Abstract
L-citrulline plays an important role in human health and nutrition and is an intermediate of the L-arginine biosynthetic pathway. L-citrulline is a by-product of L-arginine production by Corynebacterium glutamicum. In this study, C. glutamicum was engineered for overproduction of L-citrulline as major product without L-arginine being produced as by-product. To this end, L-arginine biosynthesis was derepressed by deletion of the arginine repressor gene argR and conversion of L-citrulline towards L-arginine was avoided by deletion of the argininosuccinate synthetase gene argG. Moreover, to facilitate L-citrulline production the gene encoding a feedback resistant N-acetyl L-glutamate kinase argBfbr as well as the gene encoding L-ornithine carbamoylphosphate transferase argF were overexpressed. The resulting strain accumulated 44.1 ± 0.5 mM L-citrulline from glucose minimal medium with a yield of 0.38 ± 0.01 g⋅g−1 and a volumetric productivity of 0.32 ± 0.01 g⋅l−1⋅h−1. In addition, production of L-citrulline from the alternative carbon sources starch, xylose, and glucosamine could be demonstrated.
Similar content being viewed by others
Introduction
L-citrulline is a natural non-proteinogenic amino acid whose name is derived from watermelon Citrullus lanatus (Wada [1930]). In mammalians it serves as a precursor for L-arginine. In contrast to the proteinogenic L-arginine, which is not transferred to the blood stream, when ingested, L-citrulline can be converted to L-arginine, which is then released by the kidney into the blood stream. It is applied in several medical approaches e.g. as a pharmaconutrient (Rimando and Perkins-Veazie [2005]; Curis et al. [2005]).
Currently, biocatalytic and fermentative methods to produce L-citrulline using Pseudomonas putida (Kakimoto et al. [1971]; Yamamoto et al. [1974]) or Bacillus subtilis strains exist (Okumura et al. [1966]). Additionally, extraction processes from watermelon have been established (Fish [2012]). L-citrulline is an intermediate of L-arginine biosynthesis and accumulates as a by-product of engineered L-arginine producing Corynebacterium glutamicum strains (Ikeda et al. [2009]; Schneider et al. [2011]).
C. glutamicum is a workhorse for amino acid production and is employed for the annual production of several million tons of L-glutamate and L-lysine (Wendisch [2014]). C. glutamicum has been engineered to produce a wide range of bioproducts, such as diamines, carotenoids, terpenes, proteins (Schneider and Wendisch [2010]; Schneider et al. [2012]; Heider et al. [2014a], [b]; Frohwitter et al. [2014]; Kikuchi et al. [2009]; Teramoto et al. [2011]; An et al. [2013]) and the L-glutamate family amino acids L-arginine, L-ornithine, and L-proline (Schneider et al. [2011]; Ikeda et al. [2009]; Georgi et al. [2005]; Blombach et al. [2009]; Jensen and Wendisch [2013]). However, the production of L-citrulline as the only or major product has not been published yet.
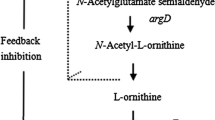
Due to its natural ability to produce L-glutamate under several eliciting conditions, C. glutamicum is a suitable producer of L-glutamate-derived products (Sato et al. [2008]; Radmacher et al. [2005]; Kim et al. [2009], [2010]; Delaunay et al. [1999]; Wendisch et al. [2014]). L-ornithine is a non-proteinogenic glutamate-family amino acid and an intermediate of L-arginine biosynthesis (Figure 1). An ornithine producer was obtained by deletion of argR, the gene encoding the genetic repressor of the arginine biosynthesis operon, and argF to prevent further processing of ornithine (Schneider et al. [2011]). The production of L-proline from L-ornithine is possible by the heterologous overexpression of ocd from Pseudomonas putida, encoding ornithine cyclodeaminase (Jensen and Wendisch [2013]). The diamine putrescine can be produced by overexpression of the Escherichia coli gene speC, which encodes ornithine decarboxylase (Schneider et al. [2012]; Schneider and Wendisch [2010]). As the arginine biosynthetic pathway is naturally regulated by feedback inhibition of N-acetylglutamate kinase (encoded by argB) by arginine, the use of feedback resistant enzyme variants in combination with deletion of argR has been described to overproduce L-arginine (Sakanyan et al. [1996]; Ikeda et al. [2009]; Schneider et al. [2011]).
L-arginine pathway in C. glutamicum (modified from (Wendisch et al.[2014])). gdh: L-glutamate dehydrogenase, cg3035: anaplerotic N-acetylL-glutamate synthase, argJ: L-ornithine N-acetyltransferase, argB: N-acetylL-glutamate kinase; argC: N-acetyl-gamma-glutamyl-phosphate reductase; argD: acetylL-ornithine aminotransferase; argE: acetylL-ornithine deacetylase; argF: L-ornithine carbamoyltransferase; argG: argininosuccinate synthetase; argH: argininosuccinate lyase. Oxoglutarate is an intermediate of the central carbon metabolism.
Biomass formation by various C. glutamicum strains. The cultivation was performed in CGXII minimal medium containing 20 g L-1 glucose, 1 mM IPTG, 750 μM L-arginine and 25 μg L-1 kanamycin. OD600 was determined of CIT0(pVWEx1) (open squares), CIT0(pVWEx1-argF) (gray circles) and CIT0(pVWEx1-argFBfbr) (black diamonds). Values and error bars represent the mean and the standard error of triplicates.
