Abstract
Background
During the first COVID-19 pandemic wave, COVID-19-associated pulmonary aspergillosis (CAPA) has been reported in up to 11–28% of critically ill COVID-19 patients and associated with increased mortality. As new SARS-CoV-2 variants emerged, the characteristics of critically ill COVID-19 patients have evolved, particularly in the era of Omicron. The purpose of this study is to investigate the characteristics of CAPA in the era of new variants.
Methods
This is a prospective multicenter observational cohort study conducted in France in 36 participating intensive care units (ICU), between December 7th, 2021 and April 26th 2023. Diagnosis criteria of CAPA relied on European Confederation of Medical Mycology (ECMM)/International Society for Human & Animal Mycology (ISHAM) consensus criteria.
Results
566 patients were included over the study period. The prevalence of CAPA was 5.1% [95% CI 3.4–7.3], and rose to 9.1% among patients who required invasive mechanical ventilation (IMV). Univariable analysis showed that CAPA patients were more frequently immunosuppressed and required more frequently IMV support, vasopressors and renal replacement therapy during ICU stay than non-CAPA patients. SAPS II score at ICU admission, immunosuppression, and a SARS-CoV-2 Delta variant were independently associated with CAPA in multivariable logistic regression analysis. Although CAPA was not significantly associated with day-28 mortality, patients with CAPA experienced a longer duration of mechanical ventilation and ICU stay.
Conclusion
This study contributes valuable insights into the prevalence, characteristics, and outcomes of CAPA in the era of Delta and Omicron variants. We report a lower prevalence of CAPA (5.1%) among critically-ill COVID-19 patients than previously reported, mainly affecting intubated-patients. Duration of mechanical ventilation and ICU stay were significantly longer in CAPA patients.
Similar content being viewed by others
Introduction
The societal and individual consequences of pneumonia caused by respiratory viruses, notably due to influenza virus and SARS-CoV-2, are well established. Patients with severe pneumonia may develop acute respiratory failure and require admission to the intensive care unit (ICU). Replication of a respiratory virus in the lower respiratory tract and severe inflammation associated with immune cell infiltration lead to gas exchanges impairment. Viral pneumonia increases patients’ susceptibility to bacterial and fungal superinfections, including invasive pulmonary aspergillosis [1, 2]. Influenza-associated pulmonary aspergillosis (IAPA) has been reported in up to 19–25% of critically ill patients with influenza, associated with poor outcomes [2, 3]. Coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA) has similarly emerged as an important coinfection in critically ill patients with COVID-19. The diagnostic criteria for these co-infections combine clinical, radiological, mycological, and histological criteria [4]. A recent autopsy study on patients infected with influenza and COVID-19 confirmed the invasive nature of the infection and the similarity of histological lesions observed in both IAPA and CAPA cases [5]. A multicenter French study conducted during the first wave revealed that 15% of critically ill patients with COVID-19 requiring invasive mechanical ventilation (IMV) fulfilled the diagnostic criteria for CAPA, which was also associated with poor outcomes [1]. In addition to the prognostic impact of CAPA, studies conducted during the first wave identified host-related risk factors of CAPA, including age, concomitant treatment with corticosteroids and tocilizumab, and prolonged duration of mechanical ventilation [1, 6].
As various SARS-CoV-2 variants have emerged along with the epidemic waves, the characteristics of critically ill COVID-19 patients significantly evolved, especially since the Omicron era: reduced use of IMV, higher rate of immunosuppressed patients, and different treatment approaches as compared to the first COVID-19 wave [7]. The purpose of this study is to investigate the characteristics of CAPA with the emergence of Delta variant followed by Omicron and related sublineages, identify potential predictive factors and assess its prognosis impact.
Methods
Patients and clinical data
This study is a prospective multicenter observational cohort study. Patients admitted between December 7th, 2021 and April 26th, 2023 in one of the 36 participating ICUs (including 19 from the Greater Paris area) were eligible for inclusion in the SEVARVIR cohort study (NCT05162508) if they presented the following inclusion criteria: age ≥ 18 years, SARS-CoV-2 infection confirmed by a positive reverse transcriptase-polymerase chain reaction (RT-PCR) in nasopharyngeal swab samples, admission in the ICU for acute respiratory failure (i.e., peripheral oxygen saturation (SpO2) ≤ 90% and need for supplemental oxygen or any kind of ventilator support), patient or next of kin informed of study inclusion. Patients with SARS-CoV-2 infection but no acute respiratory failure or with a RT-PCR cycle threshold (Ct) value > 32 in nasopharyngeal swabs were not included. Over the study period, 47% of participating ICUs had rooms with negative pressure, accounting for a total of 37% of the rooms. The study was approved by the Comité de Protection des Personnes Sud-Méditerranée I (N° EudraCT/ID-RCB: 2021-A02914-37). Informed consent was obtained from all patients or their relatives.
