Abstract
The first case of CWD in a Norwegian red deer was detected by a routine ELISA test and confirmed by western blotting and immunohistochemistry in the brain stem of the animal. Two different western blotting tests were conducted independently in two different laboratories, showing that the red deer glycoprofile was different from the Norwegian CWD reindeer and CWD moose and from North American CWD. The isolate showed nevertheless features similar to the classical BSE (BSE-C) strain. Furthermore, BSE-C could not be excluded based on the PrPSc immunohistochemistry staining in the brainstem and the absence of detectable PrPSc in the lymphoid tissues. Because of the known ability of BSE-C to cross species barriers as well as its zoonotic potential, the CWD red deer isolate was submitted to the EURL Strain Typing Expert Group (STEG) as a BSE-C suspect for further investigation. In addition, different strain typing in vivo and in vitro strategies aiming at identifying the BSE-C strain in the red deer isolate were performed independently in three research groups and BSE-C was not found in it. These results suggest that the Norwegian CWD red deer case was infected with a previously unknown CWD type and further investigation is needed to determine the characteristics of this potential new CWD strain.
Similar content being viewed by others
Introduction
Transmissible spongiform encephalopathies (TSEs) or prion diseases are infectious, transmissible and fatal neurodegenerative disorders that affect a large range of mammalian species, including cattle (Bovine Spongiform Encephalopathy or BSE), sheep and goats (scrapie), farmed and free-living cervids (Chronic Wasting Disease or CWD) and human (Creutzfeldt Jakob Disease or CJD, kuru, Gerstmann-Sträussler-Scheinker disease or GSS and Fatal Familial Insomnia or FFI). The causal proteinaceous agent, named PrPSc or prion, is considered to be an abnormal conformer of a host-encoded cellular prion protein (PrPC), mostly present in the central nervous system (CNS). PrPSc is partially resistant to proteases, accumulates and forms aggregates in the CNS and causes neurodegeneration. PrPSc is used as a biomarker for prion diseases.
CWD has been reported in North America for over 50 years and widely affects mule deer (Odocoileus hemionus), black-tailed deer (Odocoileus hemionus columbianus), white-tailed deer (Odocoileus virginianus), North American elk (Cervus canadensis nelsoni) and, to a lesser extent, moose (Alces alces). The disease was first reported in 1967 at a Colorado research facility in a captive mule deer from where it has spread efficiently through the USA [1]. The disease was identified in Canada in 1996 through an unfortunate importation of an apparently healthy deer from the USA, and the same scenario occurred in 2000 in South Korea after the importation of infected white-tailed deer, this time from Canada [2]. CWD is now spreading in the North American continent, being diagnosed in 28 USA states and four Canadian provinces.
In 2016, CWD was detected for the first time in Europe, in a Norwegian free-ranging reindeer (Rangifer tarandus tarandus) [3]. Later, moose cases were reported in Norway [4, 5], Sweden and Finland [6, 7]. In October 2017 a red deer (Cervus elaphus atlanticus) was detected with the disease on the West coast of Norway [8]. There are now three cases of CWD in red deer in Norway.
European red deer (Cervus elaphus hippelaphus), as well as Norwegian red deer (Cervus elaphus atlanticus) are closely related to North American elk (Cervus canadensis) also called wapiti, and experimental transmission studies have established that they are susceptible to the North American CWD strain CWD1 [9] and classical BSE (BSE-C) when challenged by the oral [10] and intracerebral route [11]. In addition, several cases of CWD have been reported in farmed red deer in South Korea and the United States [12,13,14].