C. glutamicum can utilize a variety of carbon sources. In contrast to many other microorganisms used in biotechnology, simultaneous utilization of carbon sources e.g. present in mixtures such as lignocellulosic hydrolysates is a hall mark of C. glutamicum (Blombach and Seibold [2010]; Meiswinkel et al. [2013a], [b]). The natural substrate spectrum of C. glutamicum includes monosaccharides, disaccharides, and organic acids as well as alcohols (Blombach and Seibold [2010]; Arndt and Eikmanns [2008]; Peters-Wendisch et al. [1998]; Jolkver et al. [2009]; Sasaki et al. [2011]). To allow access to alternative carbon sources, C. glutamicum has also been engineered for utilization of glycerol, pentoses, and amino sugars as well as polysaccharides (Schneider et al. [2011]; Rittmann et al. [2008]; Seibold et al. [2006]; Uhde et al. [2013]; Gopinath et al. [2011]; Matano et al. [2014]).
One aim to reduce production cost is the use of complex sugar substrates for the production of biotechnological products. As an example of using a polymeric raw material without decomposition to its monomeric compounds e.g. by enzyme treatment, soluble starch could be used as a carbon source for the production of L-lysine and organic acids by engineered C. glutamicum (Seibold et al. [2006]; Tateno et al. [2007]; Tsuge et al. [2013]). However, due to the growing world population and a correlating higher demand for food, biotechnological processes based on non-food derived carbon sources are sought. Xylose is a pentose sugar compound present in the hemicellulosic fraction of agricultural wastes as for example rice straw. Glucosamine, on the other hand, is a constituent of chitin, the second most abundant biopolymer in Nature, which is accessible e.g. from shrimp shell waste accumulating in the food industry. C. glutamicum has been engineered to efficiently utilize both xylose and glucosamine as alternative carbon sources for growth and amino acid production (Gopinath et al. [2011]; Meiswinkel et al. [2013a]; Uhde et al. [2013]; Matano et al. [2014]).
In this study, the rational engineering of L-citrulline production by C. glutamicum is reported and the concept was extended to production of L-citrulline from the alternative carbon sources glucosamine, xylose, and starch.
Materials and methods
Microorganisms and growth conditions
Microorganisms and plasmids used in this study are listed in Table 1. E. coli DH5α was used for gene cloning. C. glutamicum and E. coli strains were routinely grown in lysogeny broth (LB) (10 g L−1 tryptone, 5 g L−1 yeast extract, 10 g L−1 sodium chloride) in 500-mL baffled flasks on a rotary shaker (120 rpm) at 30°C or 37°C. For growth experiments, CGXII minimal medium (Eggeling and Reyes [2005]) was used for C. glutamicum. Growth was followed by measuring the optical density at 600 nm using a V-1200 Spectrophotometer (VWR, Radnor, PA, USA). An OD600 of 1 corresponds approximately to an estimated cell dry weight of 0.25 g/L.
When necessary, the growth medium was supplemented kanamycin (25 μg mL−1), spectinomycin (100 μg mL−1), tetracycline (10 μg mL−1), isopropyl β-D-1-thiogalactopyranoside (IPTG) (1 mM) and L-arginine (750 μM). The growth behavior and L-citrulline production of recombinant C. glutamicum strains were analyzed in 500 ml baffled flasks. Briefly, a 50 mL BHI (37 g L−1) seed culture was inoculated from an agar plate and grown overnight. The cells were harvested by centrifugation (4,000 × g, 10 min) and washed twice with CGXII minimal medium lacking the carbon source. Subsequently, 50 mL CGXII medium, containing a given concentration of carbon source and necessary supplements, was inoculated to an optical density of 1.0. Detailed information on the carbon source concentrations employed are given in the Results chapter.
Molecular genetic techniques
Standard methods such as restriction digestions, and ligation were carried out as described elsewhere (Sambrook and Russell [2012]). Digested DNA was purified by using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). E. coli cells were transformed by heat shock (Sambrook and Russell [2012]) and C. glutamicum cells were transformed by electroporation (Eggeling and Reyes [2005]). Isolation of genomic DNA was performed as previously described (Jensen and Wendisch [2013]). Chromosomal changes in C. glutamicum were performed as described elsewhere (Eggeling and Reyes [2005]).
Construction of strains and plasmids
The deletion of ∆argFR in MB001 was performed by using pK19mobsacB∆argFR. Afterwards argG was deleted by using pK19mobsacB∆argG to obtain CIT0. pK19mobsacB∆argG contains the up- and downstream regions of argG in the ∆argFR strain. The plasmid was constructed by amplifying the upstream region with argG _up_f (CTTgaattc AGAAGCTGCGCCGCATG) and argG _up_r (agagacgacctaagccagtctAACGATGCGGTTAGTCATGAGG) and the downstream region with argG _down_f (agactggcttaggtcgtctctGCTAACAAGCGCGATCGC) and argG _down_r (CCTctgcag AACGACCAGCGCGCAGA). The two fragments were combined by crossover PCR using argG _up_f and argG _down_r and finally cloned into pK19mobsacB with Pst I and Eco RI.
pVWEx1-argF was constructed by amplifying argF with primers argF_f (CTTgtcgac AAGGAGATATAGATATGACTTCACAACCACAGGTTCG) and argF_r (CCTggatcc TTACCTCGGCTGGTTGGC). The PCR product was treated with Sal I and Bam HI and ligated with similarly treated pVWEx1. pVWEx1-argFG was constructed by amplifying argG with primers argG_f (GGGgtcgac GAAAGGAGGCCCTTCAGATGACTAACCGCATCGTTCTTG) and argG_r (GGGgtcgac TTAGTTGTTGCCAGCTTCGCGA). The PCR product was treated with Sal I and ligated with similarly treated pVWEx1-argF.
