Abstract
Background
In acute-on-chronic liver failure (ACLF), adequate antibiotic dosing is challenging due to changes of drug distribution and elimination. We studied the pharmacokinetics of linezolid in critically ill patients with ACLF during continuous renal replacement therapy compared to patients without concomitant liver failure (NLF).
Methods
In this prospective cohort study, patients received linezolid 600 mg bid. Linezolid serum samples were analyzed by high-performance liquid chromatography. Population pharmacokinetic modelling was performed followed by Monte-Carlo simulations of 150 mg bid, 300 mg bid, 450 mg bid, 600 mg bid, and 900 mg bid to assess trough concentration target attainment of 2–7 mg/L.
Results
Eighteen patients were included in this study with nine suffering from ACLF. Linezolid body clearance was lower in the ACLF group with mean (standard deviation) 1.54 (0.52) L/h versus 6.26 (2.43) L/h for NLF, P < 0.001. A trough concentration of 2–7 mg/L was reached with the standard dose of 600 mg bid in the NLF group in 47%, with 42% being underexposed and 11% overexposed versus 20% in the ACLF group with 77% overexposed and 3% underexposed. The highest probability of target exposure was attained with 600 mg bid in the NLF group and 150 mg bid in the ACLF group with 53%.
Conclusion
Linezolid body clearance in ACLF was markedly lower than in NLF. Given the overall high variability, therapeutic drug monitoring (TDM) with dose adjustments seems required to optimize target attainment. Until TDM results are available, a dose reduction may be considered in ACLF patients to prevent overexposure.
Similar content being viewed by others
Background
Critically ill patients with acute-on-chronic liver failure (ACLF) are at risk of acquiring infections with consecutive sepsis and septic shock, which are associated with a high mortality [1, 2]. Early empiric broad-spectrum antibiotic therapy is required to reduce mortality [3]. Linezolid is often used to cover the gram-positive spectrum, particularly in the presence of vancomycin-resistant enterococci. Acute kidney injury is the most frequent type of organ failure in ACLF patients [4] and frequently leading to the use of renal replacement therapies (RRT) [5].
Attaining sufficient antibiotic concentrations in critically ill patients is often cumbersome as these patients present with various factors influencing pharmacokinetics (PK): the volume of distribution (Vd) may increase due to a capillary leak syndrome and presence of effusions as e. g., ascites, while antibiotic clearance (CL) may be decreased due to progression of organ dysfunction. Moreover, these processes are often dynamic which can lead to observed inter-occasion variability of these PK parameters.
As per linezolid labelling, no dose adaption is required for patients with liver cirrhosis Child–Pugh A and B, but no data are available for Child–Pugh C. Nevertheless, linezolid is partially metabolized, presumably by the cytochrome P450 system, but the total metabolic pathway is not fully understood, yet. Approximately 35% of linezolid are excreted renally and linezolid and its metabolites may be dialyzed [6, 7]. To investigate the effect of ACLF on the PK of linezolid in critically ill patients requiring RRT we compared this group with critically ill patients on RRT without ACLF.
Methods
Ethics
The study was approved by the Ethics Committee of the Hamburg Chamber of Physicians, Germany (Reference: PV5415). Consent was obtained from the patients’ closest relatives or legal representatives. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Study design
The study was conducted as an open label observational prospective cohort study.
Setting and population
The study was conducted in the Department of Intensive Care, University Medical Center, Hamburg-Eppendorf with twelve intensive care units (surgical, conservative, and interdisciplinary) and a total of 140 beds. Patients were eligible if they received linezolid for clinical indication and required continuous RRT. Patients < 18 years or with an extracorporeal circuit other than the RRT were excluded. According to liver function, patients were grouped into patients with ACLF and patients without ACLF (“no liver failure”, NLF).
ACLF was defined according to the definition of the Chronic Liver Failure (CLIF) consortium [4]. Presence of liver cirrhosis was diagnosed based on a combination of characteristic clinical (e.g., ascites, caput medusae, spider angiomata, etc.), laboratory and radiological findings (typical morphological changes of the liver, signs of portal hypertension, etc. in ultrasonography or computed tomography scanning), or via histology, if available [8].