The experimental transmission of a prion disease into individuals of the same species typically reproduces the disease. Inoculation of brain material of diseased animals from one species to another is generally a less efficient process, probably due to structural incompatibilities between the host cellular prion protein (PrPC) and the infecting pathological PrP assemblies (PrPSc) and this phenomenon is referred to as the species barrier. Thus, the strain of prions is also affecting interspecies transmission [15,16,17]. Prion strains are characterized by different biological properties that include tissue distribution of PrPSc, prion kinetics of replication and spread, affected brain areas and transmissibility to other species among other traits [18, 19]. These properties are usually maintained when transmitted in different hosts [18, 19]. Prion strains are thought to be encoded by different tridimensional PrPSc structures that harbour their own biological and physicochemical properties [18, 19].
In the mid-1980s, a large epidemic of BSE-C emerged in the UK and its spread was rapidly linked to the recycling and use of meat and bone meals (MBM) containing TSE-infected bovine material as feed additives within the ruminant feed chain. Recent evidence links atypical scrapie as a plausible origin for the epidemic [20,21,22,23]. Ten years later, frightening evidence was published linking the consumption of BSE-C contaminated material to a new human prion disease, called variant CJD (vCJD) [24, 25] that has been reported to have killed 224 people, mainly in the UK [26], and is thus the only zoonotic prion recognized to date. As BSE-C prions are zoonotic, there is concern about their possible circulation in other species, including free-ranging cervids, which are hunted and consumed. Therefore, any BSE-C suspected cases in any species must be referred to the European Reference Laboratory for prion diseases (EURL).
In this study, several strain-typing strategies were applied to the 2017 Norway red deer CWD case to unravel any possible link with BSE-C. The preliminary analysis of the biomolecular characteristics of the red deer CWD prions showed similarities with BSE-C when using discriminatory Western blot (WB) and immunohistochemistry. As BSE-C could not formally be excluded, the isolate was submitted to both the EURL and the Strain Typing Expert Group (STEG) in the UK for further in vitro molecular characterization. In parallel, the red deer CWD case was intracranially inoculated in a collection of rodent models available in several research laboratories to confirm or exclude the presence of BSE-C.
The goal of the present article is to summarize the findings obtained through these in vitro and in vivo techniques that aimed at investigating whether the red deer CWD prions could contain BSE-C infectivity or not. These in vivo experiments were expected to progress slowly since usually first passages in a heterologous PrPC context are not fully successful. Nevertheless, red deer CWD prion propagation in bovine PrP transgenic mice (TgBov) was readily achieved at the first passage. Strain characterization of the transmitted prions and further subpassage in TgBov resulted in strain characteristics incompatible with BSE-C.
Materials and methods
Ethics statement
All animal experiments were performed in compliance with the European Directives 86/609/EEC and 2010/63/EU. Experiments developed in CISA-INIA-CSIC (Madrid, Spain) were approved by the Committee on the Ethics of Animal Experiments of the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria and the General Directorate of the Madrid Community Government (permit numbers: PROEX 291–19, PROEX 047.1–21). In France, the animal experiments that are part of this study were approved by the local ENVT ethics committee (permit number: APAFIS #32336–2021070714414470 v3). In Italy, the experimental protocol was approved and supervised by the Service for Biotechnology and Animal Welfare of the Istituto Superiore di Sanità (ISS) and authorized by the Italian Ministry of Health (decree number 1119/2015-PR).
In UK, all animal procedures were performed under Home Office (United Kingdom) and local ethical review committee approval and in compliance with the Animal (Scientific Procedures) Act 1986.
Prion isolates
The red deer CWD case corresponds to a 16 years old adult female red deer shot by a hunter in October 2017 in western Norway that did not show signs of disease [8]. The cattle BSE-C cases used for comparative purposes in the bioassay experiments correspond to the brainstem of one naturally BSE-C affected cow (RQ 225:PG817/00), supplied by United Kingdom’s Animal and Plant Health Agency (APHA, former Veterinary Laboratory Agency or VLA) (BSE-C1) [27] and to a naturally infected cow (case PG1199/00) supplied by United Kingdom’s Animal and Plant Health Agency (APHA, former Veterinary Laboratory Agency or VLA) (BSE-C2) [28].
