Abstract
Yak butter is one of the most important foods for the Tibetan people. Of note, its production yields waste yak milk as a by-product. In this work, waste yak milk protein hydrolysates made via Pepsin hydrolysis were shown to have antimicrobial activity. Furthermore, an innovative method of magnetic liposome adsorption combined with reversed-phase high performance liquid chromatography (RP-HPLC) was developed to screen for and purify the antimicrobial peptides. Two antimicrobial peptides were obtained and their amino acid sequences were determined by N-sequencing, namely Arg-Val-Met-Phe-Lys-Trp-Ala and Lys-Val-Ile-Ser-Met-Ile. The antimicrobial activity spectra of Arg-Val-Met-Phe-Lys-Trp-Ala included Bacillus subtilis, Staphylcoccus aureus, Listeria innocua, Escherichia coli, Enterobacter cloacae and Salmonella paratyphi, while the Lys-Val-Ile-Ser-Met-Ile peptide shows not only bacterial growth inhibition but also of fungi. Haemolytic testing suggested that these two antimicrobial peptides could be considered to have no haemolytic effect at their minimum inhibitory concentrations (MICs).
Similar content being viewed by others
Introduction
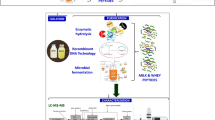
Antimicrobial peptides are advantageous due to their non-toxic nature, high antimicrobial activity and good selectivity (Xiao and Zhang 2012; Li et al. 2013; Martinez et al. 2015). Thus, they hold promise for application as antimicrobial agents in the food and medical industries (Kaur et al. 2013; Zhang et al. 2016; Zhao et al. 2016). Yaks have been domesticated for 1000 of years and primarily kept for their milk, fibre, meat and blood. It has long been believed by locals that drinking yak milk can cure intestinal and stomach inflammation (Bartels et al. 1963; Singh and Sharma 2000). Moreover, the medicinal value of yak milk is believed to be derived from the many herbs (which themselves have medicinal properties) naturally occurring in the highlands that form part of the yaks’ natural diet (Bartels et al. 1963; Singh and Sharma 2000; Xin et al. 2016). Yak milk is mainly used to produce yak butter, which is the most important food for the Tibetans. However, the remaining yak “buttermilk” after making the butter is considered a waste by-product and often poured into the environment. Therefore, developing a technological application for waste yak milk (WYM) will have environmental benefits as well as a significant improvement to the local economy. Despite the isolation of antimicrobial peptides having been reported from many other sources (Kaur et al. 2013; Martinez et al. 2015; Zhao et al. 2016), there currently exists no reports about their isolation from yak milk. In this study, antimicrobial peptides were screened and purified from the WYM left after butter processing using a novel method of magnetic liposome adsorption combined with RP-HPLC. Furthermore, an additional primary objective of this study was the characterizations of the antimicrobial peptides.
Materials and methods
Waste yak milk hydrolysates preparation
The WYM left after butter processing was provided by a local yak butter processing factory. Following the procedure reported by Tang et al. (2014), the WYM was concentrated by rotary evaporation until the concentration was ten times greater. The concentrated WYM was then hydrolysed by the addition of Pepsin, Trypsin, Neutrase, Papain or Alcalase, as described by Tomasinsig et al. (2012). Each enzymatic hydrolysis was performed for 1, 2 and 3 h. The spot-on-lawn method was applied to determine the antimicrobial activity using Staphylcoccus aureus CICC 10384 as indicator stains (Yue et al. 2013).
Nanomagnetic liposome preparation
Ferromagnetic Fe3O4 nanoparticles were prepared by the hydrothermal method (Duan et al. 2009). Egg-yolk phosphatidylcholine (EYPC, min 98% pure) and 1,2-dimyristoyl-sn-glycero-phosphatidylglycerol (sodium salt) (DMPG, min 97% pure) (2:1, w/w) were dissolved in chloroform/methanol (2:1, v/v) and nano-magnetic liposome particles were prepared by thin film dispersion (Paiva et al. 2012).
Antimicrobial peptide screening and purification
Waste yak milk protein solutions were mixed and incubated with magnetic liposomes at 37 °C for 24 h so as to adsorb the active peptides out of solution. The magnetic liposomes were then isolated at 25 °C using a magnet column where PBS (pH 7.2, 10 mM) with 50 mM NaCl served as an isocratic mobile phase. Absorbance was recorded at 215 nm using a UV/Vis DAD (Agilent 1260 series, Agilent Technologies, Santa Clara, CA, USA). Peaks were collected and analysed for their potential bactericidal effect by the spot-on-lawn method with S. aureus CICC 10384 as indicator strains (Yue et al. 2013). The protein concentration was tested by Bradford analysis (Miao et al. 2016). The fractions with the highest antimicrobial activity were collected. Experiments were repeated several times to obtain a large amount of active elute, which was then concentrated by freeze drying (Tang et al. 2014). Further analysis and purification was conducted using RP-HPLC method (Waters Symmetry C18 column, 250 × 4.6 mm, 5 μm, Dublin, Ireland) with a gradient separation (mobile phase B: 5–100%) at a flow rate of 0.5 mL/min at 25 °C. Two mobile phases were used: mobile phase (A) 0.05% (v/v) TFA and mobile phase (B) 100% acetonitrile.