Medication
Linezolid (Dr. Friedrich Eberth Arzneimittel GmbH, Germany) was given over 30 min by infusion pump at a dose of 600 mg quid 12 h via a central venous line (short-term infusion).
Renal replacement therapy
RRT was performed as continuous veno-venous hemodialysis (CVVHD) or as a postdilution continuous veno-venous hemofiltration (CVVH) as described before [9]. Both methods were performed with Multifiltrate pro® dialysis machines using an Ultraflux® AV1000S hollow-fiber hemofilter (Fresenius Medical Care, Bad Homburg, Germany) with a membrane surface area of 1.8 m2. For CVVHD, a regional citrate-calcium anticoagulation was used; and the targeted dialysate or replacement fluid dose was 30 mL/kg/h of actual body weight. CVVH was chosen in cases of severe acidosis due to the technically higher possible blood flow. No filter change occurred during the study period.
Sampling and storage
Ultrafiltrate and pre- and postfilter blood samples were obtained at the following time points: T0 as the baseline before the first monitored infusion, 1 h (T1), 2 h (T2), 4 h (T4), 6 h (T6), 8 h (T8) and 12 h (T12) after the start of infusion. T12 was obtained before the next infusion of linezolid and served as a trough concentration. Furthermore, we obtained values after 24 h (before and 30 min after end of infusion, T24 and T25) and after 48 h (T48 and T49). In cases of CVVH, postfilter samples were obtained from the extracorporeal circuit before the addition of replacement fluid. All samples were centrifuged immediately, and the supernatant stored at − 20 °C until assayed.
Assay
A well-established, validated, and accredited high performance liquid chromatography approach with diode array detection (HPLC/DAD), which has been used for routine meropenem, linezolid, piperacillin and ceftazidime analysis for more than 3 years was used for the analysis of linezolid in serum. Detailed information about the sample preparation and method settings have been previously described [10]. The method was used with the following slight modification. The assay was routinely calibrated using six linezolid standards of spiked blank human serum (0.8, 8, 12, 20, 30, 40 mg/L) with two independently prepared quality control samples (8 and 20 mg/L) included in each analytical series.
Statistics
Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, USA) was used for data management. The SPSS statistical software package (version 27, IBM Inc., Armonk, NY, USA) was used for descriptive statistical analysis. The pharmacokinetic analysis and Monte-Carlo simulation were performed with the non-linear mixed-effects modelling software NONMEM, version 7.4 (Icon Development Solutions, Ellicott City, MD, USA). Data are given as mean ± standard deviation or median and quartiles, as appropriate.
Population pharmacokinetic analysis
The integrated dialysis pharmacometric (IDP) model was used to describe linezolid pharmacokinetics [11]. The IDP model allows for simultaneous analysis of RRT-related parameters as well as pre-filter (i.e., serum), post-filter and effluent concentration samples.
The RRT clearance for the pre- and post-filter concentrations in the IDP model is estimated as follows:
where the adjusted blood flow rate (Qblood adj.) is being calculated using the blood flow (Qblood), the hematocrit (Hct) and the red blood cell-to-serum-ratio (CRBC/Cppre), with Cpre representing the pre-filter and Cpost the post-filter serum concentration.
The RRT clearance for the effluent concentration in the IDP model is estimated as follows:
with Qeffl. representing the total effluent flow rate, Qdial the dialysate flow rate, QFRR the fluid removal rate, and Ceffl. the concentration of drug in the effluent and Cpre the pre-filter serum concentration.
One- and two-compartment models with first-order elimination were evaluated to identify the model which best described linezolid concentration–time data. Between-subject variability (BSV) and between-occasion variability (BOV) of random effects were assumed to follow a log-normal distribution. The estimates of BSV and BOV were provided as percentage coefficient of variation (% CV). A combined additive and proportional error model was used to describe residual unexplained variability. First-order conditional estimation with eta-epsilon interaction (FOCE-I) was used to estimate population pharmacokinetic parameters of linezolid.
