Abstract
Cancer is an aging-related disease, while aging plays an important role in the development process of tumor, thus the two are inextricably associated. Telomere attrition is one of the recognized hallmark events of senescence. Hence, targeting telomerase which could extends telomere sequences to treat tumors is widely favored. Cancer cells rely on high activity of telomerase to maintain a strong proliferative potential. By inhibiting the expression or protein function of telomerase, the growth of cancer cells can be significantly suppressed. In addition, the human immune system itself has a defense system against malignant tumors. However, excessive cell division results in dramatic shortening on telomeres and decline in the function of immune organs that facilitates cancer cell evasion. It has been shown that increasing telomerase activity or telomere length of these immune cells can attenuate senescence, improve cellular viability, and enhance the immunosuppressive microenvironment of tumor. In this paper, we review the telomerase-targeting progress using different anti-tumor strategies from the perspectives of cancer cells and immune cells, respectively, as well as tracking the preclinical and clinical studies of some representative drugs for the prevention or treatment of tumors.
Similar content being viewed by others
Background
Replicative senescence is an irreversible state of cell growth arrest, also known as “Hayflick Limit” [1], which is caused by multiple mechanisms including telomere attrition, DNA damage response activation and epigenetic modifications [2]. The pathological development of cancer is highly associated with aging process, therefore several clinical therapeutics for malignant tumors are based on triggering senescence to inhibit growth and expansion of cancer cells [3]. Also in recent years, immunotherapy as CAR-T has been favored by scientists and clinical experts, and is considered as the fourth pillar of oncology treatment [4]. Effective immune system could kill cancer cells to protect organs from tumorigenesis. Age-related decline in immunity (also termed immunosenescence) has been linked to increase cancer risk in patients [5].
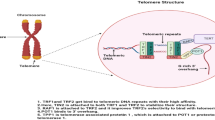
Human telomeres consist TTAGGG repeats and shelterin complex with dynamic structures at the ends of chromosomes, which can maintain genomic stability and integrity [6, 7]. Since the end replication problem, telomeres would be shortened like a mitotic clock during cell cycle process [8]. As a key hallmark of aging, telomere attrition is recognized to lead in DNA damage response activation and limitation of cell proliferation capacity [9]. However, telomeres could be elongated by telomerase in stem cells and cancer cells to maintain robust proliferation capacity. Human telomerase contains catalytic telomerase reverse transcriptase (TERT) and telomerase RNA subunit (TR) as the template for telomere replication [10]. Over 80% tumors extend telomeres depending on telomerase [11]. Hence, human telomerase activity or telomerase reverse transcriptase (TERT) is regarded as a diagnostic and therapeutic biomarker in cancer cells [12].
Telomerase, a pivotal enzyme involved in cellular processes, is subject to intricate regulatory mechanisms. One of the primary steps is to control telomerase activity in the transcription level of the human telomerase reverse transcriptase (hTERT) gene. Several transcription factors, such as c-Myc, hypoxia-inducible factor (HIF-1), Vitamin D receptor (VDR), and Sp1 et al., have been identified to bind to the core promoter region of hTERT, thereby modulating its transcriptional activity [13]. The hTERT gene spans an impressive 42 kilobases and comprises 16 exons. Notably, the mRNA derived from hTERT is subject to site-selective splicing, resulting in various spliced variants [14]. It is worth noting that, aside from the full-length transcripts, some spliced hTERT versions lack reverse transcriptase activity and, consequently, the capacity to extend telomeres [15]. Intriguingly, environmental factors have been revealed to influence the splicing pattern of hTERT, further emphasizing the complexity of its regulation [16].
The modulations of telomerase activity play fundamental roles in immunosenescence which is an essential phenotype during organism aging. Immune-related NF-κB pathway has been reported to involve in regulation of telomerase activity in leukemia cells [17] and inflammatory cells [18]. Antigen activated lymphocytes also express high-level telomerase to compensate for end loss problem after massive expansion [19]. TERT transcription would be temporarily rebooted in activated CD28+ T cells [20]. Moreover, several studies demonstrated short telomeres in peripheral blood mononuclear cells (PBMC) may relate to the risks of oncogenesis [21,22,23]. Therefore, modulating telomere length in immune cells has clinical implications for the treatment of hematologic or immune-related diseases.
Here we are summarizing telomere and telomerase regulation towards on cancer therapeutics mainly from three aspects. First one is based on the common inhibition of cellular telomerase activity to suppress tumors, and the second is to reverse or delay the immunosuppressive microenvironment of malignant tumors by targeting telomerase. Lastly, we propose several potential therapies that target non-canonical roles of telomerase.
Telomerase inhibition in cancers
Telomere, as a replication clock of cell, limits the frequency of cell division, and most cancer cells would reactivate telomerase for maintaining telomere length to achieve replicative immortality. Early generations of TERC−/− mouse seem to have epithelial cancer resistance, indicating that telomerase is associated with cancer development which depends on telomere length [24]. The tumor formation rate and survival rate in mouse with dual inactivation of TERC and INK4a were higher than those in the control group, and re-expression of TERC can complement this phenotype [25]. Many statistical data indicate that the activation of telomerase in cancer cells often symbolizes clinical malignancy in diagnosis, staging, and prognostic significance. A study on telomerase activity of breast cancer samples found that 92.5% of breast cancer lesions were telomerase positive, while almost no telomerase activity was detected in adjacent non-malignant lesions [26]. And the break fine needle aspiration samples from different patients also show that telomerase is related to malignant diseases: most of the samples with confirmed breast cancer have telomerase activity, while the patients who are underdiagnosis with telomerase positive samples will be diagnosed with cancer later [27]. This correlation exists in the diagnosis of lung cancer, pancreatic cancer, renal cancer and so on [28,29,30]. Knocking out TERC in mouse can induce cell cycle arrest and apoptosis of leukemia stem cells, while knocking down TERT in AML cells isolated from leukemia patients can gain similar results [31].
Therefore, inhibiting telomerase to induce replicative senescence and cell death is a feasible anticancer strategy. Compared with traditional chemotherapy drugs, telomerase inhibitors can theoretically target cancers in a specific manner. Based on the properties of telomerase, some designed inhibitors are identified and synthesized for cancer therapy research (Table 1).