The plasmid vector pEKEx-argBfbr (argBA49VM54V (Schneider et al. [2011])) was digested with Bam HI and Kpn I and the DNA fragment with a size of 0.9 kb harboring the argBfbr gene was cloned into the Bam HI/Kpn I digested vector pVWEx1-argF.
Determination of amino acid and carbohydrate concentrations
For the quantification of extracellular amino acids and carbohydrates, a high-performance liquid chromatography system was used (1200 series, Agilent Technologies Deutschland GmbH, Böblingen, Germany). Samples were withdrawn from the cultures, centrifuged (13,000 × g, 10 min), and the supernatant used for analysis.
Glucose and xylose were analyzed on a normal phase column (organic acid resin 300 × 8 mm, 10 μm particle size, 25 Å pore diameter; Chromatographie Service GmbH, Langerwehe, Germany) using 5 mM sulfuric acid as the mobile phase at a flow rate of 1 mL min−1 and were detected with a refractive index detector (RID G1362A, 1200 series, Agilent Technologies). Amino acids were automatically modified by precolumn derivatisation with ortho-phthalaldehyde and separated as described previously (Georgi et al. [2005]). L-ornithine was quantified using a pre-column (LiChrospher 100 RP18 EC-5 μ (40 × 4 mm), CS-Chromatographie Service GmbH, Langerwehe, Germany) and a reversed phase column (LiChrospher 100 RP18 EC-5 μ (125 × 4 mm), CS Chromatographie) as a main coulumn and detected with a fluorescence detector at excitation at 230 nm and 450 nm emission (FLD G1321A, 1200 series, Agilent Technologies). For the determination of L-citrulline, a reverse-phase (RP) LiChrospher 100 RP8 EC-5 μ precolumn (40 × 4.6 mm) and a RP8 EC-5 μ (125 × 4.6 mm) main column (CS Chromatographie, Langerwehe, Germany) were used. 100 μM L-Asparagine was used as an internal standard. The mobile phases used were in case of RP8 A: 0.25% Na-acetate pH 6, B: methanol. The gradient used was: 0 min 30% B, 1 min 30% B, 6 min, 70% B, 11 min 90% B, 14 min 70% B, 16 min 30% B. In case of RP18, the mobile phases used were A:0.1 M Na-acetate pH 7.2, B: methanol. The gradient used was: 0 min 20% B, 0.5 min 38% B, 2.5 min 46% B, 3.7 min 65% B, 5.5 min 70% B, 6 min 75% B, 6.2 min 85% B, 6.7 min 20% B.
Results
Engineering a prophage-free C. glutamicum strain for L-citrulline production
C. glutamicum has recently been cured of prophage sequences to yield MB001 (Baumgart et al. [2013]). This strain was used as the parental strain because it can be transformed easily and plasmid-based gene overexpression is more efficient (Baumgart et al. [2013]). As C. glutamicum ATCC 13032, this strain does not accumulate L-citrulline, an intermediate of L-arginine biosynthesis (Figure 1). The deletion of three genes of the L-arginine operon (L-ornithine carbamoyltransferase (EC 2.1.3.3) argF, argininosuccinate synthetase (EC 6.3.4.5) argG, and L-arginine biosynthesis operon repressor gene argR) in C. glutamicum MB001 yielded the L-arginine auxotrophic strain CIT0 (Table 1). When supplemented with 0.75 mM L-arginine, C. glutamicum CIT0 accumulated 25.2 ± 2.6 mM L-ornithine from 2% glucose (Table 2). The deletion of argF and argG could be complemented by plasmid-borne expression of these genes since the complemented strain CIT0(pVWEx1-argFG) grew without L-arginine supplement while the empty vector carrying control CIT0(pVWEx1) did not (data not shown). Comparable growth rates and biomass concentrations were observed.
To enable L-citrulline accumulation, two plasmids were constructed and used to transform C. glutamicum CIT0. While pVWEx1-argF only carries argF encoding L-ornithine carbamoyltransferase, pVWEx1-argFBfbr in addition carries argBfbr encoding feedback-resistant N-acetyl L-glutamate kinase (NAGK, EC 2.7.2.8). When grown in minimal medium with 2% glucose and 0.75 mM L-arginine C. glutamicum CIT0(pVWEx1-argF) grew to a higher OD than CIT0(pVWEx1) (Figure 2) and did not accumulate notable concentrations of L-citrulline. As opposed to CIT0(pVWEx1), CIT0(pVWEx1-argF) did not produce L-ornithine (Figure 3). By contrast, the combined overexpression of argF and argBfbr entailed L-citrulline production and the respective strain was named CIT1. C. glutamicum CIT1 accumulated 44.1 ± 0.5 mM L-citrulline in minimal medium with 2% glucose (Figure 4).