Demographics, clinical and laboratory variables were recorded upon ICU admission and during ICU stay. Patients’ frailty was assessed using the Clinical Frailty Scale [8]. The severity of the disease upon ICU admission was assessed using the World Health Organization (WHO) 10-point ordinal scale [9], the sequential organ failure assessment (SOFA) score [10], and the simplified acute physiology score (SAPS) II score [11]. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition [12]. CAPA diagnosis work-up was at the initiative of the attending clinician in our study (i.e., targeted sampling strategy). The diagnosis and classification (i.e., proven, probable or possible) of CAPA relied on ECMM/ISHAM (European Confederation of Medical Mycology, the International Society for Human and Animal Mycology) international consensus criteria (Additional file 2: Table S1) [4].
SARS-CoV-2 variant determination
Full-length SARS-CoV-2 genomes from all included patients were sequenced by means of next-generation sequencing. Briefly, viral RNA was extracted from nasopharyngeal swabs in viral transport medium using NucliSENS® easyMAG kit on EMAG device (bioMérieux, Marcy-l’Étoile, France). Sequencing was performed with the Illumina® COVIDSeq Test (Illumina, San Diego, California), which uses 98-target multiplex amplifications along the full SARS-CoV-2 genome. The libraries were sequenced with NextSeq 500/550 High Output Kit v2.5 (75 Cycles) on a NextSeq 500 device (Illumina). The sequences were demultiplexed and assembled as full-length genomes by means of the DRAGEN COVIDSeq Test Pipeline on a local DRAGEN server (Illumina). Lineages and clades were interpreted using Pangolin and NextClade, before being submitted to the GISAID international database (https://www.gisaid.org).
Statistical analyses
Descriptive results are presented as means (± standard deviation [SD]) or medians (1st–3rd quartiles) for continuous variables, and as numbers with percentages for categorical variables. Two-tailed p values < 0.05 were considered statistically significant. Unadjusted comparisons according to CAPA status (CAPA patients vs. non CAPA patients) were performed using Chi-square or Fisher’s exact tests for categorical variables, and t-tests or Mann–Whitney tests for continuous variables, as appropriate.
Multivariable logistic regression models were performed to identify the parameters most associated with CAPA, entering variables associated with a p-value < 0.20 in univariable analysis and those previously shown to be potential confounding factors, including age and gender, then applying a stepwise backward approach by retaining only variables statistically significant at a relaxed p < 0.10 level. Adjusted odds ratios (aORs) along with their 95% confidence intervals (CI) were computed.
To assess the potential effect of CAPA occurrence on subsequent prognosis, 90-day overall survival was estimated using the Simon–Makuch method [13] and was compared using the Mantel–Byar test between those patients having developed a CAPA and those who had not, considering CAPA occurrence as a time-dependent variable. Cox proportional hazards regression modelling was used to compute Hazard ratios (HR) and their corresponding CIs, with CAPA as a time-dependent covariate and further adjusting for important prognostic factors (i.e., age, gender, baseline SOFA score and immunosuppressive status). Landmark analyses of 90-day overall survival by CAPA status were also conducted as sensitivity analyses. To do so, participants who died or were censored before a 5-days landmark point (as the median time of CAPA occurrence) were excluded from the landmark analyses at day 5 of, allowing a better control of the so called ‘immortal-time bias’ (i.e., patients dying early in the study have a limited time to develop CAPA thus guaranteeing poorer outcomes in the patients unexposed to CAPA) and yielding potentially more accurate results by increasing the number of CAPA patients at risk when starting at a 5-day time point compared to earlier time points when the number of CAPA patients at risk are usually smaller.