Non-nucleosidic compound BIBR1532 is based on the structure of telomerase RNA binding domain (TRBD), which is a unique domain of hTERT protein. It can tightly bind FVYL motif on the thumb domain, selectively block the binding of the CR4/5 ring of hTR to hTERT, thereby inhibiting the activity of telomerase holoenzyme [32]. Many in vitro studies have demonstrated that BIBR1532 exhibits promising telomerase inhibitory ability in different cancer cells and it is expected to be developed as a universal anti-cancer drug [33,34,35,36]. However, BIBR1532 was also found to have strong cytotoxicity, which is consistent with the reported properties of the quinoline derivatives [37]. Nevertheless, BIBR1532 is still being accepted by researchers as an effective telomerase inhibitor to restrain the growth and progression of tumor cells. This inhibition can also be further enhanced by combining BIBR1532 with other tumor therapies, potentially providing improved treatment outcomes [38, 39]. Although no relevant clinical research data has been published, the preclinical study evaluated the effect of BIBR1532 in suppressing malignant tumor. In the model of feline oral squamous cell carcinoma, BIBR1532 can inhibit telomerase modulation and interfere with signal pathway of cell proliferation and survival, and exert a multi-level capabilities in anti-cancer [34]. However, telomerase highly expressed germ cell carcinoma is usually treated with cisplatin in clinical practice. Combined with BIBR1532 for long-term treatment (300PD), telomere shortening could be observed in cell models [40], but the related data has not yet been published in more complicated animal models. It also suggests that even telomerase inhibitors that perform well at the laboratory level may not be effective in vivo, possibly due to extensive reserved telomeres.
Imetelstat is also a relatively successful synthetic telomerase antagonist which is based on the lipid conjugation optimization of GRN163, thus also known as GRN163L [41]. This small molecule is characterized by 13 bases with the sequence 5’-TAGGGTTAGACAA-3’ that competitively binds to hTR [42]. Imetelstat has been found to delay G2/M checkpoint progression in telomerase positive cells by inhibiting telomerase activity [43]. Long-term Imetelstat treatment to cancer cells can accelerate telomere attrition and induce cellular senescence [44, 45]. Similar to BIBR1532, this small molecule can also be combined with other anti-cancer drugs, for example, with PARP inhibitors to treat cancer cells, ameliorating drug resistance and improving the suppressive efficacy [45].
In preclinical studies, Imetelstat exerted a potent and specific telomerase inhibition in several kinds of tumors including myeloma [46], glioblastoma [47], hepatoma [41]. For the indications of myelofibrosis, myelodysplastic syndromes and thrombocythemia, Imetelstat has active performance and have been proceeded to clinical trails phase III and II respectively. Most of the Imetelstat-related trials registered in ClinicalTrials.gov database areinvolved in the clinical treatment of breast cancer, lung cancer, multiple myeloma and other solid tumors.
In addition, there are several natural small molecules obtained from plant or food sources that have been found to inhibit telomerase activity through modulating telomerase component expression, holoenzyme assembly or blocking enzyme activity. Our lab performed a screening in TERT-P2A-GFP reporter cell line and found a natural product SC as a telomerase inhibitor for multiple cancer types [48]. Epigallocatechin gallate (EGCG), one of the main components of green tea, inhibits telomerase expression and functions depending on epigenetic modification [49]. Further, scientists synthesized MST-199 and MST-312 reagents based on the structure of EGCG, which exhibited stronger telomerase inhibitory efficacy [50]. Quercetin is a natural polyphenol, which can be used for cancer treatment and prevention by down-regulating hTERT expression [51]. Furthermore, quercetin in combination with EGCG could eliminate cancer stem cells [52]. One of the recently synthesized shikonin N-benzyl matrinic acid ester derivatives, PMMB-302, can inhibit telomerase expression and lung cancer cell proliferation [53].
Likewise, siRNAs targeting TERT is an emerging strategy for telomerase inhibition [54]. Using nanoparticles to deliver TERT siRNAs into cells can enhance the cancer suppression efficacy [55, 56]. AAV-mediated gene therapy targeting to telomerase also displays remarkable outcomes in animal models [57]. Resnomics, a biopharmaceutical company from Korea, is pushing forward with its hTERT-targeted adenovirus (RZ-001) therapeutic programs [58], including a preclinical study for glioblastoma and a clinical phase I program for hepatocellular carcinoma. Telomelysin (OBP-301) is a telomerase-specific oncolytic adenovirus, in which an hTERT promoter is used to increase expression of adenovirus early in regions associated with an internal ribosome entry site (IRES) sequence [59]. This construct cause OBP-301 replicates better in tissues transcriptionally expressing high levels of hTERT such as cancer [60]. With the rapid advancement of gene editing technology, more genetic therapeutics may potentiate telomerase as a target of anti-tumor drugs in the future.
Telomeric G-quandruplex is formed by its guanine-rich sequences. G4 ligands can stabilize this motif, resulting in the inability of telomerase to be recruited to elongate telomeres. As a G4 stabilizer, telomestatin was identified to inhibit telomerase activity and trigger telomere shortening in cancer cells [61,62,63], which was registered for clinical trials of esophageal cancer, melanoma and hepatocellular carcinoma. Also, synthetic oxazolyl-type telomestain derivatives have also been developed and their therapeutic efficacy was validated in preclinical cancer models [64]. Pidnarulex (also known as CX-5461) is undergoing clinical trials for hematologic cancer patients [65].
In the process of tumorigenesis, the transcriptional regulation of hTERT undergoes a pattern of “off to on” changes. While hTERT promoter activation mutations occur in some of cancer cells [66], the mechanism of wild-type hTERT promoter reactivation in a majority of cancer cells is not clear. Akıncılar S.C. and Tergaonkar V. have recently provided a new answer to this question. They identified hTERT interaction region 2 in primary colorectal cancer as an essential chromatin region necessary to regulate the reactivation of wild-type hTERT, facilitating the formation of stable complexes by associated transcriptional regulators to initiate hTERT expression [67]. This action model, in turn, allows us to design more cancer-specific telomerase inhibitors.
Targeted-telomerase immunotherapies in cancers
Human immune system includes both innate and adaptive immunity, which are together responsible for recognizing exotic matters and ultimately protect body from infections. Telomere dysfunction in tissues and organs would accelerate systemic aging, activate inflammatory responses and reduce the innate immunity. Moreover, telomere stress could be sensed by innate immune cells and in turn activate CD8+ T cells for tumor killing [68]. Here, however, we are discussing the impact of telomeres or telomerase modulationon tumor immunotherapy, especially on lymphocytes.
Adaptive immune response is a result of substantial and directed expansion of lymphocytes (T and B cells) after invasion by exogenous pathogens. T lymphocytes are categorized into CD4+ T cells and CD8+ T cells. Of these, CD8+ T cells are cytotoxic T cells, which are responsible for killing infected cells or cancer cells.