Biomass formation and production of ornithine and citrulline on glucose by various C. glutamicum strains: cell dry weight (hatched bars), L-ornithine concentration (open bars) and L-citrulline concentration (filled bars). The cultivation was performed in CGXII minimal medium containing 20 g/L glucose, 1 mM IPTG, 750 μM L-arginine and 25 μg/L kanamycin. The amino acid concentrations in the supernatant were determined after the consumption of glucose. Values and error bars represent the mean and the standard error of triplicates.
Amino acid production by various C. glutamicum strains. L-ornithine production by C. glutamicum CIT0(pVWEx1) (filled squares) (A) and L-citrulline accumulation (filled squares) and glucose consumption (open triangles) by strain CIT0(pVWEx1-argFBfbr) (B). The experiments were performed in CGXII minimal medium with 20 g/L glucose, 1 mM IPTG, 25 μg/L kanamycin and supplemented with 750 μM L-arginine. Values and error bars represent the mean and the standard error of triplicates.
When comparing the growth of C. glutamicum CIT0(pVWEx1) to that of CIT0(pVWEx1-argF), similar growth rates (0.37 ± 0.01 h−1 and 0.35 ± 0.04 h−1, respectively) were obtained, whereas L-citrulline formation by CIT0(pVWEx1-argFBfbr) was accompanied by a reduced growth rate (0.15 ± 0.01 h−1) (Figure 1). Moreover, the final OD600 of CIT0(pVWEx1-argFBfbr) was 20 ± 1 as compared to an OD600 of 26 ± 1 of CIT0(pVWEx1). By contrast, C. glutamicum CIT0(pVWEx1-argF) grew to a higher biomass concentration with a final OD600 of 35 ± 1. As shown in Figure 3, the lower growth rates of CIT0(pVWEx1) and CIT0(pVWEx1-argFBfbr) correlated inversely with the formation of the respective amino acids L-ornithine and L-citrulline, whereas C. glutamicum CIT0(pVWEx1-argF) reaches a higher final biomass and neither produces L-ornithine nor L-citrulline.
Production of L-citrulline from alternative carbon sources
Due to the high demand of biotechnological processes of using complex sugar substrates derived from raw materials and industrial wastes, the L-citrulline producer strain CIT1 was enabled to utilize the alternative carbon sources starch (as an example of a high molecular weight carbohydrate), xylose, and glucosamine (as an example of a carbohydrates, derived from forestry and food industrial wastes).
To enable C. glutamicum CIT1 to consume starch, the gene amyA from Streptomyces griseus was overexpressed. The combined overexpression of xylA from Xanthomonas campestris and endogenous xylB allowed the utilization of xylose by C. glutamicum CIT1. The endogenous nagB was overpressed ectopically to facilitate the consumption of glucosamine. The resulting strains were tested for growth and L-citrulline production.
When cultured in CGXII medium supplemented 0.75 mM L-arginine all strains engineered for alternative carbon source consumption grew with their respective substrate (Table 1). The empty vector carrying strain CIT1(pEKEx3) neither grew in xylose or glucosamine minimal medium nor consumed these substrates. By contrast, the recombinant strain CIT1(pEKEx3-xylAB) grew in xylose minimal medium with a growth rate of 0.03 ± 0.01 h−1 and reached a final OD600 of 6 ± 1. In glucosamine minimal medium, C. glutamicum CIT1(pEKEx3-nagB) grew to a final OD600 of 3 ± 1 with a growth rate of 0.02 ± 0.01 h−1. In minimal medium containing 1% starch and 0.25% glucose as carbon sources, the empty vector harbouring strain CIT1(pEC-XT99A) formed roughly one third of the biomass as compared to C. glutamicum CIT1(pAmy). Growth of CIT1(pEC-XT99A) was slower (growth rate of 0.10 ± 0.01 h−1) than that of CIT1(pAmy) (growth rate of 0.21 ± 0.01 h−1). While strain CIT1(pEC-XT99A) only utilized glucose, but not starch, CIT1(pAmy) was able to consume both, glucose and starch.
The strains engineered for utilization of xylose and glucosamine, respectively, also produced L-citrulline from these carbon sources (Figure 5). C. glutamicum CIT1(pEKEx3-nagB) accumulated 2.6 ± 0.3 mM L-citrulline which corresponds to a yield of 0.045 ± 0.002 g/g since glucosamine was utilized completely. Similarly, after complete utilization of xylose by C. glutamicum CIT1(pEKEx3-xylAB) 6.4 ± 0.1 mM L-citrulline accumulated corresponding to a yield of 0.075 ± 0.001 g per g xylose.
L-citrulline concentration in the engineered strains after the consumption of the respective carbon source. CIT1(pEC-XT99A), CIT1(pAmy) with 10 g/L soluble starch, 2,5 g/L glucose after 31 h. CIT1(pEKEx2-xylAB) with 15 g/L xylose after xylose consumption. CIT1(pEKEx3-nagB) with 10 g/L glucosamine after glucosamine consumption. Values and error bars represent the mean and the standard error of triplicates.