An exploratory unsupervised clustering analysis was achieved allowing to explore the heterogeneity of the population using the Kohonen’s self-organized map (SOM) methodology [14], allowing us to build 2-dimensional maps from multidimensional datasets. In a nutshell, each map is divided into districts in which patients are located by the SOM algorithm on the basis of their characteristics: patients with similar features are closely located on the maps, while patients with distinct profiles are farther from each other, hence allowing to identify key differences or similarities among them by drawing visual comparisons of unique or overlapping patient characteristics and disease subtypes. Clinical or biological variables considered as relevant were included in this analysis. The SOMs were obtained with the Numero package framework for the R statistical platform [15] after principal component analysis adapted for mixtures of qualitative and quantitative variables was applied (PCAMix) [16, 17].
All measurements were taken from distinct samples. Variables with missing information used for the evaluation of the risk factors of CAPA by logistic regression, for the evaluation of the effect of CAPA occurrence on overall survival by Cox proportional hazards regression modelling and for the exploratory clustering analysis by self-organizing maps, were imputed using the k-nearest neighbors (k-NN) approach. Analyses were performed using Stata V16.1 statistical software (StataCorp, College Station, TX, USA), and R 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Population
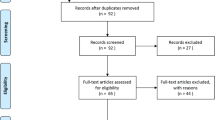
Over the study period, a total of 566 patients were admitted in one of the 36 participating ICUs and included in the study, including 242 patients requiring IMV during ICU stay. Twenty-nine patients (5.1% [95% CI 3.4–7.3]) fulfilled the diagnosis criteria for CAPA (Fig. 1). The prevalence of CAPA was higher in patients who required IMV (N = 22/242, 9.1%) than in those who did not (N = 7/324, 2.2%).
Baseline characteristics
Compared to non-CAPA patients, those who fulfilled CAPA diagnosis criteria, did not show statistically significant differences regarding age, gender and comorbidities, except for more frequent immunosuppression (N = 15/29, 54% vs. N = 174/537, 34%, p = 0.03) (Table 1). Although the proportion of vaccinated patients did not significantly differ according to CAPA status, CAPA patients had more frequently negative anti-S SARS-CoV-2 IgG antibodies than non-CAPA patients, possibly linked to the higher proportion of immunosuppressed patients in the former than in the latter group. The median delay between the first symptoms of disease and ICU admission was significantly longer in CAPA than in non-CAPA patients (8 [6–11] vs. 7 [3–10] days; p = 0.03). The viral load of SARS-CoV-2 in the upper respiratory tract (estimated with the cycle threshold of RT-PCR) did not significantly differ between groups (Table 1). There was no significant difference according to CAPA status regarding the severity of the disease at ICU admission, as reflected by the SOFA and SAPS II scores and the WHO 10-point ordinal scale (Table 1).
CAPA patients tended to require more frequent IMV support within 24 h of ICU admission (N = 11/29, 38% vs. N = 124/537, 23%, p = 0.2). All three patients requiring extracorporeal membrane oxygenation support upon ICU admission were non-CAPA patients.
ICU management and outcomes
During ICU stay, CAPA patients required significantly more IMV support (N = 22/29, 76% vs. N = 220/537, 41%, p = 0.0002) and prone positioning (N = 18/29, 64% vs. N = 153/537, 30%, p = 0.0002) than their counterparts (Table 2). Of note, IMV duration was significantly longer in CAPA than in non-CAPA patients (28 [17–34] vs. 10 [5–20] days, p = 0.0001) and CAPA patients had more frequent ventilator-acquired pneumonia (VAP) episodes. Median time between start of IMV and the first episode of VAP was 6 [2–10] days globally, and 11 [6–20] days in those patients with CAPA. There were also significant differences between groups regarding need for other organ supports: CAPA patients required significantly more vasopressors and renal replacement therapy. Regarding COVID-19 specific management, there were no significant between-group differences with 83% of patients (N = 415/566) who received dexamethasone, 33% (N = 165/566) tocilizumab, and 15% (N = 74/566) monoclonal antibodies. There was no difference in day-28 mortality according to CAPA status (N = 10/29, 34% vs. N = 151/537, 29%, p = 0.5), but duration of ICU stay was significantly longer in CAPA patients (28 [16–44] vs. 8 [4–17] days, p < 0.0001).