All TSE samples and healthy controls used in the PMCA experiments done in Nottingham University were provided by the AHVLA biological archive or through EURL. Samples isolate numbers: ovine scrapie 1, 2, 3 were PG0678/03, PG0013/17 and PG0014/17, respectively; caprine scrapie PG0848/02; red deer CWD 103, white-tailed deer CWD PG0047/18; reindeer CWD PG0043/18; elk CWD PG0044/18; experimental red deer BSE 312; experimental goat BSE PG1150/08; experimental ovine BSE PG1693/03; cattle BSE SE0253/005; uninfected red deer, reindeer, white-tailed deer and elk samples were PG0016/18, PG0015/17, PG0053/18 and PG0048/18, respectively; the red deer CWD isolate is the subject of this investigation.
Rodent models
Several rodent models (including transgenic mouse lines expressing PrP proteins from several mammal species) were used in this work to evaluate similarities and differences between red deer CWD prions and cattle BSE-C prions transmission properties (Table 1).
ELISA test
The TeSeE™ ELISA test (Bio-Rad, Marnes-la-Coquette, France) was used to detect PrPSc in red deer brain and lymphoid tissues according to the manufacturer’s instructions.
Red deer CWD prion immunohistochemistry (IHC)
Immunohistochemical labeling of PrPSc was performed in the brain, tonsil and lymph node as described previously [29]. Briefly, tissue sections on positive-charged glass slides (poly-l-lysine glass) were deparaffinized, rehydrated, immersed in formic acid 98% for 30 min, rinsed in water, then in Tris Buffer before boiled by hydrated autoclaving at 121 °C in 0.01 M citric acid, pH 6.1 for 30 min and cooled for 30 min. A commercially available kit (EnVisionTM + System HRP (AEC) DAKO, Glostrup, Denmark) was utilized using the monoclonal antibodies (mAbs) 12B2 (89-WGQGG-93 epitope of the human-PrPC sequence) [30] from Wageningen Bioveterinary Research (Lelystad, Netherlands), L42 (145-YEDRYY-150 epitope of the human-PrPC sequence) [31] from R-Biopharm (Darmstadt, Germany), or F99/97.6 (F99, 220-QYQRES-225 epitope of mule deer, conserved in Rocky Mountain elk, domestic sheep, and cattle) [32] from VMRD, Inc. (Pullman, WA, USA), at dilutions 1:5000, 1:2000 and 1:2000 respectively at 37 °C for 30 min. The sections were counterstained with hematoxylin. In each run, tissues from CWD-negative cervids were added as negative controls.
Bioassay
Six-to-seven-week-old individually identified female mice were anesthetized with isoflurane and inoculated with 2 mg equivalent of brain homogenate in the right parietal lobe by using a 25-gauge disposable hypodermic needle. Six-to-eight-week-old individually identified bank voles (BVs) were inoculated with 20 μL of 10% brain homogenates into the left cerebral hemisphere, under ketamine anesthesia (ketamine, 0.1 μg/g). Rodents were observed daily and their neurologic status was assessed twice a week. When the progression of a TSE disease was evident (before the severity of neurological impairment compromised their welfare, in particular, their ability to drink and feed adequately) or at the established experimental endpoint (> 650 days post-inoculation for mice, > 1000 days post-inoculation for BVs), animals were euthanized for ethical reasons. Then necropsy was performed and the brain collected and divided sagittally. Part of the brain was fixed by immersion in neutral-buffered 10% formalin (4% 2-formaldehyde) and used for histopathology studies while the rest of the tissue was frozen at −20 °C and used for proteinase K–resistant PrPSc (PrPres) detection by WB. Survival times were calculated as the time from inoculation to culling or death as mean days post inoculation ± standard deviation (dpi ± SD) for all the mice and the bank voles that scored positive for PrPres. Attack rate was defined as the proportion of animals that scored positive for PrPres divided by the number of inoculated animals. For primary transmissions, animals found dead or culled for intercurrent disease before 200 dpi and scoring negative at postmortem were excluded from analyses. Brain homogenates from PrPres-positive animals were used for further passaging. When all animals scored negative for PrPres on the primary passage, all PrPres-negative brains were pooled and used for the second passage.