As the determination of the starch concentration by HPLC was not possible, residual starch content was assayed by the use of Lugols solution. However, as it is known that overexpression of amyA in C. glutamicum results in high molecular mass degradation products of starch, which remain in the medium and are not detectable by Lugols solution (Seibold et al. [2006]), the L-citrulline concentration was measured until no change in OD600, starch content and L-citrulline concentration was observed. The starch utilizing strain CIT1(pAmy) was able to produce 11.9 ± 0.5 mM L-citrulline which corresponds to a yield of 0.167 g/g.
Discussion
C. glutamicum was engineered to accumulate L-citrulline as major product, both from glucose as well as from the alternative carbon sources starch, glucosamine and xylose.
Feedback insensitive N-acetyl L-glutamate kinase (encoded by argBfbr; (A49VM54V)) was required for production of L-citrulline since CIT0(pVWEx1-argF) did not produce L-citrulline, while CIT0(pVWEx1-argFBfbr) produced L-citrulline. It is unlikely that addition of L-arginine to CIT0(pVWEx1-argF) inhibited generation of L-ornithine, a precursor of L-citrulline, because strain CIT0(pVWEx1) produced L-ornithine when supplemented with L-arginine. However, it is possible that intracellular L-citrulline affects arginine biosynthesis. As overexpression of argBfbr entailed L-citrulline formation, we assume that L-citrulline inhibits the NAGK of C. glutamicum, but this has not yet been described. As expected due to its structural similarity to L-arginine, L-citrulline inhibits NAGK of other microorganisms (Farago and Denes [1967]; Haas and Leisinger [1975]). In Chlamydomonas reinhardtii, NAGK is inhibited by several L-arginine structure analogs, including L-citrulline, however, inhibition was less pronounced than L-arginine inhibition (Farago and Denes [1967]). NAGK from Pseudomonas aeruginosa lost two thirds of its activity in the presence of 2.5 mM L-citrulline which was claimed to be too weak under physiologic conditions (Haas and Leisinger [1975]). However, it is conceivable that inhibition of NAGK by L-citrulline may play a role in recombinant C. glutamicum strains engineered for L-citrulline production, thus, possibly explaining the finding that L-citrulline production required overexpression argBfbr encoding NAGK feedback resistant to L-arginine. Commensurate with this notion, simultaneous production of L-arginine and L-citrulline resulted from argBfbr overexpression in a ∆argR background (Ikeda et al. [2009]). In this argBfbr overexpressing strain, the ratio of L-citrulline to L-arginine was higher than by classically obtained strains, which solely contain native argB (Ikeda et al. [2009]). Currently, it remains to be studied if L-citrulline inhibits NAGK from C. glutamicum and if (some) variants feed-back resistant to L-arginine are also desensitized to L-citrulline.
Notably, about two fold more L-citrulline (about 7.7 g/L) was produced by strain CIT1 than L-ornithine was produced (about 3.3 g/L) by the isogenic strain CIT0(pVWEx1). Both, overexpression of argF and argBfbr may have contributed to this effect. It is more likely that argBfbr is responsible as L-arginine supplementation may have limited flux in the arginine biosynthesis pathway of strain CIT0(pVWEx1) especially in the beginning of the cultivation. In C. glutamicum CIT1, only feedback-resistant NAGK is present and additionally a gene dosage effect due ectopic overexpression of argBfbr might have contributed to increase L-citrulline production.
Glucose, glucosamine, xylose, and starch were shown to be suitable substrates for the production of L-citrulline. Strain construction was based on previously established engineering strategies (Seibold et al. [2006]; Uhde et al. [2013]; Meiswinkel et al. [2013a]; Gopinath et al. [2011]). The achieved L-citrulline concentrations on these substrates were lower than with glucose as carbon source. However, L-citrulline production from xylose (6.44 ± 0.12 mM) by CIT1(pEKEx3-xylAB) was lower, but in a similar range as production of L-ornithine (19.6 ± 1.9 mM) and putrescine (15.1 ± 1.2 mM), respectively, from the same xylose concentration by the respective recombinant C. glutamicum strains (Meiswinkel et al. [2013a]). Similarly, product yields with glucosamine as carbon source were lower for L-citrulline (0.067 g/g) than for putrescine (0.112 g/g) (Uhde et al. [2013]). Unexpectedly and hitherto not understood, the growth rate (0.02 ± 0.01 h−1) and, thus, productivity by CIT1(pEKEx3-nagB) were very low. By contrast, a putrescine producing strain carrying pEKEx3-nagB showed only a slightly decreased growth rate (Uhde et al. [2013]).
C. glutamicum strains carrying pAMY co-utilized starch with glucose (Seibold et al. [2006]). Substrate co-utilization is observed with C. glutamicum WT as well as recombinant strains for almost all mixtures of carbon sources (Blombach and Seibold [2010]). A L-lysine producing strain carrying pAMY showed increased biomass formation by addition of 10 g/L starch to 10 g/L glucose, whereas L-lysine production increased only upon addition of higher starch concentrations (Seibold et al. [2006]).
In this study, the additional presence of starch increased the growth rate of CIT1 (from 0.15 to 0.21 h−1) as well as L-citrulline production. Production of L-citrulline by CIT1(pAMY) from a starch glucose mixture was higher (11.95 ± 0.48 mM) than that by the empty vector carrying control strain (4.83 ± 0.4 mM) demonstrating that starch contributed to production of L-citrulline. It has to be noted that starch cannot be utilized completely by C. glutamicum strains overexpressing the α-amylase gene amyA because high-molecular-weight carbohydrates are generated from starch and remain unutilized in the medium (Seibold et al. [2006]).