Structural characterization
The purified antimicrobial peptides were sequenced by N-amino acid sequencing (Procise491, ABI, USA). Next, a BLAST analysis of their sequences was performed using the NCBI database (http://www.ncbi.nlm.nih.gov/). Physicochemical properties were predicted using a bioinformatics tool (ProtParam in Expasy ProtParam) (Chaparro and Da Silva Junior 2016). The Hyperchem 7.5 software was used to calculate and predict the lowest energy state 3D model structure (Tang et al. 2014).
Synthesis of antimicrobial peptide
Antimicrobial peptides were synthesized by Sengong Bioengineering Ltd. Co. (Shanghai, China) (Yue et al. 2013). MALDI-TOF/MS and HPLC were used to confirm peptide purity and identity.
Antimicrobial activity
Activity spectra (100 μg/ml) were determined with the selected indicator strains as shown in Table 1. Minimum inhibitory concentrations (MICs) were determined by testing the OD600 of bacteria suspensions treated with different dilutions of antimicrobial peptides (Yue et al. 2013).
Haemolytic testing
Haemolytic testing was conducted with the mice red blood cells (Babl/c, SPF) (provided by Northwest A & F University, Shaanxi, China) with PBS (10 mM, pH 7.2) as negative control and 0.1% Triton X-100 as a positive control (Lin et al. 2013).
Statistics
Data were analyzed by ANOVA using SPSS 16.0 software (Tang et al. 2014). The data are presented as the mean ± standard deviation. The statistical significance was defined as a P value of less than 0.05.
Results
Activity of Waste yak milk hydro lysates
Fragments hydrolysed by Pepsin for 2 and 3 h exhibited the highest antimicrobial activities (Fig. 1). In the following experiments, yak blood fragments Pepsin hydrolysed for 2 h were used for subsequent antimicrobial peptide purification.
Purification of antimicrobial peptides
Two fractions were eluted from the magnetic liposomes (Fig. 2a). Antimicrobial activity testing suggested that elution #2 showed the highest antimicrobial activity (Fig. 2b). These fractions were then further purified by RP-HPLC. As shown in Fig. 2c (Elution #1) and Fig. 2d (Elution #2), one signal peak can be observed, signifying a high purification of the antimicrobial peptide.
Structural characterization of antimicrobial peptides
The amino acid sequences of these two antimicrobial peptides were successfully identified as Arg-Val-Met-Phe-Lys-Trp-Ala and Lys-Val-Ile-Ser-Met-Ile. However, no similarity with any known protein could be detected after performing BLAST analysis (http://web.expasy.org/blast/). Structural characterization and theoretical structures are predicted in Fig. 3. The theoretical minimum energy state for “RVMFKWA” is Energy = − 0.8208; Gradient = 0.0957, while that of “KVISMI” is Energy = 4.3906; Gradient = 0.0996.
Antimicrobial activity of peptides
The activity spectra and MIC of these two antimicrobial peptides are listed in Table 1. “RVMFKWA” had growth inhibitory activity towards Bacillus subtilis, Staphylcoccus aureus, Listeria innocua, Escherichia coli, Pseudomonas aeruginosa, Serratia marcescens, Enterobacter cloacae, Salmonella paratyphi and Shigella dysenteriae. “KVISMI” was not only able to inhibit bacterial growth, but also the growth of some fungi, such as Candida albicans and Saccharomyces cerevisiae.
Haemolytic activity
The haemolytic activities of these two antimicrobial peptides are shown in Fig. 4. Nearly undetectable haemolysis is observed at the MIC. When concentrations more than 256 μg/ml were used, 12.63 and 20.81% haemolysis was observed using “RVMFKWA” and “KVISMI” respectively. These data demonstrate that these two antimicrobial peptides have no haemolytic activity near their MICs.
Discussion
Many studies have investigated the isolation of high activity antimicrobial peptides from hydrolysates obtained via different enzyme treatments (Yang et al. 2012; Kobbi et al. 2015; Sun et al. 2016). For example, Bah reported the use of proteases to hydrolyse deer, sheep, pig and cattle red blood cell fractions to successfully obtain antimicrobial peptides (Bah et al. 2016).
Traditional peptide purification procedures have many disadvantages; they are lengthy and complex to perform, exhibit a low recovery of desired products and a loss of activity (Kandula and Terli 2013; Goh and Philip 2015; Panagiota et al. 2016; Wang et al. 2016). In this study, the adsorption onto magnetic liposomes combined with RP-HPLC was able to quickly screen and isolate potential antimicrobial components.