Histologic examination and paraffin-embedded tissue blotting of transgenic mice samples
For samples obtained from transgenic mice, all procedures comprising the histopathologic analysis of mouse brains were performed as previously described [33]. Briefly, mouse brains were fixed in neutral-buffered 10% formalin (4% 2-formaldehyde) and embedded in paraffin. After deparaffinization, 4-μm-thick tissue slices were stained with hematoxylin and eosin and brain lesion profiles were represented according to published methods [34]). Paraffin-embedded tissue (PET) blots were conducted as previously described [2] using the Sha31 mAb [35]. Sha31 recognizes the 145-WEDRYYRE-152 epitope of the human-PrPC sequence, which is conserved in mouse, ovine and bovine sequences.
Western blotting
The original red deer case was analyzed by the ISS BSE Discriminatory WB to compare it with BSE-C. The ISS BSE Discriminatory WB is a discriminatory assay validated by EURL for the discrimination of BSE-C, BSE-L and BSE-H types in bovines. The principle of discrimination is based on the molecular weight (measured on the core mAb blot by comparison with molecular weight standard run in three lanes of each gel), differential N-terminal cleavage by PK (determined by using mAb 12B2) and the different glycosylation profile as determined with a core mAb. Brain homogenates were prepared at 10% w/v in 100 mM Tris–HCl pH 7.4 and 2% sarcosyl and treated with proteinase K at the final concentration of 200 μg/mL for 1 h at 38 °C. Protease treatment was stopped with 6 mmol/L PMSF (Sigma-Aldrich). Aliquots of samples were added with an equal volume of isopropanol/butanol (1:1 vol/vol) and centrifuged at 20 000 g for 10 min. The pellets were resuspended in denaturing sample buffer (NuPAGE LDS Sample Buffer; Life Technologies) and heated for 10 min at 90 °C. Samples were loaded in two replica gels (12% bis–Tris, Invitrogen) for electrophoresis with subsequent WB on polyvinylidene fluoride membranes using the Trans-Blot Turbo Transfer System (Bio-Rad) according to the manufacturer’s instructions. The blots were processed with L42 [31] and 12B2 mAb [30], by using the SNAP i.d. 2.0 system (Millipore, Burlington, MA, USA) according to the manufacturer’s instructions. After incubation with horseradish peroxidase–conjugated anti–mouse immunoglobulin (Pierce Biotechnology, Waltham, MA, USA) at 1:20000, the PrP bands were detected by using enhanced chemiluminescent substrate (SuperSignal Femto; Pierce Biotechnology) and ChemiDoc imaging system (Bio-Rad). The chemiluminescence signal was quantified by using Image Lab 5.2.1 (Bio-Rad).
For samples obtained from transgenic mice, frozen brain tissues (175 ± 20 mg) were homogenated in 5% glucose in distilled water in grinding tubes (Bio-Rad) adjusted to 10% (w/v) by using a TeSeE Precess 48TM homogenizer (Bio-Rad). Brain PrPres presence was WB detected by using the reagents of the ELISA commercial test TeSeE (Bio-Rad) as previously described [36] in 12% Bis–Tris Gel (Bio-Rad). PK treatment consisted of a final PK concentration of 40 ug/mL for 15 min at 37 °C. Proteins were electrophoretically transferred onto polyvinylidene fluoride membranes (Millipore,) and blocked overnight with 3% bovine serum albumin blocking buffer. We incubated membranes with Sha31 [35] and 12B2 [30] mAbs at a concentration of 1 μg/mL. Immunocomplexes were detected by membrane incubation for 1 h with horseradish peroxidase-conjugated anti-mouse IgG (GE Healthcare Amersham Biosciences) and development with enhanced chemiluminescence in ECL Select (GE Healthcare Amersham Biosciences). Images were captured using the ChemiDoc XRS + System and processed by using Image Lab 5.2.1 software (both Bio-Rad).