Taken together, production of L-citrulline as major product from glucose, starch, glucosamine, and xylose by recombinant C. glutamicum strains was achieved.
References
An SJ, Yim SS, Jeong KJ: Development of a secretion system for the production of heterologous proteins in Corynebacterium glutamicum using the Porin B signal peptide. Protein Expr Purif 2013, 89(2):251–257. doi:10.1016/j.pep.2013.04.003 10.1016/j.pep.2013.04.003
Arndt A, Eikmanns BJ: Regulation of carbon metabolism in Corynebacterium glutamicum . In Corynebacteria: genomics and molecular biology. Edited by: Burkovski A. Caister Academic Press, Wymondham, UK; 2008:155–182.
Baumgart M, Unthan S, Ruckert C, Sivalingam J, Grunberger A, Kalinowski J, Bott M, Noack S, Frunzke J: Construction of a prophage-free variant of Corynebacterium glutamicum ATCC 13032 for use as a platform strain for basic research and industrial biotechnology. Appl Environ Microbiol 2013, 79(19):6006–6015. doi:10.1128/AEM. 01634–13 10.1128/AEM.01634-13
Blombach B, Seibold GM: Carbohydrate metabolism in Corynebacterium glutamicum and applications for the metabolic engineering of L-lysine production strains. Appl Microbiol Biotechnol 2010, 86(5):1313–1322. doi:10.1007/s00253–010–2537-z 10.1007/s00253-010-2537-z
Blombach B, Hans S, Bathe B, Eikmanns BJ: Acetohydroxyacid synthase, a novel target for improvement of L-lysine production by Corynebacterium glutamicum . Appl Environ Microbiol 2009, 75(2):419–427. doi:10.1128/AEM. 01844–08 10.1128/AEM.01844-08
Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L: Almost all about citrulline in mammals. Amino Acids 2005, 29(3):177–205. doi:10.1007/s00726–005–0235–4 10.1007/s00726-005-0235-4
Delaunay S, Gourdon P, Lapujade P, Mailly E, Oriol E, Engasser JM, Lindley NL, Goergen JL: An improved temperature triggered process for glutamate production with Corynebacterium glutamicum . Enz Microb Biotechnol 1999, 25: 762–768. 10.1016/S0141-0229(99)00120-9
Eggeling L, Reyes O: Experiments. In Handbook of Corynebacterium glutamicum . Edited by: Eggeling L, Bott M. CRC Press, Boca Raton, USA; 2005:3535–3566.
Farago A, Denes G: Mechanism of arginine biosynthesis in Chlamydomonas reinhardti . II. Purification and properties of N-acetylglutamate 5-phosphotransferase, the allosteric enzyme of the pathway. Biochim Biophys Acta 1967, 136(1):6–18. 10.1016/0304-4165(67)90315-7
Fish WW: Process for the production of L-citrulline from watermelon flesh and rind. US 8173837 B1. 2012.
Frohwitter J, Heider SA, Peters-Wendisch P, Beekwilder J, Wendisch VF (2014) Production of the sesquiterpene (+)-valencene by metabolically engineered Corynebacterium glutamicum. J Biotechnol. doi:10.1016/j.jbiotec.2014.05.032
Georgi T, Rittmann D, Wendisch VF: Lysine and glutamate production by Corynebacterium glutamicum on glucose, fructose and sucrose: roles of malic enzyme and fructose-1,6-bisphosphatase. Metab Eng 2005, 7(4):291–301. 10.1016/j.ymben.2005.05.001
Gopinath V, Meiswinkel TM, Wendisch VF, Nampoothiri KM (2011) Amino acid production from rice straw and wheat bran hydrolysates by recombinant pentose-utilizing Corynebacterium glutamicum. Appl Microbiol Biotechnol. doi:10.1007/s00253–011–3478-x
Haas D, Leisinger T: N-acetylglutamate 5-phosphotransferase of Pseudomonas aeruginosa . Catalytic and regulatory properties. Eur J Biochem 1975, 52(2):377–393. 10.1111/j.1432-1033.1975.tb04004.x
Hanahan D: Studies on transformation of Escherichia coli with plasmids. J Mol Biol 1983, 166(4):557–580. 10.1016/S0022-2836(83)80284-8
Heider SA, Peters-Wendisch P, Netzer R, Stafnes M, Brautaset T, Wendisch VF: Production and glucosylation of C50 and C 40 carotenoids by metabolically engineered Corynebacterium glutamicum . Appl Microbiol Biotechnol 2014, 98(3):1223–1235. doi:10.1007/s00253–013–5359-y 10.1007/s00253-013-5359-y
Heider SA, Peters-Wendisch P, Wendisch VF, Beekwilder J, Brautaset T: Metabolic engineering for the microbial production of carotenoids and related products with a focus on the rare C50 carotenoids. Appl Microbiol Biotechnol 2014, 98(10):4355–4368. doi:10.1007/s00253–014–5693–8 10.1007/s00253-014-5693-8
Ikeda M, Mitsuhashi S, Tanaka K, Hayashi M: Reengineering of a Corynebacterium glutamicum L-arginine and L-citrulline producer. Appl Environ Microbiol 2009, 75(6):1635–1641. doi:10.1128/AEM. 02027–08 10.1128/AEM.02027-08
Jensen JV, Wendisch VF: Ornithine cyclodeaminase-based proline production by Corynebacterium glutamicum . Microb Cell Factories 2013, 12: 63. doi:10.1186/1475–2859–12–63 10.1186/1475-2859-12-63
Jolkver E, Emer D, Ballan S, Kramer R, Eikmanns BJ, Marin K: Identification and characterization of a bacterial transport system for the uptake of pyruvate, propionate, and acetate in Corynebacterium glutamicum . J Bacteriol 2009, 191(3):940–948. doi:10.1128/JB.01155–08 10.1128/JB.01155-08
Kakimoto T, Shibatani T, Nishimura N, Chibata I: Enzymatic production of L-citrulline by Pseudomonas putida. Appl Microbiol 1971, 22(6):992–999.