Mammals are one of the most important sources of antimicrobial peptides (Expósito et al. 2006; Stanford et al. 2009). Expósito et al. (2006), identified bovine antibacterial peptides. Bu et al. (2011), reported the discovery of an antimicrobial peptide from Kulun donkey blood. However, until now there has been no study regarding the isolation of antimicrobial peptides from WYM. These two antimicrobial peptides both have a low molecular mass (937.17 and 689.91 Da). Earlier studies implied that antimicrobial peptides are generally low-molecular-weight, such as SP-1 (MW 878.97 Da) (Sun et al. 2016), and Glu-Leu-Ala–Ala-Ala-Cys (MW 162.1 Da) (Kobbi et al. 2015), showed strong antimicrobial activity.
Ma et al. (2016), reported that antimicrobial peptides isolated from whey were able to inhibit the growth of Streptococcus agalactiae, Staphylococcus aureus, and Escherichia coli, but not fungi. “KVISMI” demonstrated antifungi activity that was not seen in Ma et al. (2016). Compared with the antimicrobial activity of the bovine-derived peptides reported by Expósito et al. (2006), “KVISMI” showed higher potency against Staphylococcus aureus and Escherichia coli.
Typically, antimicrobial peptides exhibiting no/low haemolytic activity can be considered for practical application (Wang et al. 2016). For example, Ma reported that their antimicrobial peptide derived from swine blood was able to induce 2.4% haemolysis at the MICs (Ma et al. 2016). The results in this study indicated that the two antimicrobial peptides could be considered to have no haemolytic at MICs.
Abbreviations
- RP-HPLC:
-
reversed-phase high performance liquid chromatography
- K:
-
Lys
- Y:
-
Tyr
- G:
-
Gly
- N:
-
Asn
- L:
-
Leu
- S:
-
Ser
- R:
-
Arg
- I:
-
Ile
- F:
-
Phe
- A:
-
Ala
- V:
-
Val
- M:
-
Met
- W:
-
Trp
References
Bah CS, Carne A, Mcconnell MA, Mros S, Ael-D B (2016) Production of bioactive peptide protein solutions from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem 202:458–466
Bartels H, Hilpert P, Barbey K (1963) Respiratory functions of blood of the yak, llama, camel, dybowski deer, and African elephant. Am J Physiol 205:331–336
Bu RE, Wu JH, Lu YS (2011) Antibacterial activity of antibacterial peptides from Kulun Donkey Blood leukocytes against the major pathogens responsible for bovine mastitis. J Pathog Biol 6(10):721–723
Chaparro E, Da Silva Junior PI (2016) Lacrain: the first antibacterial peptide from the body extract of the Brazilian centipede Scolopendra viridicornis. Int J Antimicrob Ag 48(3):277–285
Duan L, Jia S, Wang Y, Duan LF, Jia SS, Wang YJ, Chen J, Zhao LG (2009) Synthesis of Fe3O4 polyhedra by hydrothermal method: using l-arginine as precipitator. J Mater Sci 44:4407
Expósito LI, Minervini F, Amigo L, Recio I (2006) Identification of antibacterial peptides from bovine kappa-casein. J Food Prot 69(12):2992–2997
Goh HF, Philip K (2015) Isolation and mode of action of bacteriocin BacC1 produced by nonpathogenic Enterococcus faecium C1. J Dairy Sci 98(8):5080–5090
Kandula S, Terli R (2013) Production, purification and characterization of an antibacterial compound from marine Streptomyces coeruleorubidus BTSS-301. J Pharm Res 7(5):397–403
Kaur G, Singh TP, Malik RK (2013) Antibacterial efficacy of Nisin, Pediocin 34 and Enterocin FH99 against Listeria monocytogenes and cross resistance of its bacteriocin resistant variants to common food preservatives. Braz J Microbiol 14(44):63–71
Kobbi S, Balti R, Bougatef A, Flem GL, Firdaous L, Bigan M (2015) Antibacterial activity of novel peptides isolated from protein protein solutions of rubisco purified from green juice alfalfa. J Funct Foods 18:703–713
Li L, Shi Y, Cheserek MJ, Su G, Le G (2013) Antibacterial activity and dual mechanisms of peptide analog derived from cell-penetrating peptide against Salmonella typhimurium and Streptococcus pyogenes. Appl Microbiol Biot 97(4):1711–1723
Lin MC, Hui CF, Chen JY, Wu JL (2013) Truncated antibacterial peptides from marine organisms retain anticancer activity and antibacterial activity against multidrug-resistant Staphylococcus aureus. Peptides 44:139–148
Ma Y, Liu J, Shi H, Wu J, Yu L (2016) Isolation and characterization of anti-inflammatory peptides derived from whey protein. J Dairy Sci 99(9):6902–6912