Telomerase activation is an essential part of T cell immune response, which would be regulated at multiple levels. Although TERT mRNA can be expressed, there is no detectable enzymatic activity in resting T cells [69]. Activated T cells re-express telomerase activity upon antigen presentation in order to satisfy the need of adaptive immune system to complete a rapid expansion [20]. Telomerase could be also activated in synaptic stimulated T cells to develop memory T cells [70]. Furthermore, telomere length is also heterogeneous in different subsets of T cells [71]. Shorter telomeres could be detected in senescent T cells. Overall, the complicated regulation of telomerase plays an important role in the T cell development. Ectopic expression of TERT in human CD4+ and CD8+ T cells by retrovirus-mediated infection can induce high levels of telomerase activity and maintain telomere length [72, 73], but no proliferation advantage was seen in TERT-transduced CD8+ cells [74]. TERT-immortalized T cells were injected back into both rodent [75] and non-human primate [76,77,78] models, and showed comparable proliferation ability with no loss of antigen recognition, creating a potential new tool for immunotherapy. Interestingly, in cancer cells TERT could activate endogenous retrovirus and induce interferon response which also promote to establish a immunosuppression tumor microenvironment by inhibiting different kinds of T cell populations [79]. Not only that, telomerase is also important for natural killer cells (NK cells) function. Acute myeloid leukemia (AML) is an aggressive malignancy with highly active telomerase, but some evidence suggests that NK cytotoxicity is impaired in AML patients [80]. Dizaji Asl K et al. proposed a double-edged role of BIBR1532 in cancer cell killing and in negative effect on the NK cell activity [81]. It may be worthwhile to try to prove that increasing telomerase activity in hematopoietic progenitor stem cells could contribute to an enhanced anti-tumor immune microenvironment.
A recent study showed that antigen contacted T cells extend telomeres relying on APC cells to deliver telomeric fragments rather than depending on telomerase function [82]. Such ALT-like telomere repair mechanism may in turn provide a novel approach to enhance tumor T-cell therapy.
Though there is no novel pharmaceutical development targeting increased telomerase levels and tumor-killing properties in immune effective cells, substantial evidence in vivo indicated that telomere length and telomerase activity directly impacts immunity against cancer or other diseases. Centenarians with relatively longer telomere length possessed lymphocyte populations which were more sensitive to the immune response [83]. Abnormal telomere shortening usually displays heterogeneous growth and functional abnormalities in multiple organs, which are described synthetically as short telomere syndrome (STS). In pediatric patients with STS, it is more likely to have bone marrow failure and tumors; while in adult patients, the malignant diseases including myelodysplastic syndrome, myelofibrosis, and hepatic and pulmonary fibrosis are more prevalent [84, 85].
Moreover, Armanios M.‘s team has recently found that most STS patients were susceptible only for squamous cell carcinoma of the head and neck, anus or skin, which was associated with immunodeficiency rather than genomic instability, and proved in mice that this susceptibility of STS patients to solid cancers was a result of T-cell exhaustion and impairment of tumor surveillance ecology [86]. Telomere elongation could also promote immunological memory of T cells [82]. Here we summarize several potential telomere elongation modulators in tumor prevention or adjuvant therapy in Table 2.
Telomerase activator 65 (TA-65) is a small molecule telomerase activator extracted from the roots of Astragalus membranaceus, which has shown anti-aging and lifespan-prolonging potential [87]. Oral administration of TA-65 were found to extend the average telomere length of leukocytes, increase cytotoxic T cells, as well as help the body to reinforcethe immune system [88]. In addition, cycloastragenol (CAG), as a component of TA65, is also considered to be a potential telomerase activator with anti-aging effects [89]. Studies have shown that CAG, by binding to its target protein cathepsin B, inhibits the lysosomal degradation of major tissue-compatible complex I, promotes the aggregation of MHC-I to the cell membrane and accelerates the presentation of tumor antigens. The combination of CAG and PD-1 antibody can also effectively enhance the killing ability of CD8+ T cells in cell-derived xenograft (CDX) mice and colorectal cancer organoids [90]. Remarkably, the activation of telomerase in senescent cells and telomere dysfunctional cells did not increase the risk of oncogenesis. For this point, Blasco M.A.’s lab has already proved the safety of overexpressing TERT in mice models [91, 92].
L-Ascorbic acid (Vitamin C, Vc) can promote tissue regeneration mediated by mesenchymal stem cells via increasing their telomerase activity [93]. It was found that oncoprotein E6 can activate telomerase in HeLa cells, while p53 inhibits telomerase activity by down-regulating the transcription of hTERT gene. Vc was found to reduce telomerase activity in HeLa cells by restoring cell redox potential, inhibiting E6 gene and promoting p53 gene expression [94]. These studies seems controversial on Vc’s role in telomere regulation. Recent studies have suggested that Vc can be used to treat cancer by targeting weaknesses common to many cancer cells, namely redox imbalances, epigenetic reprogramming, and oxygen-sensing regulation [95]. Clinical data show that Vc can provide support for cancer prevention or treatment, and the exact role of Vc in preventing cancer progression remains to be demonstrated by more clinical trials in the future.
Acting as a tumor antigen itself, overexpression of hTERT can activate the immune response in vivo. Increased intracellular hTERT expression activates human endogenous retroviral genes, and triggers anti-cancer efficacy through a cascade effect that activates virus-associated innate immunity [96]. More interestingly, cancer cells could process endogenous hTERT and then present immunogenic hTERT-derived peptides on the cytotoxic T cells surface via MHC-I and MHC-II, and finally kill cancer cells [97]. hTERT-related vaccines were summarized in Table 3.
RIAVAX (also known as GV1001) is an anticancer vaccine derived from the sequence of human telomerase active site [98]. It has been reported that RIAVAX not only penetrates the cytoplasm to reduce HSP levels, but also decreases the expression of HIF-1α and VEGF in cancer cells under hypoxic conditions [99, 100]. RIAVAX is the first vaccine to be tested in non-randomized clinical trials for the treatment of different types of cancer, including advanced pancreatic cancer, non-small cell lung cancer and melanoma [97]. Clinical results demonstrated that RIAVAX significantly prolongs survival in pancreatic cancer patients who respond to CD8+ T cells [101].
Yet the general vaccine effect was not high and had no statistical significance on prolonging patient survival in combination therapy. As a result, UV1 was developed as a second-generation telomerase targeted peptide vaccine. UV1 performed well in clinical trials of metastatic hormone-naive prostate cancer [102] and received FDA fast track designation for use in combination with anti-PD1 therapies for the treatment of advanced malignant melanoma.