Kikuchi Y, Itaya H, Date M, Matsui K, Wu LF: TatABC overexpression improves Corynebacterium glutamicum Tat-dependent protein secretion. Appl Environ Microbiol 2009, 75(3):603–607. doi:10.1128/AEM. 01874–08 10.1128/AEM.01874-08
Kim J, Hirasawa T, Sato Y, Nagahisa K, Furusawa C, Shimizu H: Effect of odhA overexpression and odhA antisense RNA expression on Tween-40-triggered glutamate production by Corynebacterium glutamicum . Appl Microbiol Biotechnol 2009, 81(6):1097–1106. doi:10.1007/s00253–008–1743–4 10.1007/s00253-008-1743-4
Kim J, Fukuda H, Hirasawa T, Nagahisa K, Nagai K, Wachi M, Shimizu H: Requirement of de novo synthesis of the OdhI protein in penicillin-induced glutamate production by Corynebacterium glutamicum . Appl Microbiol Biotechnol 2010, 86(3):911–920. doi:10.1007/s00253–009–2360–6 10.1007/s00253-009-2360-6
Kirchner O, Tauch A: Tools for genetic engineering in the amino acid-producing bacterium Corynebacterium glutamicum . J Biotechnol 2003, 104(1–3):287–299. 10.1016/S0168-1656(03)00148-2
Matano C, Uhde A, Youn JW, Maeda T, Clermont L, Marin K, Kramer R, Wendisch VF, Seibold GM: Engineering of Corynebacterium glutamicum for growth and L-lysine and lycopene production from N-acetyl-glucosamine. Appl Microbiol Biotechnol 2014, 98(12):5633–5643. doi:10.1007/s00253–014–5676–9 10.1007/s00253-014-5676-9
Meiswinkel TM, Gopinath V, Lindner SN, Nampoothiri KM, Wendisch VF: Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine. Microb Biotechnol 2013, 6(2):131–140. doi:10.1111/1751–7915.12001 10.1111/1751-7915.12001
Meiswinkel TM, Rittmann D, Lindner SN, Wendisch VF: Crude glycerol-based production of amino acids and putrescine by Corynebacterium glutamicum . Bioresour Technol 2013, 145: 254–258. doi:10.1016/j.biortech.2013.02.053 10.1016/j.biortech.2013.02.053
Okumura S, Shibuya M, Shimpachi K, Teruo S, Noboru K: Method of producing citrulline by bacterial fermentation. US 3282794 A. 1966.
Peters-Wendisch PG, Kreutzer C, Kalinowski J, Patek M, Sahm H, Eikmanns BJ: Pyruvate carboxylase from Corynebacterium glutamicum : characterization, expression and inactivation of the pyc gene. Microbiology 1998, 144(Pt 4):915–927. 10.1099/00221287-144-4-915
Peters-Wendisch PG, Schiel B, Wendisch VF, Katsoulidis E, Mockel B, Sahm H, Eikmanns BJ: Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum . J Mol Microbiol Biotechnol 2001, 3(2):295–300.
Radmacher E, Stansen KC, Besra GS, Alderwick LJ, Maughan WN, Hollweg G, Sahm H, Wendisch VF, Eggeling L: Ethambutol, a cell wall inhibitor of Mycobacterium tuberculosis , elicits L-glutamate efflux of Corynebacterium glutamicum . Microbiology 2005, 151(Pt 5):1359–1368. 10.1099/mic.0.27804-0
Rimando AM, Perkins-Veazie PM: Determination of citrulline in watermelon rind. J Chromatogr A 2005, 1078(1–2):196–200. 10.1016/j.chroma.2005.05.009
Rittmann D, Lindner SN, Wendisch VF: Engineering of a glycerol utilization pathway for amino acid production by Corynebacterium glutamicum . Appl Environ Microbiol 2008, 74(20):6216–6222. doi:10.1128/AEM. 00963–08 10.1128/AEM.00963-08
Sakanyan V, Petrosyan P, Lecocq M, Boyen A, Legrain C, Demarez M, Hallet JN, Glansdorff N: Genes and enzymes of the acetyl cycle of arginine biosynthesis in Corynebacterium glutamicum : enzyme evolution in the early steps of the arginine pathway. Microbiology 1996, 142(Pt 1):99–108. 10.1099/13500872-142-1-99
Sambrook J, Russell D: Molecular cloning. A laboratory manual. 4th edition. Cold Spring Harbor Laboratoy Press, Cold Spring Harbor, NY; 2012.