The overall biochemical approach to PrPres typing in BVs was as previously described [37] with minor modifications. PK was added at a final concentration of 100 μg/mL, and then the samples were incubated for 1 h at 55 °C with gentle shaking. After electrophoresis on 12% bis–Tris polyacrylamide gels (Invitrogen) and WB on polyvinylidene fluoride membranes using the Trans-Blot Turbo Transfer System (Bio-Rad), the blots were processed with anti-PrP mAbs by using the SNAP i.d. 2.0 system (Millipore). PrPres was detected with mAbs SAF84 (which recognizes the 163–169 amino acid residues of BV PrPC sequence) [38] and 12B2 [30]. PrP was visualized by enhanced chemiluminescent substrate and the ChemiDoc imaging system (Bio-Rad) by Image Lab5.2.1 Lab software (Bio-Rad). The chemiluminescent signal was quantified by Image Lab software (Bio-Rad).
Protein Misfolding Cyclic Amplification (PMCA)
The PMCA method was applied to the red deer isolate in 4 different laboratories (University of Nottingham, CIC bioGUNE, ENVT and CISA) using slightly different protocols (Table 2). CISA and Nottingham University protocols are further detailed because of their wider use in the experiments presented.
CISA protocol was as follows:
Brains from TgBov, TgOv-ARQ, TgOv-VRQ, TgMet129, TgPo [39] and Tga20 [40] mice were used to prepare the PMCA substrates. PMCA was performed as previously described [20, 41]. Briefly, PMCA reactions (50 μL final volume) were seeded with 5 μL of sample to be tested, diluted at 10–2 in the correspondent substrate. PMCA reactions were then subjected to 1 to 9 amplification rounds (depending on the experiment), each round comprising 48 cycles (20 s sonication, 29 min and 40 s incubation at 37 °C) in a Qsonica700 device. After each round, reaction products (1 volume) were mixed with fresh substrate (9 volumes) to seed the following round. The PMCA reaction products were analyzed by WB for the presence of PrPres as previously described [20, 41]. Unseeded controls (2 unseeded controls for 8 seeded reactions) were also included in each PMCA round.
University of Nottingham was as follows:
Briefly, each 100 μL sPMCA reaction was set up by adding the test sample at a 1-in-10 dilution into AHQ/AHQ sPMCA brain homogenate substrate. Within 0.2-mL PCR tubes, samples were sonicated (model 4000; Misonix) at 37 °C for 40 s at 200 W and repeated once every 30 min for 24 h (one sPMCA round). Samples were then diluted 1 in 3 with fresh substrate brain homogenate of the VRQ/VRQ genotype to a final volume of 100 μL, and subjected to another round of sPMCA. A total of 5 rounds of sPMCA were performed. AHQ/AHQ brain substrate was used at rounds 1, 3, and 5 and VRQ/VRQ substrate at rounds 2 and 4. Reaction products were digested with proteinase K (PK) and then analyzed by WB. PrPSc was detected using Sha31 mAb at a dilution of 1/80000 (Cayman Chemicals), then probed using a secondary goat anti-mouse horseradish peroxidase (HRP) conjugate at a 1/20000 dilution (Dako). Bands were visualized using an HRP chemiluminescent substrate (Geneflow) and a Photek imaging system. Gel images were analyzed with ImageJ software to estimate PrPSc signal values. Lanes on WB were defined manually, the area corresponding to the PrPSc bands defined and the pixel densities determined. A cut-off value for a positive PrPSc signal was estimated using the mean value plus 3 standard deviations (SDs) of the Sha31 mAb blots of negative-control sPMCA samples. Confirmatory analysis for samples that were positive for PrPSc was carried out by the digestion of samples with PK followed by the detection of prions by WB using the antibody P4 (89-WGQGGSH-95 epitope of human-PrPC sequence) at 1/2000 dilution (R-Biopharm). Each blot was additionally loaded with 2 μL of 10% ovine brain homogenate from scrapie isolate and/or BSE-C isolate as blotting control.