Model building was guided by the drop in the objective function value (ΔOFV; proportional to -2 log-likelihood), inspection of goodness-of-fit plots and overlay plots of individually predicted vs. observed PK measurements and visual predictive checks (VPC). A decrease in OFV of more than 3.84 between two nested models after the addition of one parameter was considered significant (corresponds to p < 0.05). Data visualization and evaluation of NONMEM output during the model development process were conducted with PsN 5.0.0 [12], and R (version 4.2.1, R Foundation for statistical computing, Vienna, Austria). The individual PK parameters were used to assess any potential differences between patients with ACLF and NLF patients. The differences in PK parameters between the two groups were tested by comparing the mean parameter values using the t-test.
Simulations
The final model was used to perform Monte-Carlo simulations in 1000 virtual patients in presence or absence of ACLF receiving linezolid in the following doses, each given twice daily: 150 mg, 300 mg, 450 mg, 600 mg, and 900 mg. In the simulation, RRT flow rates of 120 ml/min, 150 ml/h and 2200 ml/h for blood, ultrafiltration, and dialysate were used, respectively. Day 2 trough concentrations were simulated and evaluated against the target range of 2–7 mg/L [13].
Results
The linezolid serum concentration–time data used in this analysis were obtained in 18 critically ill patients on RRT, with 9 of these patients diagnosed with ACLF. A total of 334 serum concentrations and 120 effluent concentrations were available for analysis. Out of the 334 serum concentrations, 167 were pre-dialysis filter samples and 167 post-dialysis filter samples. The detailed patient demographics, and RRT modes and flow rates are depicted in Table 1. The flow rates differed between subjects and within subjects over the duration of the study. The linezolid measurements are given in the Additional file 1: Table S1.
Structural model and parameter estimates
Linezolid serum PK was best described by a two-compartment model with first-order elimination. The estimated typical linezolid body clearance for a patient without liver disease was 6.17 L/h while RRT clearance was 1.65 L/h. Individual predicted linezolid concentrations are shown in Figs. 1, 2. No adsorption to the membrane was estimable. The final parameter estimates are presented in Table 2 and a VPC showing suitable fit is shown in Fig. 3.
Individual predicted linezolid concentrations versus time after the first dose (0–12 h). The blue line represents the pre-filter serum concentrations, the black line represents the post-filter serum concentrations, the green line represents the effluent concentrations, and the dots are the observed concentrations. NLF no liver failure, ACLF acute-on-chronic liver failure
Individual predicted linezolid concentrations versus time after the first dose (12–50 h) on a normal scale. The blue line represents the pre-filter serum concentrations, the black line represents the post-filter serum concentrations, the green line represents the effluent concentrations, and the dots are the observed concentrations. NLF no liver failure, ACLF acute-on-chronic liver failure
Prediction-corrected visual predictive check of linezolid concentrations versus time after the first dose. Stratified by serum and effluent concentrations between groups. The solid and dashed lines represent the 5th, 50th, and 95th percentiles of the observed data, while the shaded areas represent the model-predicted 95% confidence intervals for the same percentiles. The dots are the observed concentrations. NLF no liver failure, ACLF acute-on-chronic liver failure
Patients with ACLF exhibited a body clearance of 1.32 L/h, corresponding to a 79% reduction in body clearance when compared to patients without ACLF (ΔOFV = − 12.4, p < 10–4). This was further confirmed by an analysis of the empirical Bayesian estimates of the individual PK parameters using the t-test which found body clearance as the most significantly different PK parameter between the two groups. The results are presented in Table 3.