The Simon-Makuch estimates of overall survival from ICU admission to day-90 is depicted in Fig. 2. No statistically significant association was found between CAPA occurrence and mortality, whether considering Mantel-Byar test (p = 0.926) or Cox time-dependent analyses (HR 0.97 (0.56–1.70), p = 0.927; adjusted HR after missing data imputation 0.79 (0.45–1.41), p = 0.425; adjusted HR on raw data 0.76 (0.42–1.40), p = 0.382). Sensitivity analyses considering a 5-day timepoint yielded similar results (Mantel-Byar p = 0.943; HR 1.02 [0.58–1.79], p = 0.944; adjusted HR after missing data imputation 0.90 [0.51–1.60], p = 0.719; adjusted HR on raw data 0.83 [0.45–1.53], p = 0.551).
Characteristics of CAPA patients
According to CMM/ISHAM CAPA definitions, 24 patients (83%) fulfilled the criteria of proven/probable CAPA and 5 patients (17%) were classified as possible CAPA (Table 3). The detailed diagnostic criteria are shown in Additional file 1: Figure S1. The diagnosis of CAPA was made a median of 5 [2–16] days after ICU admission and 6 [2–13] days after tracheal intubation (among the CAPA patients requiring IMV). Half of the patients were immunosuppressed: 10 (66%) had an onco-hematological malignancy, and 4 (27%) had received an organ transplant. Among the 29 CAPA patients, 28 (97%) received an antifungal treatment during ICU stay. Voriconazole and isavuconazole were the two most frequently administered antifungal drugs (Table 3). To better characterize the phenotypic differences between CAPA and non-CAPA patients, an exploratory analysis using the SOM method was performed to plot 2-D maps of patients grouped according to their characteristics (Fig. 3). SOM analysis depicted the observed differences according to CAPA status. As shown in the figure, CAPA patients tended to cluster in the upper left area of the map, where the highest frequencies of immunosuppression, the highest values of the SOFA and SAPS II scores also clustered. Patients with the highest rates of day-28 mortality and use of IMV during ICU stay clustered in the same area of the map than CAPA patients.
Unsupervised analysis of the clinical and biological characteristics of the 566 critically-ill COVID-19 patients by self-organized maps (SOMs). Unsupervised analysis by SOM automatically located patients with similar clinical and paraclinical parameters within 1 of 40 small groupings (“districts”) throughout the map. The more similar the patients, the closer on the map. Each individual map shows the mean values or proportions per district for each characteristic: blue indicates the lowest average values, red the highest, with numbers shown for a selection of representative districts in each SOM. For instance, immunosuppressed patients were more frequently located in the upper districts and also had higher serum urea levels, less frequent Delta variant infection, higher SAPS II and SOFA scores and day-28 mortality rates. WHO World Health Organization, SOFA Sequential Organ Failure Assessment, SAPS II Simplified Acute Physiology Score II, MV mechanical ventilation
Factors associated with CAPA
In multivariable analysis after missing data imputation, four factors associated with higher risk of CAPA were retained after stepwise analysis: increased SAPS II score (aOR 1.03 [95% CI 1.003–1.05], p = 0.028), immunosuppression (aOR 2.65 [1.13–6.20], p = 0.025), a SARS-CoV-2 Delta variant (aOR 2.72 [1.12–6.58], p = 0.027), and to a lower extent an increased delay between the first symptoms and ICU admission (aOR 1.03 [0.997–1.06], p = 0.077) (Table 4). Age and IMV support during ICU stay were not significantly associated with CAPA at the p < 0.10 level. Results of the multivariable analysis on raw data are presented in Additional file 3: Table S2.
Discussion
To the best of our knowledge, we herein report the largest cohort study investigating the prevalence and the characteristics of CAPA among critically-ill COVID-19 patients in the era of Delta and Omicron SARS-CoV-2 variants. The main results of our study are the following: (i) the prevalence of CAPA in the whole cohort was 5.1%, and 9.1% among patients requiring IMV; (ii) CAPA patients were more frequently immunosuppressed, required more frequently IMV, vasopressors and renal replacement therapy during ICU stay; (iii) CAPA was not associated with day-28 mortality, but with a longer duration of mechanical ventilation support and ICU stay; and (iv) SAPS II score at ICU admission and the delay between the first symptoms and ICU admission were independently associated with CAPA.