Thermostability assay
Brain sample heat treatment were performed as previously described [42]. Original red deer CWD and cattle BSE-C isolates as well as brain homogenates of TgBov mice infected with either red deer CWD prions or cattle BSE-C prions were aliquoted (500 μL), placed in safe-lock tubes (Eppendorf) and heated at 98 °C for 2 h in a thermocycler (Primus 96 Plus Thermal Cycler, MWG AG Biotech). Samples were removed, allowed to cool gradually to room temperature and harvested at −20 °C till use. Measurement of prion templating activity of heated samples and non-heated controls was performed by PMCA technique above described in a series of tenfold dilutions using TgBov brain substrate.
Results
The analysis on the red deer brain by TeSeE ELISA indicated that PrPSc was mostly detectable in the brainstem (data not shown) and to a lesser extent the midbrain whereas other brain regions were negative and no PrPSc was detected by ELISA in lymphoid tissue (data not shown), as previously reported for Norwegian moose [8]. The morphology of the obex was unfortunately not optimal, rendering the identification of the other anatomical areas speculative.
Red deer CWD prion characterization by IHC
In the medulla oblongata, the general IHC staining was quite pronounced using F99 and L42 mAbs (Figure 1). In some areas, only the intraneuronal staining, (as reported in Norwegian moose brain tissues [7]) was observed (Figure 1A), while in other areas both punctate, coarse granular and intra-neuronal staining were observed. Indeed, stained and non-stained neurons were detected next to each other (Figure 1B). With 12B2 mAb, the intraneuronal staining was absent, as it is characteristic of BSE-C [11].
Red deer medulla oblongata, IHC staining of PrPres. A Intraneuronal staining in one neuron (arrow) using L42mAb, × 40. B Stained (arrow) and unstained (arrowheads) neurons, and coarse granular deposits in the neuropil using L42mAb, × 60. C Intraneuronal, coarse granular staining in the neuropil and astrocyte-like deposits (empty arrow heads) using F99mAb, × 20 D No staining is to be observed in the tonsil using F99mAb, × 4.
Furthermore, astrocyte-like staining was observed in part of the medulla oblongata (Figure 1C) and in some limited areas in the frontal cortex. In the inner part of the cerebral cortex, F99 and 12B2 stained few PrPSc aggregates next to structures that might be considered as vessels. No staining was observed by IHC using F99, L42 or 12B2 mAbs in tonsils (Figure 1D), or popliteal, submandibular, retropharyngeal, and parotid lymph nodes (data not shown).
Red deer CWD prion characterization by WB
Preliminary WB analysis of brain PrPres from the red deer case revealed a classical three-band pattern characterized by a predominance of the di-glycosylated band, low molecular weight of the bands, and the absence of the 12B2 mAb epitope and no other additional fragments (data not shown).
As low molecular weight fragments, absence of the 12B2 mAb epitope, absence of an additional 14 kDa C-terminal PrPres fragment and high glycosylation status are all features which characterize BSE-C PrPres in cattle and other species (i.e. in small ruminants), we analyzed the red deer case along with reindeer and moose isolates from Norway by the ISS BSE Discriminatory WB to compare it with BSE-C (Figure 2).