Simulation
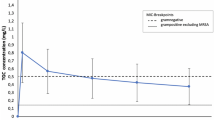
The probability to attain the target of a trough concentration of 2–7 mg/L for patients receiving the standard linezolid dose of 600 mg twice daily in the group without liver disease was 47%, while 42% were underexposed and 11% were overexposed. In the ACLF group, 77% of the simulated patients were overexposed, 20% were in the target range, and only 3% were underexposed. The distribution of the trough concentration values is presented in Fig. 4. The highest probability of exposure in the recommended range of 2–7 mg/L was attained in the NLF group at the standard dose of 600 mg twice daily and at 150 mg twice daily in the ACLF group.
Target attainment. Target attainment (labels) for minimum linezolid concentration (Cmin) between 2 and 7 mg/L (dashed lines) calculated using Monte Carlo Simulations with the developed pharmacometric model using twice daily dosing of 150–900 mg. NLF no liver failure, ACLF acute-on-chronic liver failure
Discussion
In this study, we used the IDP model to characterize the PK of linezolid in critically ill patients undergoing RRT and assessed the impact of ACLF on PK and PTA of linezolid [11]. The model identified a 79% reduction in linezolid body clearance in patients with ACLF. The decreased linezolid clearance in patients with ACLF is similar to the decreased linezolid clearance reported in patients with severe liver cirrhosis (50%) [14] and in patients after liver transplantation (60%) [15]. In patients without organ dysfunction, 35% of linezolid are excreted renally, 50% are metabolized into the two major inactive metabolites PNU-142586 and PNU-142300, and 10% are excreted with the feces [7]. The metabolism is unrelated to the typical cytochrome P450 isoenzymes 1A2, 2C9, 2C19, 2D6, 2E1, and 3A4 that play a major role in drug interactions [16] and therefore, linezolid metabolism was believed to be entirely unrelated to the cytochrome system. However, recent data indicate that the two cytochrome isoenzymes CYP2J2 and CYP4F2 metabolize linezolid [17] which may be influenced by liver failure and thus could explain the reduced body clearance in ACLF as shown in our study. Linezolid and its main metabolites may be removed by hemodialysis and a clearance of 1.88 L/h by CVVH has been determined in critically ill patients which is similar to our results [18]. A systematic review found clearances of 1.2–2.3 L/h for CVVH, 0.9–2.2 L/h for CVVHDF and 2.3 L/h for CVVHD while highlighting a wide variability of PK parameters [19]. As in our study, the observed linezolid population PK parameters were highly variable. Total clearance and volume of distribution values varied widely. Our IDP model did not detect any adsorption of linezolid to the hemofilter membrane. This finding is supported by previous experimental data, showing no absorption to hemofilter membranes [20] or extracorporeal membrane oxygenators [21].
Concerning the mechanism of action, linezolid binds to the 50S subunit of the prokaryotic ribosome, preventing formation of the initiation complex for protein synthesis [22]. Under clinical circumstances, linezolid exhibits only gram-positive activity due to efflux pumps prevalent in most gram-negative bacteria [22]. Time-kill curves indicate a bacteriostatic effect against S. aureus and Enterococcus spp. [23] which may explain data suggesting an inferior clinical efficacy compared to beta-lactams or glycopeptides [24]. However, the latter also may be explained by insufficient target attainment because no TDM was performed in that study. Moreover, inconsistent clinical targets with the following clinical efficacy PK/PD targets have been proposed [13, 25, 26]: a trough concentration of 2–7 mg/L, an AUC0–24 h/MIC of 80–120 mg*h and a time above MIC of ≥ 85%. Trough concentrations above 7 mg/L have been linked to an increase of toxicity, thus making precise targeting particularly important for linezolid. In our study, we evaluated the appropriateness of the standard dosing regimen of 600 mg twice daily based on the developed pharmacometric model with regard to attainment of a trough concentration range of 2–7 mg/L [13]. The most marked finding was that 77% of the simulated patients with liver disease were overexposed. This could also be demonstrated in a very recent clinical trial showing a significantly higher risk for overexposure in patients with severe liver failure [27]. Too high concentrations of linezolid have been associated with thrombocytopenia [28]. Linezolid use in patients with liver failure has also been previously linked to thrombocytopenia, but no TDM was performed in this study and we suggest that the higher incidence of thrombocytopenia may be explained by undetected linezolid overexposure [29]. Furthermore, mitochondrial ribosomal inhibition may lead to lactic acidosis [30, 31], although prolonged therapy and not the peak concentration may be the main risk factor [32]. Interestingly, even in our group without liver disease, solely 47% of the patients were in the target range with approximately 40% being underdosed. These cases are prone to therapy failure and would require an increase of the linezolid dose.