Martinez RCR, Staliano CD, Vieira ADS, Villarreal MLM, Todorov SD, Saad SMI, Franco MGBD (2015) Bacteriocin production and inhibition of Listeria monocytogenes by Lactobacillus sakei subsp. sakei 2a in a potentially symbiotic cheese spread. Food Microbiol 48:143–152
Miao JY, Guo HX, Chen FL, Zhao LC, He LP, Ou YW, Huang MM, Zhang Y, Guo BY, Cao Y, Huang QR (2016) Antibacterial effects of a cell-penetrating peptide isolated from Kefir. J Agric Food Chem 64:3234–3242
Paiva AD, Irving N, Breukink E, Mantovani HC (2012) Interaction with lipid II induces conformational changes in bovicin HC5 Structure. Antimicrob Agents Chemother 56(9):4586–4593
Panagiota K, Kyriakou EB, Kristiansen PE, Kaznessis YN (2016) Interactions of a class IIb bacteriocin with a model lipid bilayer, investigated through molecular dynamics simulations. BBA Biomembranes 1858(4):824–835
Singh M, Sharma KB (2000) A note on blood biochemical profile of Himalayan Yak. Indian Vet J 78(6):492–493
Stanford WS, Kalli A, Håkansson K, Kastrantas J, Orugunty RS, Smith L (2009) Oxidation of lanthionines renders the lantibiotic nisin inactive. Appli Environ Microbiol 75(75):1381–1387
Sun Y, Chang R, Li Q, Li B (2016) Isolation and characterization of an antibacterial peptide from protein solutions of Spirulina platensis. Eur Food Res Technol 242:685
Tang WT, Zhang H, Wang L (2014) New cationic antibacterial peptide screened from boiled-dried anchovies by immobilized bacterial membrane liposome chromatography. J Agr Food Chemi 62(7):1564–1571
Tomasinsig L, Skerlavaj B, Scarsini M, Guida F, Piccinini R, Tossi A, Zanetti M (2012) Comparative activity and mechanism of action of three types of bovine antibacterial peptides against pathogenic Prototheca spp. J Pept Sci 18:105–113
Wang Y, Zhang Y, Lee WH, Zhang Y, Zhang Y (2016) Novel peptides from skins of amphibians showed broad-spectrum antibacterial activities. Chem Biol Drug Des 87(3):419–424
Xiao JH, Zhang H (2012) Antibacterial peptide isolated from ovalbumin hydrolysate by immobilized liposome-binding extraction. J Biome Screen 17:752–760
Xin DU, Xiao L, Cheng LI, Wen X, Tian T, Zhou HL (2016) Optimization of fermentation conditions for preparation of antioxidant peptides from WYM antioxidant peptides with bacillus subtilis. Food Machin 32(3):165–168
Yang X, Lee WH, Zhang Y (2012) Extremely abundant antibacterial peptides existed in the skins of nine kinds of Chinese odorous frogs. J Proteome Res 11(1):306–319
Yue TL, Pei JJ, Yuan YH (2013) Purification and characterization of anti-Alicyclobacillus bacteriocin produced by Lactobacillus rhamnosus. J Food Protect 76:1575–1581
Zhang Y, Guo BY, Cao Y, Huang QR (2016) Antibacterial effects of a cell-penetrating peptide isolated from Kefir. J Agri Food Chem 64:3234–3242
Zhao SG, Han JZ, Bie XM, Lu ZX, Zhang C, Lv FX (2016) Purification and characterization of plantaricin JLA-9: a novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a traditional Chinese fermented cabbage. J Agri Food Chem 64:2754–2764
Authors’ contributions
JP, HJ and WJ carried out the experiments; JP and YT wrote the paper; XL and YT designed the research; JP obtained the funding. All authors read and approved the final manuscript.
Acknowledgements
We thank the subjects for participating in the study. The authors gratefully acknowledge the generous support of the Shaanxi University of Technology and Northwest Institute of Plateau Biology, Chinese Academy of Sciences.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data on which the conclusions are made are all presented in this paper.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This study was funded by Research Foundation of Science and Technology Bureau of Shaanxi, China (2015SZS-15-05), Collaborative innovation center Research Foundation (QBXT-Z(P)-15-28), the China Scholarship Council (CSC) Foundation (201608615024), Special Scientists’ Foundations of Shaanxi University of Technology (SLGKYQD2014-2-18) and Research Foundations of Shaanxi University of Technology (SLGQD13-13).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pei, J., Jiang, H., Li, X. et al. Antimicrobial peptides sourced from post-butter processing waste yak milk protein hydrolysates. AMB Expr 7, 217 (2017). https://doi.org/10.1186/s13568-017-0497-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-017-0497-8