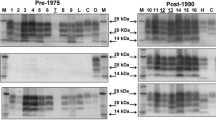
ISS BSE Discriminatory WB analysis of brain PrPres of TSE cases. Samples, including reindeer, moose and red deer CWD as well as cattle BSE-C and sheep scrapie, were loaded in two replica gels, probed with mAbs L42 and 12B2, as indicated on the left of the blots. Both, moose (lanes 2 and 6) and bovine (lanes 3 and 5) samples have been loaded at different concentrations, in order to maximize the chance of having PrPSc levels directly comparable to that of the red deer. The proportion of diglycosylated, monoglycosylated and unglycosylated PrPres bands in each sample were as follows: 50:36:14 for reindeer CWD; 40:32:28 for moose CWD; 61:29:10 for BSE-C; 69:23:8 for red deer CWD and 47:35:18 for sheep scrapie. Concentrations of samples (mg eq/lane) were indicated at the bottom of the blots. Protein standards are loaded in lanes indicated as “M” (10, 15, 20, 25, and 37 kDa).
The discriminatory WB basically confirmed previous observations. Indeed, both moose and red deer PrPres showed a molecular weight lower than scrapie and reindeer PrPres and similar to BSE-C; accordingly, they also showed loss of signal with 12B2 mAb. Interestingly, this analysis showed that red deer CWD prions were highly glycosylated, another feature shared with BSE-C prions. Red deer CWD was slightly more glycosylated than BSE-C, while ovine classical scrapie, reindeer CWD and moose CWD samples were much less glycosylated, with only 50% or less of PrPres being diglycosylated.
Red deer CWD prion characterization by PMCA
In order to characterize red deer CWD prions, the original isolate was subjected to PMCA, and although the laboratories used slightly different protocols and different substrates, the results were similar. In summary, in vitro propagation in bovine substrate (perfused cow brain homogenate) did result in efficient prion propagation of experimental red deer inoculated BSE-C, porcine BSE-C and sheep BSE-C as well as human vCJD (Additional file 1). By contrast, the original red deer CWD prions were not able to be amplified in bovine substrate (Additional file 1). Application of a serial PMCA assay (sPMCA) [43] using ovine substrate, alternating through rounds with distinct PrP genotypes (AHQ/AHQ and VRQ/VRQ) detected C-BSE from bovine, ovine, caprine and cervid sources but did not amplify the red deer CWD isolate (Additional file 2). In line with these results, red deer CWD prions were not able to be amplified in substrate coming from transgenic mouse TgBov and TgOv-ARQ, but amplification was achieved in substrate coming from transgenic mouse Tga20 (data not shown).
Red deer CWD prion characterization by bioassay
As part of a strain-typing assay for European CWD strain typing, the red deer CWD isolate was intracranially inoculated in a collection of rodent models susceptible to prion diseases including transgenic mouse lines expressing PrP proteins from different mammal species and BV (Table 3). Rodent models known to be highly susceptible (TgBov and TgOv-ARQ), medium susceptible (TgOv-VRQ, TgPo and TgMet129 lines) or not susceptible to BSE-C (BVs and TgVal129) were challenged, and the outcome compared with BSE-C.
The first passages of the red deer CWD isolate into different rodent models did not transmit the disease, contrary to BSE-C, except in the TgBov mice (Table 3). By contrast, the first passage in BV resulted in successful transmission for the red deer CWD prions (Table 3). Second passages for all these transmissions are currently ongoing. Focusing on the results in TgBov mice, red deer CWD prions were readily transmitted reaching 100% attack rates even at the first passage (Table 3). A mean survival time of 437 ± 41 dpi was obtained for this transmission. The second and third passages were also very efficient with a mean survival time shortened to 265 ± 10 and 223 ± 6 dpi respectively (Table 3). PrPres signature of the original red deer CWD isolate was characterized by predominance of the di-glycosylated band as well as a 19 kDa molecular mass of the non-glycosylated band (Figure 2). Such configuration was not detected by the 12B2 mAb (Figure 2) as is the case of cattle BSE-C prions and other prion strains like Scandinavian moose atypical/sporadic CWD cases. After transmission in TgBov transgenic mice, PrPres signature shared the same biochemical features with cattle BSE-C prions transmitted in TgBov both in the first and second passage (Figure 3A). Brain PrPres signature in the inoculated BV (Figure 4) was different from the brain PrPres signature found in the original red deer CWD isolate, as vole PrPres was rather well detectable with 12B2, implying a more N-terminal PK cleavage in BVs than in red deer (see Figure 2 for comparison). SAF84 mAb detected low amounts of CTF-13 (Figure 4), a feature reported for Nordic CWD moose transmission in this model [5].