Expressing hTERT and lysosomal-associated membrane protein 1 (LAMP1) in dendritic cells induces an increased degradation of hTERT by lysosomes into small tumor antigenic peptides, which in turn activate downstream Cytotoxic T Lymphocyte (CTL) responses via antigen presentation. Based on this principle, two dendritic cell- based vaccines, GRNVAC1 and GRNVAC2, have been generated. These vaccines have both presented good tumor suppression and tolerability in clinical trials [101, 103, 104].
GX301 and Vx-001 are also the TERT peptide vaccines in clinical trials for treating cancer. The ingredients of GX301 are TERT540 − 548, TERT611 − 626, TERT672 − 686, and TERT766 − 780 peptide segments, as well as two adjuvants named Montanide ISA-51 and Imiquimod. In clinical trials, GX301 can induce specific immune responses in patients with prostate cancer and kidney cancer, extending the progression free survival and overall survival of treatment group [105]. Although multiple immunizations tend to pose a risk of T cell exhaustion, considering that GX301 has not shown serious adverse reactions of patients in multiple dosing regimens, it is advisable to use more doses of vaccine to improve immune response when facing intractable tumors [106]. Vx-001 includes multiple injection cycles of TERT572 peptide and TERT572Y peptide. General treatment is to first inoculate with MHC-I restricted TERT572Y peptide to generate TERT specific CD8+ T cells; and T cells targeting natural TERT antigens were screened by later vaccination with TERT572 peptide [107]. The conducted experiments have shown that Vx-001 can prolong the progression free survival and overall survival of patients with advanced solid tumors, but there are significant individual differences in clinical outcomes within the groups [108,109,110]. For non-small cell lung cancer (NSCLC), the efficacy of Vx-001 can be predicted by the number of tumor-infiltrating lymphocytes (TIL): Patients with high levels of CD3+-infiltrating lymphocytes, CD8+-infiltrating lymphocytes, and GZMB+- infiltrating lymphocytes are not suitable for immunotherapy with Vx-001 [111].
Related therapies that target non-canonical roles of telomerase
Targeting telomerase activity seems to be an attractive therapy, however, this approach was failed in clinical trials due to possible side effects on stem cells. Thus an alternative strategy could be considered to target the molecules involved in the non-canonical functions of telomerase components.
TERT can also directly binds to promoters with TCF elements and promote transcription, such as c-Myc and cyclin D1, which are highly expressed in cancer stem cells. MST-312 has been found as a telomerase inhibitor that directly target the TERT and p65 binding interface, which inhibits NF-κB binding to target promoters [112]. MST-312 has been shown to inhibit the proliferation of lung cancer stem cells and reduce tumor volume in mouse model [113, 114]. Treating acute promyelocytic leukemia cells with MST-312 causes the expression of NF-κB-target genes significantly downregulated, without toxicity to PBMC at comparable dosage [114]. Diverse choice of DNA damage response pathways is one of the main causes for radiation/chemotherapy resistance. Liu Y. et al. found that TERT can inhibit non-homologous end-joining and prefer a more accurate homologous recombination pathway for DNA damage repair. Using TERT covalent inhibitor NU-1 to relieve the inhibition of non-homologous end-joining can induce immune infiltration and reduce tumor volume in CDX mice models [115].
Notably, Nagpal N. et al. conducted large-scale screening to identify PAPD5 inhibitors as TERC boosters and restored telomere length in DC cells [116]. Lab of Agarwal S. also found thymidine nucleotide metabolism controls human telomere length [117]. Interestingly, TERC was found to suppress PD-L1 expression by downregulating RNA binding protein HuR. Small compound AS1842856, a Foxo1 activator, inhibited the upregulation of PD-L1 induced by chemotherapy drugs [118]. These findings provide new insights for telomerase-targeting tumor therapy.
Conclusion
Aging plays a critical role in the development of cancers. Scientists have also tried to synergistically link cell senescence with tumors, such as mitochondrial stress and inflammatory response, hoping to target these senescence-related pathways to prevent or treat cancer. Telomere attrition is one of the recognized biomarkers of senescence. Cancer cells rely on high expression of telomerase activity to escape senescence. Researchers have found many telomere inhibitors, which tend to perform well against cancer cell growth in vitro or in vivo. Tumor immunotherapy is currently one of the most promising approaches to significantly improve survival cycles and even achieve a cure for cancer patients. Either anti-immune cell senescence or re-establishing the immunosuppressive microenvironment of tumors by activating T-cell killing effect through TERT antigenic peptides could effectively intensify therapy. Currently there is no anti-tumor drug approved in the market by targeting telomeres or telomerase, and there are many potential telomerase inhibitors with unsatisfactory prognosis in preclinical and clinical trials. However, with the rapid development of technology, gene editing and epigenetic editing are expected to break through the barriers of small molecule inhibitors and achieve potent telomerase inhibition and tumor suppression.
Abbreviations
- CAR-T:
-
Chimeric Antigen Receptor T-Cell Immunotherapy
- TR:
-
Telomerase RNA
- hTERT:
-
Human telomerase reverse transcriptase
- HIF-1:
-
Hypoxia inducible factor-1
- VDR:
-
Vitamin D receptor
- IGF-1:
-
Insulin-like growth factor 1
- PBMC:
-
Peripheral blood mononuclear cell
- AML:
-
Acute myeloid leukemia
- TRBD:
-
Telomerase RNA binding domain
- hTR:
-
Human Telomerase RNA
- PARP:
-
Poly(ADP-ribose) polymerases
- EGCG:
-
Epigallocatechin gallate
- AAV:
-
Adeno-associated virus
- IRES:
-
Internal Ribosome Entry Site
- MM:
-
Multiple myeloma
- GBM:
-
Glioblastoma multiforme
- HCC:
-
Hepatoma carcinoma cell
- CDX:
-
Cell-derived xenograft
- ICIs:
-
Immune checkpoint inhibitors
- mRNA:
-
Messenger RNA
- NK:
-
Cell Natural killer cell
- APC:
-
Professional antigen presenting cell
- STS:
-
Short telomere syndrome
- TA-65:
-
Telomerase Activator 65
- MHC-I:
-
Major histocompatibility complex class I
- PD-1:
-
Programmed cell death protein 1
- Vc:
-
Vitamin C / L-Ascorbic acid
- MHC-II:
-
Major histocompatibility complex class I
- HSP:
-
Heat shock protein
- HIF-1α:
-
Hypoxia-inducible factor 1 subunit alpha
- VEGF:
-
Vascular endothelial growth factor
- FDA:
-
Food and drug administration
- LAMP1:
-
Lysosomal-associated membrane protein 1
- CTL:
-
Cytotoxic T lymphocyte
- NSCLC:
-
Non-small cell lung cancer
- TIL:
-
Tumor-infiltrating lymphocytes
- GZMB:
-
Granzyme B
- CAG:
-
Cycloastragenol
- TCF:
-
T-cell factor
- PD-L1:
-
Programmed cell death protein1 ligand 1
References
Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621.
Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–33.
Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular Senescence: aging, Cancer, and Injury. Physiol Rev. 2019;99(2):1047–78.
Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21(3):e168–78.
Lian J, Yue Y, Yu W, Zhang Y. Immunosenescence: a key player in cancer development. J Hematol Oncol. 2020;13(1):151.
Meyne J, Ratliff RL, Moyzis RK. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989;86(18):7049–53.
Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97(4):503–14.
Yuan X, Dai M, Xu D. Telomere-related markers for Cancer. Curr Top Med Chem. 2020;20(6):410–32.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–78.
Ghanim GE, Fountain AJ, van Roon AM, Rangan R, Das R, Collins K, et al. Structure of human telomerase holoenzyme with bound telomeric DNA. Nature. 2021;593(7859):449–53.
Barthel FP, Wei W, Tang M, Martinez-Ledesma E, Hu X, Amin SB, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49(3):349–57.
Guterres AN, Villanueva J. Targeting telomerase for cancer therapy. Oncogene. 2020;39(36):5811–24.
Ramlee MK, Wang J, Toh WX, Li S. Transcription regulation of the human telomerase reverse transcriptase (hTERT) gene. Genes (Basel) 2016, 7(8).
Hrdlicková R, Nehyba J, Bose HR. Jr. Alternatively spliced telomerase reverse transcriptase variants lacking telomerase activity stimulate cell proliferation. Mol Cell Biol. 2012;32(21):4283–96.
Saebøe-Larssen S, Fossberg E, Gaudernack G. Characterization of novel alternative splicing sites in human telomerase reverse transcriptase (hTERT): analysis of expression and mutual correlation in mRNA isoforms from normal and tumour tissues. BMC Mol Biol. 2006;7:26.
Radan L, Hughes CS, Teichroeb JH, Vieira Zamora FM, Jewer M, Postovit LM, et al. Microenvironmental regulation of telomerase isoforms in human embryonic stem cells. Stem Cells Dev. 2014;23(17):2046–66.
Ghaffari SH, Momeny M, Bashash D, Mirzaei R, Ghavamzadeh A, Alimoghaddam K. Cytotoxic effect of arsenic trioxide on acute promyelocytic Leukemia cells through suppression of NFkβ-dependent induction of hTERT due to down-regulation of Pin1 transcription. Hematology. 2012;17(4):198–206.
Gizard F, Heywood EB, Findeisen HM, Zhao Y, Jones KL, Cudejko C, et al. Telomerase activation in Atherosclerosis and induction of telomerase reverse transcriptase expression by inflammatory stimuli in macrophages. Arterioscler Thromb Vasc Biol. 2011;31(2):245–52.
Buchkovich KJ, Greider CW. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell. 1996;7(9):1443–54.
Huang EE, Tedone E, O’Hara R, Cornelius C, Lai TP, Ludlow A, et al. The maintenance of telomere length in CD28 + T cells during T lymphocyte stimulation. Sci Rep. 2017;7(1):6785.
Zhang C, Doherty JA, Burgess S, Hung RJ, Lindström S, Kraft P, et al. Genetic determinants of telomere length and risk of common cancers: a mendelian randomization study. Hum Mol Genet. 2015;24(18):5356–66.
Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, Bowden J, et al. Association between Telomere length and risk of Cancer and Non-neoplastic Diseases: a mendelian randomization study. JAMA Oncol. 2017;3(5):636–51.
Rode L, Nordestgaard BG, Bojesen SE. Long telomeres and cancer risk among 95 568 individuals from the general population. Int J Epidemiol. 2016;45(5):1634–43.
González-Suárez E, Samper E, Flores JM, Blasco MA. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat Genet. 2000;26(1):114–7.
Greenberg RA, Chin L, Femino A, Lee KH, Gottlieb GJ, Singer RH, et al. Short dysfunctional telomeres impair tumorigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell. 1999;97(4):515–25.
Bednarek AK, Sahin A, Brenner AJ, Johnston DA, Aldaz CM. Analysis of telomerase activity levels in Breast cancer: positive detection at the in situ breast carcinoma stage. Clin Cancer Res. 1997;3(1):11–6.
Hiyama E, Saeki T, Hiyama K, Takashima S, Shay JW, Matsuura Y, et al. Telomerase activity as a marker of breast carcinoma in fine-needle aspirated samples. Cancer. 2000;90(4):235–8.
Arai T, Yasuda Y, Takaya T, Ito Y, Hayakawa K, Toshima S, et al. Application of telomerase activity for screening of primary Lung cancer in broncho-alveolar lavage fluid. Oncol Rep. 1998;5(2):405–8.
Hiyama E, Kodama T, Shinbara K, Iwao T, Itoh M, Hiyama K, et al. Telomerase activity is detected in Pancreatic cancer but not in benign tumors. Cancer Res. 1997;57(2):326–31.
Orlando C, Gelmini S, Selli C, Pazzagli M. Telomerase in urological malignancy. J Urol. 2001;166(2):666–73.
Bruedigam C, Bagger FO, Heidel FH, Paine Kuhn C, Guignes S, Song A, et al. Telomerase inhibition effectively targets mouse and human AML stem cells and delays relapse following chemotherapy. Cell Stem Cell. 2014;15(6):775–90.
Bryan C, Rice C, Hoffman H, Harkisheimer M, Sweeney M, Skordalakes E. Structural basis of telomerase inhibition by the highly specific BIBR1532. Structure. 2015;23(10):1934–42.
Pascolo E, Wenz C, Lingner J, Hauel N, Priepke H, Kauffmann I, et al. Mechanism of human telomerase inhibition by BIBR1532, a synthetic, non-nucleosidic drug candidate. J Biol Chem. 2002;277(18):15566–72.
Altamura G, Degli Uberti B, Galiero G, De Luca G, Power K, Licenziato L, et al. The small molecule BIBR1532 exerts potential anti-cancer activities in preclinical models of feline oral squamous cell Carcinoma through Inhibition of Telomerase Activity and Down-Regulation of TERT. Front Vet Sci. 2020;7:620776.
Doğan F, Özateş NP, Bağca BG, Abbaszadeh Z, Söğütlü F, Gasımlı R, et al. Investigation of the effect of telomerase inhibitor BIBR1532 on Breast cancer and Breast cancer stem cells. J Cell Biochem. 2019;120(2):1282–93.
