 0.77; p < 0.001) or skilled nursing facility (25.3% vs 31.6%; adjusted OR: 0.63; 95% CI 0.47 to 0.84; p = 0.002). There was a trend toward higher hospice utilization in the intervention arm versus control during the 6-month follow-up (12.6% vs 6.8%; adjusted OR: 1.67; 95% CI 0.82 to 3.36; p = 0.15).
As previously reported, the cost per patient who received the intervention (N = 547) was $169.88, which included costs associated with the 12-h communication skills training course and regular site visits by a QI implementation specialist throughout the intervention phase [26].
Discussion
We previously reported that the PARTNER intervention improved surrogates’ ratings of the patient- and family centeredness of care in the ICU, decreased ICU and hospital length of stay, and did not impact surrogates’ psychological symptom burden at 6-month follow-up [26]. In the present analysis, we found that the PARTNER intervention decreased patients’ hospitalization costs, an effect mediated largely by decreased costs among patients who died in the hospital, possibly related to decreased ICU length of stay in that group (4.4 days vs. 6.8 days, as reported previously). In addition, among patients who survived the index hospitalization, the intervention resulted in significantly fewer patients readmitted to acute care hospitals and skilled nursing facilities over 6-month follow-up, with a trend toward increased hospice utilization.
Interventions to support surrogate decision-makers in ICUs have had variable impact on end-of-life treatment intensity and costs. Interventions that have either been brief or focused on providing surrogates better decision-relevant information have generally not impacted index hospitalization costs or healthcare utilization [23, 38, 39]. Conversely, observational data suggests that multifaceted system-level interventions focused on improving serious illness communication may decrease both costs and healthcare use [40, 41]. To the best of our knowledge, only one other family support intervention has demonstrated reduced treatment intensity at the end of life and hospitalization costs in a randomized control trial [42]. That intervention consisted of a trained facilitator who longitudinally supported the family and mediated conflict during the ICU stay. The PARTNER intervention that we tested was also conceptually and practically focused on providing longitudinal emotional and psychological support to surrogates. When taken in context of prior studies, our findings add to the evidence suggesting that interventions focused on providing longitudinal emotional and psychosocial support may be a promising strategy to decrease utilization of invasive burdensome treatments at the end of life. This hypothesis fits with research on surrogates’ experiences that reveals the intense psychological difficulty many experience during the process of making decisions to forego life support for an incapacitate loved one [16, 17, 19].
What may explain that the PARTNER intervention- which was delivered only during the ICU stay- decreased subsequent readmission rates and non-statistically significant trend toward more hospice utilization among survivors through 6-month follow-up? Although our data cannot establish a mechanism, one possibility is that the intervention helped surrogates come to terms with the gravity of their loved one’s illness, such that when the patient experienced a subsequent clinical deterioration, surrogates were more prepared to shift the goals of care to comfort-focused treatment. Another possibility is that the intervention enhanced the readiness of surrogates and patients to engage in advance care planning, which may have resulted in more decisions by patients to forego life support and enter hospice.
In this trial, we included only patients at high risk of death or severe functional impairment, which has implications for how health systems should think about how to apply the trial results to their patient populations. We observed that most of the cost savings of the PARTNER intervention accrued among decedents, but that improvements in the patient- and family-centeredness of care accrued to both survivors and decedents. Therefore, health systems interested in improving patient- and family-centeredness of care might plausibly choose to adopt the intervention among a broader cohort of patients, while acknowledging that the net cost savings would likely be smaller than observed in the trial because doing so would likely require a larger investment in training and increasing nursing staffing.
We also expect that the effect of the PARTNER intervention may be augmented or diminished depending on ICUs’ existing care practices related to family support. For example, the effect of the intervention may be greater in ICUs with less intensive physician staffing models, infrequent interdisciplinary family meetings, and limited integration of specialty palliative care consultants. Conversely, the effect of the intervention may be smaller in ICUs with high intensity intensivist staffing models, protocols for frequent family meetings, and extensive integration of palliative care consultants into patients’ care.
As previously reported, the PARTNER intervention cost approximately $170 USD per patient to deploy, which included the costs of training and ongoing implementation support during the trial [26]. The trial was successfully conducted without increasing nurse staffing in the study ICUs. However, it is possible that ICUs with different nurse staffing models may need to increase staffing to allow nurses the needed time to engage with families. Nonetheless, even under very conservative estimates (e.g., adding 4 h of nursing time for each enrolled patient), the intervention would still result in substantially lower direct variable and total controllable costs.
This study has several strengths. First, the intervention was explicitly designed to be deployed by ICU clinicians, which increases its scalability and lessens the overall costs compared to adding new staff to the ICU team. Second, we leveraged the UPMC health systems activity-based costing system to calculate costs, which arguably provides a more accurate quantification compared to other costing methods. We assessed the impact of the intervention on both costs of the index hospitalization and on utilization of acute inpatient care, skilled nursing and rehabilitation facilities, and hospice after hospital discharge, which provides insight about costs from both the hospital perspective and the payer perspective.