The Vd was lower in the ACLF group. We cannot give a conclusive explanation for this finding as protein binding of LZD is only 31% and should not relevantly influence the Vd. Ascites which is often present in ACLF patients may increase and not decrease Vd as has been shown for meropenem [10]. However, the lower Vd may be a contributing factor to the more frequent overexposure to linezolid in the ACLF group. Due to this lower Vd and in connection with the reduced body clearance as outlined above, a reduction of the linezolid dose in ACLF patients seems reasonable before TDM results are available. Because the Vd and the reduced body clearance are independent of RRT, we suggest that a dose reduction should also be considered in ACLF patients that do not receive RRT.
Our study has the following limitations. The sample size included only 9 patients per groups, but this is a typical number of patients in PK studies [9, 10, 33, 34]. We did not systematically evaluate thrombocytopenia or lactic acidosis as common adverse effects of linezolid because both findings are typical in the included population of critically ill patients, which prevents correct causal attribution. We included different modes of continuous RRT with more patients in the ACLF group receiving CVVH, but our results from the IDP model indicate that type of RRT did not have an influence on the linezolid clearance.
Conclusion
We developed a PK model for linezolid in critically ill patients receiving continuous RRT with and without ACLF. Linezolid body clearance in patients with ACLF was markedly lower compared to patients without liver failure while the central volume of distribution was slightly decreased. Given the overall high variability in the present cohort, TDM with dose adjustments seems required to reach target attainment. Until results from TDM are available, a reduction of linezolid dose may be considered in ACLF patients to prevent overdosing due to the low body clearance and lower Vd.
Availability of data and materials
The data are available from the corresponding author upon reasonable request.
References
Fernandez J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martinez J, Saliba F, Jalan R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67(10):1870–80.
Hubener P, Braun G, Fuhrmann V. Acute-on-chronic liver failure: a diagnostic and therapeutic challenge for intensive care. Med Klin Intensivmed Notfmed. 2018;113(8):649–57.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–247.
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–37.
Staufer K, Roedl K, Kivaranovic D, Drolz A, Horvatits T, Rasoul-Rockenschaub S, Zauner C, Trauner M, Fuhrmann V. Renal replacement therapy in critically ill liver cirrhotic patients-outcome and clinical implications. Liver Int. 2017;37(6):843–50.
Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK, O’Grady M, Hopkins NK, Jungbluth GL. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother. 2003;47(9):2775–80.
Slatter JG, Stalker DJ, Feenstra KL, Welshman IR, Bruss JB, Sams JP, Johnson MG, Sanders PE, Hauer MJ, Fagerness PE, et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C]linezolid to healthy human subjects. Drug Metab Dispos. 2001;29(8):1136–45.
Drolz A, Horvatits T, Rutter K, Landahl F, Roedl K, Meersseman P, Wilmer A, Kluwe J, Lohse AW, Kluge S, et al. Lactate improves prediction of short-term mortality in critically ill patients with cirrhosis: a multinational study. Hepatology. 2019;69(1):258–69.
Grensemann J, Pfaffendorf C, Wicha SG, König C, Roedl K, Jarczak D, Iwersen-Bergmann S, Manthey C, Kluge S, Fuhrmann V. Voriconazole pharmacokinetics are not altered in critically ill patients with acute-on-chronic liver failure and continuous renal replacement therapy: an observational study. Microorganisms. 2021. https://doi.org/10.3390/microorganisms9102087.