El-Daly H, Kull M, Zimmermann S, Pantic M, Waller CF, Martens UM. Selective cytotoxicity and telomere damage in Leukemia cells using the telomerase inhibitor BIBR1532. Blood. 2005;105(4):1742–9.
Pandya VA, Crerar H, Mitchell JS, Patani R. A non-toxic concentration of telomerase inhibitor BIBR1532 fails to reduce TERT expression in a Feeder-Free Induced Pluripotent Stem Cell Model of Human Motor Neurogenesis. Int J Mol Sci 2021, 22(6).
Ding X, Cheng J, Pang Q, Wei X, Zhang X, Wang P, et al. BIBR1532, a selective telomerase inhibitor, enhances radiosensitivity of Non-small Cell Lung Cancer through increasing Telomere Dysfunction and ATM/CHK1 inhibition. Int J Radiat Oncol Biol Phys. 2019;105(4):861–74.
Shi Y, Sun L, Chen G, Zheng D, Li L, Wei W. A combination of the telomerase inhibitor, BIBR1532, and paclitaxel synergistically inhibit cell proliferation in Breast cancer cell lines. Target Oncol. 2015;10(4):565–73.
Mueller S, Hartmann U, Mayer F, Balabanov S, Hartmann JT, Brummendorf TH, et al. Targeting telomerase activity by BIBR1532 as a therapeutic approach in germ cell tumors. Invest New Drugs. 2007;25(6):519–24.
Djojosubroto MW, Chin AC, Go N, Schaetzlein S, Manns MP, Gryaznov S, et al. Telomerase antagonists GRN163 and GRN163L inhibit Tumor growth and increase chemosensitivity of human hepatoma. Hepatology. 2005;42(5):1127–36.
Fragkiadaki P, Renieri E, Kalliantasi K, Kouvidi E, Apalaki E, Vakonaki E et al. Τelomerase inhibitors and activators in aging and cancer: a systematic review. Mol Med Rep 2022, 25(5).
Thompson CAH, Gu A, Yang SY, Mathew V, Fleisig HB, Wong JMY. Transient telomerase inhibition with Imetelstat impacts DNA damage signals and cell-cycle kinetics. Mol Cancer Res. 2018;16(8):1215–25.
Hochreiter AE, Xiao H, Goldblatt EM, Gryaznov SM, Miller KD, Badve S, et al. Telomerase template antagonist GRN163L disrupts telomere maintenance, Tumor growth, and Metastasis of Breast cancer. Clin Cancer Res. 2006;12(10):3184–92.
Burchett KM, Etekpo A, Batra SK, Yan Y, Ouellette MM. Inhibitors of telomerase and poly(ADP-ribose) polymerases synergize to limit the lifespan of Pancreatic cancer cells. Oncotarget. 2017;8(48):83754–67.
Shammas MA, Koley H, Bertheau RC, Neri P, Fulciniti M, Tassone P, et al. Telomerase inhibitor GRN163L inhibits Myeloma cell growth in vitro and in vivo. Leukemia. 2008;22(7):1410–8.
Marian CO, Cho SK, McEllin BM, Maher EA, Hatanpaa KJ, Madden CJ, et al. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and Tumor growth. Clin Cancer Res. 2010;16(1):154–63.
Yan S, Lin S, Chen K, Yin S, Peng H, Cai N et al., . Natural Product Library Screens Identify Sanguinarine Chloride as a Potent Inhibitor of Telomerase Expression and Activity. Cells 2022, 11(9)
Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008;103(2):509–19.
Seimiya H, Oh-hara T, Suzuki T, Naasani I, Shimazaki T, Tsuchiya K, et al. Telomere shortening and growth inhibition of human cancer cells by novel synthetic telomerase inhibitors MST-312, MST-295, and MST-1991. Mol Cancer Ther. 2002;1(9):657–65.
Avci CB, Yilmaz S, Dogan ZO, Saydam G, Dodurga Y, Ekiz HA, et al. Quercetin-induced apoptosis involves increased hTERT enzyme activity of leukemic cells. Hematology. 2011;16(5):303–7.
Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit Prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal. 2010;5:14.
Han H, He C, Chen X, Luo Y, Yang M, Wen Z, et al. Shikonin N-benzyl matrinic acid ester derivatives as novel telomerase inhibitors with potent activity against Lung cancer cell lines. Bioorg Med Chem Lett. 2022;57:128503.
Cheng W, Wei Z, Gao J, Zhang Z, Ge J, Jing K, et al. Effects of combined siRNA-TR and -TERT on telomerase activity and growth of bladder transitional cell cancer BIU-87 cells. J Huazhong Univ Sci Technolog Med Sci. 2010;30(3):391–6.
Zhao R, Jin X, Li A, Xu B, Shen Y, Wang W, et al. Precise Diabetic Wound Therapy: PLS nanospheres eliminate senescent cells via DPP4 targeting and PARP1 activation. Adv Sci (Weinh). 2022;9(1):e2104128.
Ghareghomi S, Ahmadian S, Zarghami N, Hemmati S. hTERT-molecular targeted therapy of Ovarian cancer cells via folate-functionalized PLGA nanoparticles co-loaded with MNPs/siRNA/wortmannin. Life Sci. 2021;277:119621.
Quazi S. Telomerase gene therapy: a remission toward cancer. Med Oncol. 2022;39(6):105.
Han SR, Lee CH, Im JY, Kim JH, Kim JH, Kim SJ, et al. Targeted Suicide gene therapy for Liver cancer based on ribozyme-mediated RNA replacement through post-transcriptional regulation. Mol Ther Nucleic Acids. 2021;23:154–68.
Kawashima T, Kagawa S, Kobayashi N, Shirakiya Y, Umeoka T, Teraishi F, et al. Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res. 2004;10(1 Pt 1):285–92.
Heo J, Liang JD, Kim CW, Woo HY, Shih IL, Su TH, et al. Safety and dose escalation of the targeted oncolytic adenovirus OBP-301 for refractory advanced Liver cancer: phase I clinical trial. Mol Ther. 2023;31(7):2077–88.
Seimiya H, Nagasawa K, Shin-Ya K. Chemical targeting of G-quadruplexes in telomeres and beyond for molecular cancer therapeutics. J Antibiot (Tokyo). 2021;74(10):617–28.
Hasegawa D, Okabe S, Okamoto K, Nakano I, Shin-ya K, Seimiya H. G-quadruplex ligand-induced DNA damage response coupled with telomere dysfunction and replication stress in glioma stem cells. Biochem Biophys Res Commun. 2016;471(1):75–81.
Long S, Argyle DJ, Gault EA, Nasir L. Inhibition of telomerase in canine cancer cells following telomestatin treatment. Vet Comp Oncol. 2007;5(2):99–107.