This study also has several limitations. First, our sample was limited to one region of the country. Second, despite randomization, there were baseline differences between groups and, although we used advanced statistical techniques to adjust for these differences, we cannot exclude the possibility of residual confounding. In particular, the intervention group was older and more comorbid, which may have diminished the true effect of the PARTNER intervention on resource utilization due to higher baseline healthcare use associated with increasing age [43]. Third, data on hospitalization cost data was not available on patients admitted before the health system put into place the activity-based costing system due to changes in how UPMC health system recorded cost data. Fourth, we ascertained rates of hospital readmission and utilization of post-acute care facilities through interviews with patients’ family caregivers, which is subject to recall errors.
In conclusion, a low-cost family support intervention delivered by the existing interprofessional ICU team resulted in significant reductions in hospitalization costs, particularly among patients who died. Among patients who survived the index hospitalization, the intervention resulted in fewer patients being subsequently admitted to an acute care hospital or skilled nursing facility and a trend toward more patients enrolling in hospice.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICU:
-
Intensive Care Medicine
- PARTNER:
-
Pairing Re-engineered ICU Teams with Nurse-Driven Emotional Support and Relationship-Building
- SAPS:
-
Simplified Acute Physiology Score
- UPMC:
-
University of Pittsburgh Medical Centre
References
Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638–43.
Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158(4):1163–7.
Sprung CL, Cohen SL, Sjokvist P, et al. End-of-life practices in European intensive care units: the Ethicus study. JAMA. 2003;290(6):790–7.
Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–8.
Black MD, Vigorito MC, Curtis JR, et al. A multifaceted intervention to improve compliance with process measures for ICU clinician communication with ICU patients and families. Crit Care Med. 2013;41(10):2275–83.
Kodali S, Stametz R, Clarke D, et al. Implementing family communication pathway in neurosurgical patients in an intensive care unit. Palliat Support Care. 2015;13(4):961–7.
Penrod JD, Pronovost PJ, Livote EE, et al. Meeting standards of high-quality intensive care unit palliative care: clinical performance and predictors. Crit Care Med. 2012;40(4):1105–12.
Selph RB, Shiang J, Engelberg R, Curtis JR, White DB. Empathy and life support decisions in intensive care units. J Gen Intern Med. 2008;23(9):1311–7.
Curtis JR, Engelberg RA, Wenrich MD, Shannon SE, Treece PD, Rubenfeld GD. Missed opportunities during family conferences about end-of-life care in the intensive care unit. Am J Respir Crit Care Med. 2005;171(8):844–9.
White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. Prognostication during physician-family discussions about limiting life support in intensive care units. Crit Care Med. 2007;35(2):442–8.
White DB, Ernecoff N, Buddadhumaruk P, et al. Prevalence of and factors related to discordance about prognosis between physicians and surrogate decision makers of critically ill patients. JAMA. 2016;315(19):2086–94.
Chiarchiaro J, Ernecoff NC, Scheunemann LP, et al. Physicians rarely elicit critically ill patients’ previously expressed treatment preferences in intensive care units. Am J Respir Crit Care Med. 2017;196(2):242–5.
Cunningham TV, Scheunemann LP, Arnold RM, White D. How do clinicians prepare family members for the role of surrogate decision-maker? J Med Ethics. 2018;44(1):21–6.
Scheunemann LP, Cunningham TV, Arnold RM, Buddadhumaruk P, White DB. How clinicians discuss critically ill patients’ preferences and values with surrogates: an empirical analysis. Crit Care Med. 2015;43(4):757–64.
Schenker Y, Tiver GA, Hong SY, White DB. Association between physicians’ beliefs and the option of comfort care for critically ill patients. Intensive Care Med. 2012;38(10):1607–15.
Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336–46.
Schenker Y, Crowley-Matoka M, Dohan D, Tiver GA, Arnold RM, White DB. I don’t want to be the one saying ‘we should just let him die’: intrapersonal tensions experienced by surrogate decision makers in the ICU. J Gen Intern Med. 2012;27(12):1657–65.
Schenker Y, White DB, Crowley-Matoka M, Dohan D, Tiver GA, Arnold RM. “It hurts to know… and it helps”: exploring how surrogates in the ICU cope with prognostic information. J Palliat Med. 2013;16(3):243–9.
Kirchhoff KT, Walker L, Hutton A, Spuhler V, Cole BV, Clemmer T. The vortex: families’ experiences with death in the intensive care unit. Am J Crit Care. 2002;11(3):200–9.
Lynn J, Teno JM, Phillips RS, et al. Perceptions by family members of the dying experience of older and seriously ill patients. SUPPORT Investigators. Study to understand prognoses and preferences for outcomes and risks of treatments. Ann Intern Med. 1997;126(2):97–106.