The prevalence of CAPA herein reported (5.1%) is lower than previously reported in studies conducted during the first wave in mechanically ventilated ICU patients: 11–28% [1, 6, 18, 19] or in more recent studies: 16–33% [20, 21]. Several factors may explain these results. First, these studies were carried out only in mechanically ventilated patients, while more than half of the patients in our cohort did not require IMV. Indeed, in the subgroup of patients requiring IMV, the reported prevalence was higher (9.1%). Second, these studies routinely screened for diagnosis criteria for CAPA once or twice a week, whereas it was at the initiative of the attending clinician in our study (i.e., targeted sampling). Whether bronchoalveolar lavage sampling should be routinely performed to increase the sensitivity of the diagnosis criteria has been suggested [22]. We acknowledge that our study may be associated with a lower prevalence of CAPA than that reported in other studies performing routine screening [20], however our targeted sampling strategy reflects real-life practice in a nation-wide study. The most appropriate diagnostic strategy for CAPA remains to be defined. Indeed, as most of the CAPA diagnostic criteria are nonspecific (i.e., biomarkers, non-specific clinical and/or radiological signs), a systematic diagnostic approach (as opposed to targeted sampling) might be associated with a lower pre-test probability and thus a lower positive predictive value of CAPA diagnosis. Third and importantly, the clinical phenotype of critically-ill COVID-19 patients has evolved in line with the natural course of the disease, with older and frailer patients, more frequently immunosuppressed, and presenting with higher severity scores at ICU admission [7]. Yet, in the current series, the prevalence of CAPA among patients infected with Omicron (4.8%) was lower than that observed in patients infected with Delta (9.2%), and lower than that reported during the first pandemic wave [1]. These findings might point to a variant-related effect. CAPA has been associated with impaired antifungal immunity (i.e., altered integrity of the epithelial barrier, and decreased capacity to phagocytise and kill Aspergillus spores and to destroy Aspergillus hyphae) [23]. Recent findings suggested a reduced evasion of variant Omicron from innate immunity [24, 25], as compared to pre-existing variants, which might be associated with more effective antifungal immunity and hence a lower prevalence of CAPA. Another potential factor might be the inherent disorganization during the first epidemic wave, resulting in less frequent use of rooms with negative pressure in comparison with the period of this study (i.e., Delta and Omicron era).
We also describe the existence of CAPA in non-intubated patients, although occurring in only 2.2% of patients, which had rarely been reported to date. Indeed, previous studies investigating CAPA included almost exclusively patients requiring IMV [1, 18, 20, 21, 26]. However, two multicenter studies reported that a minority of CAPA occurred in non-intubated patients [6, 27], 6% and 12% of CAPA patients, respectively. Unfortunately, their design did not allow for a reliable assessment of the prevalence of CAPA in this setting. In our study, however, most of non-intubated CAPA patients had a specific underlying condition (five patients were immunocompromised (four haematological malignancies and one solid organ transplantation) and one patient had a chronic cavitary pulmonary aspergillosis, rather than a “classical” CAPA. No classical risk factor was identified for the last patient. Therefore, it appears appropriate to screen non-intubated COVID-19 patients for CAPA in case of underlying immunosuppressive status or other risk factors, rather than routinely.
Previous studies identified various factors associated with CAPA: age, long-term corticosteroids use, chronic obstructive pulmonary disease, haematological malignancy, IMV and its prolonged duration, tocilizumab treatment, especially in association with corticosteroids [1, 6, 18, 19, 28,29,30,31]. In our study, we could identify three factors independently associated with CAPA in multivariable analysis, including SAPS II score at admission, immunosuppression and a SARS-CoV-2 Delta variant. The first two factors are thus in line with previous findings; whereas the Delta variant effect has not yet been described [20]. It is important to note most of the previous studies included patients before the Omicron era, and our study is the largest one investigating CAPA in the era of Omicron.
We did not observe an association between day-28 mortality and CAPA status in our study, in contrast with previous studies that reported a high mortality associated with CAPA [1, 6, 18, 19, 26, 32]. Nevertheless, duration of mechanical ventilation and ICU stay were significantly longer in CAPA patients than non-CAPA patients. Moreover, vital status was only reported at day-28, and it is likely that CAPA status was associated with longer-term prognosis. In line with previous studies, the diagnosis of CAPA was mostly reliant on serum and/or respiratory fungal markers. Thus, the prognostic relevance of CAPA per se could be questioned: it might be considered a simple severity indicator rather than a truly invasive super-infection. A recent pathology-based studies has highlighted the invasive nature of CAPA [5], thereby supporting systematic CAPA screening of intubated patients and specific antifungal treatment.