Teng FY, Jiang ZZ, Guo M, Tan XZ, Chen F, Xi XG, et al. G-quadruplex DNA: a novel target for drug design. Cell Mol Life Sci. 2021;78(19–20):6557–83.
Xu H, Hurley LH. A first-in-class clinical G-quadruplex-targeting drug. The bench-to-bedside translation of the fluoroquinolone QQ58 to CX-5461 (Pidnarulex). Bioorg Med Chem Lett. 2022;77:129016.
Vinagre J, Almeida A, Pópulo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185.
Akıncılar SC, Chua JYH, Ng QF, Chan CHT, Eslami SZ, Chen K, et al. Identification of mechanism of cancer-cell-specific reactivation of hTERT offers therapeutic opportunities for blocking telomerase specifically in human Colorectal cancer. Nucleic Acids Res. 2023;51(1):1–16.
Mender I, Zhang A, Ren Z, Han C, Deng Y, Siteni S, et al. Telomere stress potentiates STING-Dependent anti-tumor immunity. Cancer Cell. 2020;38(3):400–411e406.
Liu K, Schoonmaker MM, Levine BL, June CH, Hodes RJ, Weng NP. Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes. Proc Natl Acad Sci U S A. 1999;96(9):5147–52.
Akbar AN, Beverley PC, Salmon M. Will telomere erosion lead to a loss of T-cell memory? Nat Rev Immunol. 2004;4(9):737–43.
Burns JB, Lobo ST, Bartholomew BD. In vivo reduction of telomere length in human antigen-reactive memory T cells. Eur J Immunol. 2000;30(7):1894–901.
Hooijberg E, Ruizendaal JJ, Snijders PJ, Kueter EW, Walboomers JM, Spits H. Immortalization of human CD8 + T cell clones by ectopic expression of telomerase reverse transcriptase. J Immunol. 2000;165(8):4239–45.
Rufer N, Migliaccio M, Antonchuk J, Humphries RK, Roosnek E, Lansdorp PM. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood. 2001;98(3):597–603.
Migliaccio M, Amacker M, Just T, Reichenbach P, Valmori D, Cerottini JC, et al. Ectopic human telomerase catalytic subunit expression maintains telomere length but is not sufficient for CD8 + T lymphocyte immortalization. J Immunol. 2000;165(9):4978–84.
Verra NC, Jorritsma A, Weijer K, Ruizendaal JJ, Voordouw A, Weder P, et al. Human telomerase reverse transcriptase-transduced human cytotoxic T cells suppress the growth of human Melanoma in immunodeficient mice. Cancer Res. 2004;64(6):2153–61.
Loffredo JT, Rakasz EG, Giraldo JP, Spencer SP, Grafton KK, Martin SR, et al. Tat(28–35)SL8-specific CD8 + T lymphocytes are more effective than gag(181–189)CM9-specific CD8 + T lymphocytes at suppressing simian immunodeficiency virus replication in a functional in vitro assay. J Virol. 2005;79(23):14986–91.
Andersen H, Barsov EV, Trivett MT, Trubey CM, Giavedoni LD, Lifson JD, et al. Transduction with human telomerase reverse transcriptase immortalizes a rhesus macaque CD8 + T cell clone with maintenance of surface marker phenotype and function. AIDS Res Hum Retroviruses. 2007;23(3):456–65.
Minang JT, Barsov EV, Yuan F, Trivett MT, Piatak M Jr., Lifson JD, et al. Efficient inhibition of SIV replication in rhesus CD4 + T-cell clones by autologous immortalized SIV-specific CD8 + T-cell clones. Virology. 2008;372(2):430–41.
Mao J, Zhang Q, Wang Y, Zhuang Y, Xu L, Ma X, et al. TERT activates endogenous retroviruses to promote an immunosuppressive tumour microenvironment. EMBO Rep. 2022;23(4):e52984.
Wang W, Guo H, Geng J, Zheng X, Wei H, Sun R, et al. Tumor-released Galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in Tumor Escape from NK cell Attack. J Biol Chem. 2014;289(48):33311–9.
Dizaji Asl K, Rafat A, Movassaghpour AA, Nozad Charoudeh H, Tayefi Nasrabadi H. The Effect of Telomerase Inhibition on NK Cell activity in Acute Myeloid Leukemia. Adv Pharm Bull. 2023;13(1):170–5.
Lanna A, Vaz B, D’Ambra C, Valvo S, Vuotto C, Chiurchiù V, et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat Cell Biol. 2022;24(10):1461–74.
Tedone E, Huang E, O’Hara R, Batten K, Ludlow AT, Lai TP, et al. Telomere length and telomerase activity in T cells are biomarkers of high-performing centenarians. Aging Cell. 2019;18(1):e12859.
Coukos A, Daccord C, Lazor R, Blum S, Naveiras O, Unger S, et al. [Short telomere syndrome in adults: a rare entity that should be evoked]. Rev Med Suisse. 2022;18(793):1606–13.
Shin DY, Lim KM, Park HS, Kwon S, Yoon SS, Lee DS. The importance of critically short telomere in Myelodysplastic Syndrome. Biomark Res. 2022;10(1):79.
Schratz KE, Flasch DA, Atik CC, Cosner ZL, Blackford AL, Yang W, et al. T cell immune deficiency rather than chromosome instability predisposes patients with short telomere syndromes to squamous cancers. Cancer Cell. 2023;41(4):807–817e806.
Eyolfson E, Malik H, Mychasiuk R. Sexually dimorphic behavioral and genetic outcomes Associated with Administration of TA65 (a telomerase activator) following repetitive traumatic brain Injury: a pilot study. Front Neurol. 2020;11:98.
Harley CB, Liu W, Blasco M, Vera E, Andrews WH, Briggs LA, et al. A natural product telomerase activator as part of a health maintenance program. Rejuvenation Res. 2011;14(1):45–56.
Cheng L, Zhang H, Cui H, Wang W, Yuan Q. Efficient production of the anti-aging drug Cycloastragenol: insight from two glycosidases by enzyme mining. Appl Microbiol Biotechnol. 2020;104(23):9991–10004.
Deng G, Zhou L, Wang B, Sun X, Zhang Q, Chen H et al. Targeting cathepsin B by cycloastragenol enhances antitumor immunity of CD8 T cells via inhibiting MHC-I degradation. J Immunother Cancer 2022, 10(10).
Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012;4(8):691–704.
Muñoz-Lorente MA, Martínez P, Tejera Á, Whittemore K, Moisés-Silva AC, Bosch F, et al. AAV9-mediated telomerase activation does not accelerate tumorigenesis in the context of oncogenic K-Ras-induced Lung cancer. PLoS Genet. 2018;14(8):e1007562.