Grensemann J, Busse D, Konig C, Roedl K, Jager W, Jarczak D, Iwersen-Bergmann S, Manthey C, Kluge S, Kloft C, et al. Acute-on-chronic liver failure alters meropenem pharmacokinetics in critically ill patients with continuous hemodialysis: an observational study. Ann Intensive Care. 2020;10(1):48.
Broeker A, Vossen MG, Thalhammer F, Wallis SC, Lipman J, Roberts JA, Wicha SG. An integrated dialysis pharmacometric (IDP) model to evaluate the pharmacokinetics in patients undergoing renal replacement therapy. Pharm Res. 2020;37(6):96.
Lindbom L, Pihlgren P, Jonsson N. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241–57.
Abdul-Aziz MH, Alffenaar JC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, Neely MN, Paiva JA, Pea F, Sjovall F, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper(). Intensive Care Med. 2020;46(6):1127–53.
Sasaki T, Takane H, Ogawa K, Isagawa S, Hirota T, Higuchi S, Horii T, Otsubo K, Ieiri I. Population pharmacokinetic and pharmacodynamic analysis of linezolid and a hematologic side effect, thrombocytopenia, Japanese patients. Antimicrob Agents Chemother. 2011;55(5):1867–73.
Swoboda S, Ober MC, Lichtenstern C, Saleh S, Schwenger V, Sonntag HG, Haefeli WE, Hempel G, Hoppe-Tichy T, Weigand MA. Pharmacokinetics of linezolid in septic patients with and without extended dialysis. Eur J Clin Pharmacol. 2010;66(3):291–8.
Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet. 2003;42(13):1129–40.
Obach RS. Linezolid metabolism is catalyzed by cytochrome P450 2J2, 4F2, and 1B1. Drug Metab Dispos. 2022;50(4):413–21.
Meyer B, Kornek G, Nikfardjam M, Karth GD, Heinz G, Locker G, Jaeger W, Thalhammer F. Pharmacokinetics of linezolid in critically ill patients undergoing continuous venovenous hemofiltration. Crit Care. 2004;8(1):P231.
Villa G, Di Maggio P, De Gaudio AR, Novelli A, Antoniotti R, Fiaccadori E, Adembri C. Effects of continuous renal replacement therapy on linezolid pharmacokinetic/pharmacodynamics: a systematic review. Crit Care. 2016;20(1):374.
Shiraishi Y, Okajima M, Sai Y, Miyamoto K, Inaba H. Elimination of teicoplanin by adsorption to the filter membrane during haemodiafiltration: screening experiments for linezolid, teicoplanin and vancomycin followed by in vitro haemodiafiltration models for teicoplanin. Anaesth Intensive Care. 2012;40(3):442–9.
Shekar K, Roberts JA, McDonald CI, Ghassabian S, Anstey C, Wallis SC, Mullany DV, Fung YL, Fraser JF. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care. 2015;19(1):164.
Livermore DM. Linezolid in vitro: mechanism and antibacterial spectrum. J Antimicrob Chemother. 2003;51(Suppl 2):9–16.
Wise R, Andrews JM, Boswell FJ, Ashby JP. The in-vitro activity of linezolid (U-100766) and tentative breakpoints. J Antimicrob Chemother. 1998;42(6):721–8.
Wilcox MH, Tack KJ, Bouza E, Herr DL, Ruf BR, Ijzerman MM, Croos-Dabrera RV, Kunkel MJ, Knirsch C. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis. 2009;48(2):203–12.
Rayner CR, Forrest A, Meagher AK, Birmingham MC, Schentag JJ. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin Pharmacokinet. 2003;42(15):1411–23.
Bandín-Vilar E, García-Quintanilla L, Castro-Balado A, Zarra-Ferro I, González-Barcia M, Campos-Toimil M, Mangas-Sanjuan V, Mondelo-García C, Fernández-Ferreiro A. A review of population pharmacokinetic analyses of linezolid. Clin Pharmacokinet. 2022;61(6):789–817.