Our study has some limitations. We were unable to compare these results with those from the first waves, as this prospective cohort began during Delta variant era. The relatively small number of CAPA patients included may have limited our statistical ability to show between-group differences. Non-systematic screening for CAPA may have underestimated the prevalence of CAPA. However, our study also has strengths, including the constitution of a unique prospective multicenter cohort reporting the prevalence and characteristics of CAPA in the era of Delta and Omicron SARS-CoV-2 variants.
Conclusion
To conclude, we report a lower prevalence of CAPA (5.1%) among critically-ill COVID-19 patients in the era of Delta and Omicron variants than previously reported, and mainly affecting intubated-patients. SAPS II score at ICU admission, immunosuppression status and a SARS-CoV-2 Delta variant were independently associated with CAPA. Even though CAPA was not associated with day-28 mortality, duration of mechanical ventilation and ICU stay were significantly longer in CAPA patients.
Availability of data and materials
The clinical datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request (N.D.P.)
Change history
14 June 2024
A Correction to this paper has been published: https://doi.org/10.1186/s13613-024-01318-x
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CAPA:
-
COVID-19-associated pulmonary aspergillosis
- IAPA:
-
Influenza-associated pulmonary aspergillosis
- ICU:
-
Intensive care unit
- IMV:
-
Invasive mechanical ventilation
- HR:
-
Hazard ratio
- SAPS:
-
Simplified acute physiology score
- SOFA:
-
Sequential organ failure assessment
- VAP:
-
Ventilator-associated pneumonia
- WHO:
-
World Health Organization
References
Gangneux JP, Dannaoui E, Fekkar A, Luyt CE, Botterel F, Prost ND, et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med. 2021. https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(21)00442-2/fulltext. Accessed 9 Dec 2021.
Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–92.
Vanderbeke L, Janssen NAF, Bergmans DCJJ, Bourgeois M, Buil JB, Debaveye Y, et al. Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): a randomised, open-label, proof-of-concept trial. Intensive Care Med. 2021;47(6):674–86.
Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149–62.
Vanderbeke L, Jacobs C, Feys S, Reséndiz-Sharpe A, Debaveye Y, Hermans G, et al. A pathology-based case series of influenza- and COVID-19-associated pulmonary aspergillosis: the proof is in the tissue. Am J Respir Crit Care Med. 2023. https://doi.org/10.1164/rccm.202208-1570OC.
Prattes J, Wauters J, Giacobbe DR, Salmanton-García J, Maertens J, Bourgeois M, et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2022;28(4):580–7.
de Prost N, Audureau E, Heming N, Gault E, Pham T, Chaghouri A, et al. Clinical phenotypes and outcomes associated with SARS-CoV-2 variant Omicron in critically ill French patients with COVID-19. Nat Commun. 2022;13(1):6025.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95.
WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–7.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63.
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, ARDS Definition Task Force, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35–44.
Kohonen T, Somervuo P. How to make large self-organizing maps for nonvectorial data. Neural Netw. 2002;15(8):945–52.
Gao S, Mutter S, Casey A, Mäkinen VP. Numero: a statistical framework to define multivariable subgroups in complex population-based datasets. Int J Epidemiol. 2019;48(2):369–74.
van de Velden M, Iodice D’Enza A, Markos A. Distance-based clustering of mixed data. WIREs Comput Stat. 2019;11(3): e1456.
Chavent M, Kuentz-Simonet V, Labenne A, Saracco J. Multivariate analysis of mixed data: the R package PCAmixdata. arXiv; 2022. http://arxiv.org/abs/1411.4911. Accessed 4 Aug 2023.
Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021;73(11):e3606–14.
White PL, Donnelly JP. PCR of plasma and BAL fluid for diagnosing invasive aspergillosis. Clin Infect Dis. 2023;77:1291–3.
Feys S, Lagrou K, Lauwers HM, Haenen K, Jacobs C, Brusselmans M, et al. High burden of COVID-19-associated pulmonary aspergillosis in severely immunocompromised patients requiring mechanical ventilation. Clin Infect Dis. 2023;78:361–70.
Hurt W, Youngs J, Ball J, Edgeworth J, Hopkins P, Jenkins DR, et al. COVID-19-associated pulmonary aspergillosis in mechanically ventilated patients: a prospective, multicentre UK study. Thorax. 2023. https://thorax.bmj.com/content/early/2023/09/01/thorax-2023-220002. Accessed 31 Oct 2023.