Wei F, Qu C, Song T, Ding G, Fan Z, Liu D, et al. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J Cell Physiol. 2012;227(9):3216–24.
Reddy VG, Khanna N, Singh N. Vitamin C augments chemotherapeutic response of cervical carcinoma HeLa cells by stabilizing P53. Biochem Biophys Res Commun. 2001;282(2):409–15.
Ngo B, Van Riper JM, Cantley LC, Yun J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer. 2019;19(5):271–82.
Liu T, Li S, Xia C, Xu D. TERT promoter mutations and methylation for telomerase activation in urothelial carcinomas: new mechanistic insights and clinical significance. Front Immunol. 2022;13:1071390.
Mizukoshi E, Kaneko S. Telomerase-targeted Cancer Immunotherapy. Int J Mol Sci 2019, 20(8).
Jafri MA, Ansari SA, Alqahtani MH, Shay JW. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016;8(1):69.
Kim BK, Kim BR, Lee HJ, Lee SA, Kim BJ, Kim H, et al. Tumor-suppressive effect of a telomerase-derived peptide by inhibiting hypoxia-induced HIF-1α-VEGF signaling axis. Biomaterials. 2014;35(9):2924–33.
Kim H, Seo EH, Lee SH, Kim BJ. The telomerase-derived anticancer peptide vaccine GV1001 as an Extracellular Heat shock protein-mediated cell-penetrating peptide. Int J Mol Sci 2016, 17(12).
Brower V. Telomerase-based therapies emerging slowly. J Natl Cancer Inst. 2010;102(8):520–1.
Lilleby W, Gaudernack G, Brunsvig PF, Vlatkovic L, Schulz M, Mills K, et al. Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive Prostate cancer. Cancer Immunol Immunother. 2017;66(7):891–901.
Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8 + and CD4 + T cell responses in patients with metastatic Prostate cancer. J Immunol. 2005;174(6):3798–807.
Sioud M, Nyakas M, Sæbøe-Larssen S, Mobergslien A, Aamdal S, Kvalheim G. Diversification of Antitumour Immunity in a Patient with Metastatic Melanoma Treated with Ipilimumab and an IDO-Silenced Dendritic Cell Vaccine. Case Rep Med 2016, 2016: 9639585.
Fenoglio D, Traverso P, Parodi A, Tomasello L, Negrini S, Kalli F, et al. A multi-peptide, dual-adjuvant telomerase vaccine (GX301) is highly immunogenic in patients with prostate and renal cancer. Cancer Immunol Immunother. 2013;62(6):1041–52.
Filaci G, Fenoglio D, Nolè F, Zanardi E, Tomasello L, Aglietta M, et al. Telomerase-based GX301 cancer vaccine in patients with metastatic castration-resistant Prostate cancer: a randomized phase II trial. Cancer Immunol Immunother. 2021;70(12):3679–92.
Vetsika EK, Konsolakis G, Aggouraki D, Kotsakis A, Papadimitraki E, Christou S, et al. Immunological responses in cancer patients after vaccination with the therapeutic telomerase-specific vaccine Vx-001. Cancer Immunol Immunother. 2012;61(2):157–68.
Kotsakis A, Vetsika EK, Christou S, Hatzidaki D, Vardakis N, Aggouraki D, et al. Clinical outcome of patients with various advanced cancer types vaccinated with an optimized cryptic human telomerase reverse transcriptase (TERT) peptide: results of an expanded phase II study. Ann Oncol. 2012;23(2):442–9.
Kotsakis A, Papadimitraki E, Vetsika EK, Aggouraki D, Dermitzaki EK, Hatzidaki D, et al. A phase II trial evaluating the clinical and immunologic response of HLA-A2(+) non-small cell Lung cancer patients vaccinated with an hTERT cryptic peptide. Lung Cancer. 2014;86(1):59–66.
Gridelli C, Ciuleanu T, Domine M, Szczesna A, Bover I, Cobo M, et al. Clinical activity of a htert (vx-001) cancer vaccine as post-chemotherapy maintenance immunotherapy in patients with stage IV non-small cell Lung cancer: final results of a randomised phase 2 clinical trial. Br J Cancer. 2020;122(10):1461–6.
Pateras IS, Kotsakis A, Avgeris M, Baliou E, Kouroupakis P, Patsea E et al. Clinical activity of an hTERT-Specific Cancer vaccine (Vx-001) in Immune Desert NSCLC. Cancers (Basel) 2021, 13(7).
Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, et al. Telomerase directly regulates NF-κB-dependent transcription. Nat Cell Biol. 2012;14(12):1270–81.
Serrano D, Bleau AM, Fernandez-Garcia I, Fernandez-Marcelo T, Iniesta P, Ortiz-de-Solorzano C, et al. Inhibition of telomerase activity preferentially targets aldehyde dehydrogenase-positive cancer stem-like cells in Lung cancer. Mol Cancer. 2011;10:96.
Fatemi A, Safa M, Kazemi A. MST-312 induces G2/M cell cycle arrest and apoptosis in APL cells through inhibition of telomerase activity and suppression of NF-κB pathway. Tumour Biol. 2015;36(11):8425–37.
Liu Y, Betori RC, Pagacz J, Frost GB, Efimova EV, Wu D, et al. Targeting telomerase reverse transcriptase with the covalent inhibitor NU-1 confers immunogenic radiation sensitization. Cell Chem Biol. 2022;29(10):1517–1531e1517.
Nagpal N, Wang J, Zeng J, Lo E, Moon DH, Luk K, et al. Small-molecule PAPD5 inhibitors restore telomerase activity in patient stem cells. Cell Stem Cell. 2020;26(6):896–909e898.
Mannherz W, Agarwal S. Thymidine nucleotide metabolism controls human telomere length. Nat Genet. 2023;55(4):568–80.
Jin H, Chen Y, Ren J, Huang J, Zhao Y, Liu H. TERC suppresses PD-L1 expression by downregulating RNA binding protein HuR. Sci China Life Sci. 2022;65(12):2505–16.
Acknowledgements
We thank the colleagues of Prof. Zhou Songyang’s lab.
Funding
This work was funded by the National Natural Science Foundation (82271598, 81871109, 82071587).
Author information
Authors and Affiliations
Contributions
S Yan and Y Huang wrote the original manuscript. S Lin, H Qiu, X Wang, Y He and C Wang provided advices and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest regarding the publication of this review.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, S., Lin, S., Qiu, H. et al. Regulation of telomerase towards tumor therapy. Cell Biosci 13, 228 (2023). https://doi.org/10.1186/s13578-023-01181-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-023-01181-6