Sasaki M, Teramoto H, Inui M, Yukawa H: Identification of mannose uptake and catabolism genes in Corynebacterium glutamicum and genetic engineering for simultaneous utilization of mannose and glucose. Appl Microbiol Biotechnol 2011, 89(6):1905–1916. doi:10.1007/s00253–010–3002–8 10.1007/s00253-010-3002-8
Sato H, Orishimo K, Shirai T, Hirasawa T, Nagahisa K, Shimizu H, Wachi M: Distinct roles of two anaplerotic pathways in glutamate production induced by biotin limitation in Corynebacterium glutamicum . J Biosci Bioeng 2008, 106(1):51–58. 10.1263/jbb.106.51
Schneider J, Wendisch VF: Putrescine production by engineered Corynebacterium glutamicum . Appl Microbiol Biotechnol 2010, 88(4):859–868. doi:10.1007/s00253–010–2778-x 10.1007/s00253-010-2778-x
Schneider J, Niermann K, Wendisch VF: Production of the amino acids L-glutamate, L-lysine, L-ornithine and L-arginine from arabinose by recombinant Corynebacterium glutamicum . J Biotechnol 2011, 154(2–3):191–198. doi:10.1016/j.jbiotec.2010.07.009 10.1016/j.jbiotec.2010.07.009
Schneider J, Eberhardt D, Wendisch VF: Improving putrescine production by Corynebacterium glutamicum by fine-tuning ornithine transcarbamoylase activity using a plasmid addiction system. Appl Microbiol Biotechnol 2012, 95(1):169–178. doi:10.1007/s00253–012–3956–9 10.1007/s00253-012-3956-9
Seibold G, Auchter M, Berens S, Kalinowski J, Eikmanns BJ: Utilization of soluble starch by a recombinant Corynebacterium glutamicum strain: growth and lysine production. J Biotechnol 2006, 124(2):381–391. 10.1016/j.jbiotec.2005.12.027
Stansen C, Uy D, Delaunay S, Eggeling L, Goergen JL, Wendisch VF: Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production. Appl Environ Microbiol 2005, 71(10):5920–5928. 10.1128/AEM.71.10.5920-5928.2005
Tateno T, Fukuda H, Kondo A: Production of L-Lysine from starch by Corynebacterium glutamicum displaying alpha-amylase on its cell surface. Appl Microbiol Biotechnol 2007, 74(6):1213–1220. doi:10.1007/s00253–006–0766-y 10.1007/s00253-006-0766-y
Teramoto H, Watanabe K, Suzuki N, Inui M, Yukawa H: High yield secretion of heterologous proteins in Corynebacterium glutamicum using its own Tat-type signal sequence. Appl Microbiol Biotechnol 2011, 91(3):677–687. doi:10.1007/s00253–011–3281–8 10.1007/s00253-011-3281-8
Tsuge Y, Tateno T, Sasaki K, Hasunuma T, Tanaka T, Kondo A: Direct production of organic acids from starch by cell surface-engineered Corynebacterium glutamicum in anaerobic conditions. AMB Express 2013, 3(1):72. doi:10.1186/2191–0855–3-72 10.1186/2191-0855-3-72
Uhde A, Youn JW, Maeda T, Clermont L, Matano C, Kramer R, Wendisch VF, Seibold GM, Marin K: Glucosamine as carbon source for amino acid-producing Corynebacterium glutamicum . Appl Microbiol Biotechnol 2013, 97(4):1679–1687. doi:10.1007/s00253–012–4313–8 10.1007/s00253-012-4313-8
Wada M: Über Citrullin, eine neue Aminosäure im Presssaft der Wassermelone, Citrullus vulgaris Schrad. Biochem Z 1930, 224: 420–429.
Wendisch VF: Microbial production of amino acids and derived chemicals: synthetic biology approaches to strain development. Curr Opin Biotechnol 2014, 30C: 51–58. doi:10.1016/j.copbio.2014.05.004 10.1016/j.copbio.2014.05.004
Wendisch VF, Eberhardt D, Herbst M, Jensen JVK (2014) Amino acids and nucleotides. In: Bicas J (ed) Biotechnological production of natural ingredients for food industry. Bentham eBooks.
Yamamoto K, Sato T, Tosa T, Chibata I: Continuous production of L-citrulline by immobilized Pseudomonas putida cells. Biotechnol Bioeng 1974, 16(12):1589–1599. doi:10.1002/bit.260161203 10.1002/bit.260161203
Acknowledgment
This work was partially supported by the Bundesministerium für Bildung und Forschung (BMBF, grant. no. 0316017) and by the program ZIM (grant. no. KF2969003SB2). We acknowledge support for the Article Processing Charge by the Deutsche Forschungsgemeinschaft and the Open Access Publication Fund of Bielefeld University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DE designed experiments, performed experiments, analysed results and drafted the manuscript. JVKJ designed experiments, performed experiments and analysed results. VFW coordinated the study, designed experiments, analysed results and wrote the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eberhardt, D., Jensen, J.V. & Wendisch, V.F. L-citrulline production by metabolically engineered Corynebacterium glutamicum from glucose and alternative carbon sources. AMB Expr 4, 85 (2014). https://doi.org/10.1186/s13568-014-0085-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-014-0085-0