Sharma RK, Freedman VA, Mor V, Kasper JD, Gozalo P, Teno JM. Association of racial differences with end-of-life care quality in the United States. JAMA Intern Med. 2017;177(12):1858–60.
Somogyi-Zalud E, Zhong Z, Hamel MB, Lynn J. The use of life-sustaining treatments in hospitalized persons aged 80 and older. J Am Geriatr Soc. 2002;50(5):930–4.
Cox CE, White DB, Hough CL, et al. Effects of a personalized web-based decision aid for surrogate decision makers of patients with prolonged mechanical ventilation: a randomized clinical trial. Ann Intern Med. 2019. https://doi.org/10.7326/m18-2335. (In Eng).
Riley GF, Lubitz JD. Long-term trends in medicare payments in the last year of life. Health Serv Res. 2010;45(2):565–76.
Hogan C, Lunney J, Gabel J, Lynn J. Medicare beneficiaries’ costs of care in the last year of life. Health Aff. 2001;20(4):188–95.
White DB, Angus DC, Shields AM, et al. A randomized trial of a family-support intervention in intensive care units. N Engl J Med. 2018;378(25):2365–75. https://doi.org/10.1056/NEJMoa1802637.
Davidson JE, Aslakson RA, Long AC, et al. Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med. 2017;45(1):103–28.
Kon AA, Davidson JE, Morrison W, Danis M, White DB. Shared decision making in ICUs: an American college of critical care medicine and American thoracic society policy statement. Crit Care Med. 2016;44(1):188–201.
Truog RD, Campbell ML, Curtis JR, et al. Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American Academy of Critical Care Medicine. Crit Care Med. 2008;36:953–63.
Power TE, Swartzman LC, Robinson JW. Cognitive-emotional decision making (CEDM): a framework of patient medical decision making. Patient Educ Couns. 2011;83(2):163–9.
Pham MT. Emotion and rationality: a critical review and interpretation of empirical evidence. Rev Gen Psychol. 1997;11(2):155–78.
Liu V, Turk BJ, Ragins AI, Kipnis P, Escobar GJ. An electronic simplified acute physiology score-based risk adjustment score for critical illness in an integrated healthcare system. Crit Care Med. 2013;41(1):41–8.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Kahn JM, Rubenfeld GD, Rohrbach J, Fuchs BD. Cost savings attributable to reductions in intensive care unit length of stay for mechanically ventilated patients. Med Care. 2008;46(12):1226–33.
StataCorp. Stata statistical software: release 15. College Station: StataCorp LLC; 2017.
Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28(2):182–91. https://doi.org/10.1016/j.cct.2006.05.007.
Thompson JA, Fielding KL, Davey C, Aiken AM, Hargreaves JR, Hayes RJ. Bias and inference from misspecified mixed-effect models in stepped wedge trial analysis. Stat Med. 2017;36(23):3670–82.
Carson SS, Cox CE, Wallenstein S, et al. Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA. 2016;316(1):51–62.
SUPPORT. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274(20):1591–8.
Chang DW, Neville TH, Parrish J, et al. Evaluation of time-limited trials among critically ill patients with advanced medical illnesses and reduction of nonbeneficial ICU treatments. JAMA Intern Med. 2021;181(6):786–94.
Hui D, Huang YT, Andersen C, et al. Cost of hospitalization associated with inpatient goals-of-care program implementation at a comprehensive cancer center: a propensity score analysis. Cancers. 2024;16(7):1316.
Curtis JR, Treece PD, Nielsen EL, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med. 2016;193(2):154–62.
Kalseth J, Halvorsen T. Health and care service utilisation and cost over the life-span: a descriptive analysis of population data. BMC Health Serv Res. 2020;20(1):435.
Acknowledgements
The authors wish to acknowledge Praewpannarai Buddadhumaruk, MS, RN for assistance with statistical analysis and input on the draft manuscript.
Funding
This work was supported by a UPMC innovation award, the Greenwall foundation, and the national institutes of health (K24Hl148314). The funding bodies had no influence on the design or content of this study.
Author information
Authors and Affiliations
Consortia
Contributions
S.K.A—writing, original draft, review and editing, validation; visualization C.H.C—methodology, formal analysis; R.M.A—conceptualization, resources, writing—review and editing; C.P.—methodology, investigation J.P.D—conceptualization, resources, methodology; D.C.A—conceptualization, resources, methodology, writing—review and editing; D.B.W—supervision, conceptualization, funding acquisition, resources, methodology, writing, original draft, review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the University of Pittsburgh Institutional Review Board (IRB; approval no: PRO13020304; approval date: 2/26/2013), the UPMC Quality Improvement Committee, and the leadership of participating ICUs, and registered on ClinicalTrials.gov (NCT01844492).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andersen, S.K., Chang, CC.H., Arnold, R.M. et al. Impact of a family support intervention on hospitalization costs and hospital readmissions among ICU patients at high risk of death or severe functional impairment. Ann. Intensive Care 14, 103 (2024). https://doi.org/10.1186/s13613-024-01344-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-024-01344-9