Feys S, Hoenigl M, Gangneux JP, Verweij PE, Wauters J. Fungal fog in viral storms: necessity for rigor in aspergillosis diagnosis and research. Am J Respir Crit Care Med. 2023. https://doi.org/10.1164/rccm.202310-1815VP.
Feys S, Gonçalves SM, Khan M, Choi S, Boeckx B, Chatelain D, et al. Lung epithelial and myeloid innate immunity in influenza-associated or COVID-19-associated pulmonary aspergillosis: an observational study. Lancet Respir Med. 2022;10(12):1147–59.
Bojkova D, Widera M, Ciesek S, Wass MN, Michaelis M, Cinatl J. Reduced interferon antagonism but similar drug sensitivity in Omicron variant compared to Delta variant of SARS-CoV-2 isolates. Cell Res. 2022;32(3):319–21.
Bay P, Rodriguez C, Caruso S, Demontant V, Boizeau L, Soulier A, et al. Omicron induced distinct immune respiratory transcriptomics signatures compared to pre-existing variants in critically ill COVID-19 patients. J Med Virol. 2023;95(12): e29268.
Dellière S, Dudoignon E, Fodil S, Voicu S, Collet M, Oillic PA, et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2020;27(5):790.e1-5.
Salmanton-García J, Sprute R, Stemler J, Bartoletti M, Dupont D, Valerio M, et al. COVID-19–associated pulmonary aspergillosis, March–August 2020. Emerg Infect Dis J CDC. 2021;27:1077.
Wang J, Yang Q, Zhang P, Sheng J, Zhou J, Qu T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: a retrospective case series. Crit Care. 2020;24(1):299.
Van Biesen S, Kwa D, Bosman RJ, Juffermans NP. Detection of invasive pulmonary aspergillosis in COVID-19 with nondirected BAL. Am J Respir Crit Care Med. 2020;202(8):1171–3.
Desmedt L, Raymond M, Le Thuaut A, Asfar P, Darreau C, Reizine F, et al. Covid-19-associated pulmonary aspergillosis in mechanically ventilated patients: incidence and outcome in a French multicenter observational cohort (APICOVID). Ann Intensive Care. 2024;14(1):17.
Gioia F, Walti LN, Orchanian-Cheff A, Husain S. Risk factors for COVID-19-associated pulmonary aspergillosis: a systematic review and meta-analysis. Lancet Respir Med. 2024;12(3):207–16.
Janssen NAF, Nyga R, Vanderbeke L, Jacobs C, Ergün M, Buil JB, et al. Multinational observational cohort study of COVID-19-associated pulmonary aspergillosis1. Emerg Infect Dis. 2021;27(11):2892–8.
Acknowledgements
The authors would like to thank all study investigators, Dr Pierre-André Natella, Ms Nolwenn Bombenger for taking care of regulatory aspects, Ms Clélia Chambraud for taking care of data management, Mr Mohamed Ader for clinical data abstraction, the nurses and physicians who took care of the patients, the laboratory staff who took care of virological samples and the patients and their family for agreeing to participate in the study.