Liao R, Dong Y, Chen L, Wang T, Li H, Dong H. A standard dose of linezolid puts patients with hepatic impairment at risk of overexposure. Eur J Clin Pharmacol. 2023;79(1):149–57.
Abou Warda AE, Sarhan RM, Al-Fishawy HS, Moharram AN, Salem HF. Continuous versus intermittent linezolid infusion for critically ill patients with hospital-acquired and ventilator-associated pneumonia: efficacy and safety challenges. Pharmaceuticals. 2022. https://doi.org/10.3390/ph15030296.
Ikuta S, Tanimura K, Yasui C, Aihara T, Yoshie H, Iida H, Beppu N, Kurimoto A, Yanagi H, Mitsunobu M, et al. Chronic liver disease increases the risk of linezolid-related thrombocytopenia in methicillin-resistant staphylococcus aureus-infected patients after digestive surgery. J Infect Chemother. 2011;17(3):388–91.
Santini A, Ronchi D, Garbellini M, Piga D, Protti A. Linezolid-induced lactic acidosis: the thin line between bacterial and mitochondrial ribosomes. Expert Opin Drug Saf. 2017;16(7):833–43.
Apodaca AA, Rakita RM. Linezolid-induced lactic acidosis. N Engl J Med. 2003;348(1):86–7.
Mao Y, Dai D, Jin H, Wang Y. The risk factors of linezolid-induced lactic acidosis: a case report and review. Medicine. 2018;97(36):e12114.
Fuhrmann V, Schenk P, Jaeger W, Ahmed S, Thalhammer F. Pharmacokinetics of moxifloxacin in patients undergoing continuous venovenous haemodiafiltration. J Antimicrob Chemother. 2004;54(4):780–4.
Fuhrmann V, Schenk P, Jaeger W, Miksits M, Kneidinger N, Warszawska J, Holzinger U, Kitzberger R, Thalhammer F. Pharmacokinetics of voriconazole during continuous venovenous haemodiafiltration. J Antimicrob Chemother. 2007;60(5):1085–90.
Acknowledgements
We would like to thank the teams of our intensive care units for their support and particularly our study nurses for their valuable help in conducting the examination and taking the specimens.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported in part by the DAMP-Stiftung, Kiel, Germany, Reference No. 2017–20. Further expenses were financed by departmental funds.
Author information
Authors and Affiliations
Contributions
TJ performed the pharmacokinetic analysis and the Monte-Carlo Analysis and helped to write the manuscript, VF designed the study and interpreted the data and acquired funding, CK helped to design the study and helped to write the manuscript, DJ obtained the data and helped with data management, SIB developed the analytical method and analyzed the samples, SK helped to design the study and to interpret the data and helped to acquire funding, SW helped to perform the pharmacokinetic analysis and to interpret the data, JG designed the study and interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Hamburg Chamber of Physicians, Germany (Reference: PV5415). Consent was obtained from the patients’ closest relatives or legal surrogates. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
SK received research support from Cytosorbents and Daiichi Sankyo, lecture fees from Biotest, Daiichi Sankyo, Fresenius Medical Care, Gilead, Mitsubishi Tanabe Pharma, MSD, Pfizer and Zoll, and consultant fees from Fresenius, Gilead, MSD and Pfizer. SGW reports grants from Boehringer Ingelheim, consulting fees from Medicines for Malaria Venture, Merck KGaA, UTIlity Therapeutics, and lecture fees from GlaxoSmithKline. JG received research support and lecture fees from Infectopharm, and research support from Pfizer outside of the presented work. CK reports lecture fees from Gilead and Shionogi as well as research support from Infectopharm.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Linezolid Concentrations at Time Points.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tikiso, T., Fuhrmann, V., König, C. et al. Acute-on-chronic liver failure alters linezolid pharmacokinetics in critically ill patients with continuous hemodialysis: an observational study. Ann. Intensive Care 13, 83 (2023). https://doi.org/10.1186/s13613-023-01184-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-023-01184-z