Consortia: the SEVARVIR investigators: Keyvan Razazi, Raphaël Bellaïche, Elie Azoulay, Jean-François Timsit, Guillaume Voiriot, Nina de Montmollin, Stéphane Marot, Maxime Gasperment, Tomas Urbina, Hafid Ait Oufella, Eric Maury Djeneba Bocar Fofana, Charles-Edouard Luyt, Djillali Annane, Ferhat Meziani, Louis-Marie Jandeaux, Samira Fafi-Kremer, Cédric Darreau, Jean Thomin, Anaïs Dartevel, Sylvie Larrat, Evelyne Schvoerer, Cédric Hartard, Béatrice La Combe, Séverine Haouisee, Sami Hraeich, Pierre-Edouard Fournier, Philippe Colson, Emmanuel Canet, Berthe Marie Imbert, Guillaume Thiery, Sylvie Pillet, Rémy Coudroy, Nicolas Leveque, Clément Saccheri, Valérie Giordanengo, Kada Klouche, Edouard Tuaillon, Cécile Aubron, Adissa Tran, Jean-Marc Tadié, Jean-Christophe Plantier & Sophie Vallet
Keyvan Razazi, Raphaël Bellaïche, Elie Azoulay, Jean-François Timsit, Guillaume Voiriot, Nina de Montmollin, Stéphane Marot, Maxime Gasperment, Tomas Urbina, Hafid Ait Oufella, Eric Maury Djeneba Bocar Fofana, Charles-Edouard Luyt, Djillali Annane, Ferhat Meziani, Louis-Marie Jandeaux, Samira Fafi-Kremer, Cédric Darreau, Jean Thomin, Anaïs Dartevel, Sylvie Larrat, Evelyne Schvoerer, Cédric Hartard, Béatrice La Combe, Séverine Haouisee, Sami Hraeich, Pierre-Edouard Fournier, Philippe Colson, Emmanuel Canet, Berthe Marie Imbert, Guillaume Thiery, Sylvie Pillet, Rémy Coudroy, Nicolas Leveque, Clément Saccheri, Valérie Giordanengo, Kada Klouche, Edouard Tuaillon, Cécile Aubron, Adissa Tran, Jean-Marc Tadié, Jean-Christophe Plantier, Sophie Vallet
Funding
The SEVARVIR study has been funded by the EMERGEN consortium—ANRS Maladies Infectieuses Emergentes (ANRS0153). This study has been labeled as a National Research Priority by the National Orientation Committee for Therapeutic Trials and other researches on Covid-19 (CAPNET). The investigators would like to acknowledge ANRS|Emerging infectious diseases for their scientific support, the French Ministry of Health and Prevention and the French Ministry of Higher Education, Research and Innovation for their funding and support.
Author information
Authors and Affiliations
Consortia
Contributions
N.D.P., E.A., J.M.P., and S.F., designed the study and obtained funding; E.A. performed statistical analyses; P.B., N.D.P., N.H., T.P., M.T., A.J., S.P., R.F., C.-E.L., J.M., D.R., S.M, F.P., D.C., S.G., T.P., A.K., L.-M.J., P.G., A.G., F.T., T.D., F.D., B.S., L.P., M.H., M.E., S.J., D.A., E.A., A.M.D., included the patient and were responsible for clinical data collection; E.G., A.C., L.M.-J., M.-L.C., A.G., S.B., S.M, D.D., F.R., A.H., S.B., C.H., L.H., C.G.-S., A.P., L.H., A.M., S.H., V.T., C.R., and S.F. were responsible of the management of virological samples; J.-M.P., C.R., and S.F. were responsible of virological analyses; P.B., N.D.P., E.A., and S.F. wrote the first draft of the article; All authors revised and approved the article. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. N.D.P. is the guarantor.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Comité de Protection des Personnes Sud-Méditerranée I (N° EudraCT/ID-RCB: 2021-A02914-37). Informed consent was obtained from all patients or their relatives.
Competing interests
S.F. has served as a speaker for GlaxoSmithKline, AstraZeneca, MSD, Pfeizer, Cepheid and Moderna. J.-M.P. has served as an advisor or speaker for Abbvie, Arbutus, Assembly Biosciences, Gilead and Merck. E.A. has received fees for lectures from Alexion, Sanofi, Gilead and Pfizer. His hospital has received research grant from Pfizer, MSD and Alexion. D.D. served as an advisor for Gilead-Sciences, ViiV Health care, Janssen-Cilag et MSD. F.P. served as an advisor for Gilead; he also received research grant from Alexion. N.D.P has served as an advisor or speaker for Moderna and AstraZeneca. Other authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article has been revised: the table 1, table 2 and fig 3 legend are corrected.
Supplementary Information
Additional file 1: Figure S1.
Diagnosis criteria of CAPA (proven/probable and possible), relied on ECMM/ISHAM consensus criteria. BAL, bronchoalveolar lavage; CAPA, COVID-19-associated pulmonary aspergillosis; PCR, polymerase chain reaction.
Additional file 2: Table S1.
Criteria used for the classification of patients according to the ECMM/ISHAM consensus criteria.
Additional file 3: Table S2.
Predictors of CAPA occurrence by univariable and multivariable logistic regression models in critically ill patients with COVID-19: results on raw data (n = 566).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bay, P., Audureau, E., Préau, S. et al. COVID-19 associated pulmonary aspergillosis in critically-ill patients: a prospective multicenter study in the era of Delta and Omicron variants. Ann. Intensive Care 14, 65 (2024). https://doi.org/10.1186/s13613-024-01296-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-024-01296-0