Red deer CWD and cattle BSE-C transmission in TgBov mice. A Brain PrPres WB with Sha31 and 12B2 mAbs. Concentrations of samples (mg eq/lane) were indicated at the bottom of the blots. Protein standards are indicated as “M” (40, 30 and 20 kDa). B PET blotting images with Sha31 mAb. C Brain lesion profiles.
Red deer CWD transmission in BV. Representative WB from brains of BVs inoculated with red deer CWD. PrPres in BV brain was detected by SAF84 and 12B2 mAbs. The arrow indicates the additional C-terminal fragment (CTF13) detected by SAF84 mAb. Protein standards are indicated as “M” (25, 20, 15 and 10 kDa).
PET blotting performed on the brains of TgBov mice inoculated with red deer CWD also showed similarities with the TgBov mice inoculated with cattle BSE-C (Figure 3B). Dense deposits in the whole brain characterized the PrPres deposition pattern. In the same line of results, brain lesion profiles of TgBov mice inoculated with red deer CWD or cattle BSE-C prions were also similar (Figure 3C). Profiles are mainly characterized by intense vacuolation in the dorsal medulla, thalamus, septum, and the mesencephalic tegmentum brain areas (Figure 3C).
The striking resemblance of the red deer CWD isolate with cattle BSE-C prions in their original PrPres biochemical features as well as overlapping phenotypes after transmission in TgBov mice was worrisome. In contrast to the findings in TgBov, efficient transmission in BV (expected to be resistant to BSE-C), would suggest biological differences between the red deer CWD prions and BSE-C prions. Thus, these discrepancies needed further clarification. For that purpose, the transmission features of the red deer CWD prions and cattle BSE-C prions were investigated in a larger panel of transgenic mice expressing PrP protein from several mammal species and were compared according to their reported susceptibility to BSE-C (Table 3). Cattle BSE-C prions are known to be able to cross several species barriers maintaining their original strain features. Successful transmission of cattle BSE-C prions was obtained for the high-susceptible BSE-C models such as TgBov and TgOv-ARQ as well as in medium susceptible BSE-C models like TgOv-VRQ, TgPo, TgMet129 [4x] and TgMet129 [6x] as expected (Table 3). In the case of human PrP Met129 transgenic mice, adaptation was achieved at the completion of the second passage since a species barrier exists from cattle BSE-C prions for transmission in Met129 human PrP as was already reported [44, 45]. BSE-C prions were not transmissible in resistant BSE-C models: BV, as it was already reported for Met109 BVs [28], and TgVal129 (Table 3). TgVal129 remained uninfected after cattle BSE-C challenge since Val129 variant is a strong protector against animal BSE-C prions [45]. By contrast, red deer CWD prions were not able to directly infect any of the challenged transgenic mouse lines apart from TgBov mice and BV (Table 3), stating clear differences between red deer CWD prions and cattle BSE-C prions. Indeed, red deer CWD prions and cattle BSE-C prions clearly differ in their transmissibility to BV and other models like TgOv-ARQ, TgOv-VRQ, TgPo and TgMet129 mice lines, indicating that these strains are different (Table 3).
Characterization of the red deer CWD prions passaged into TgBov by sPMCA
Since red deer CWD prions were transmitted in TgBov very similarly to BSE-C and the phenotypes of disease were very similar, we aimed to deepen the characterization of these TgBov-adapted strains in order to determine if the same BSE-C strain was isolated from both sources. Red deer CWD prions and cattle BSE-C prions in vitro amplification properties after adaptation to TgBov were compared by in vitro sPMCA. Red deer CWD prions and cattle BSE-C prions were subjected to several PMCA rounds using brains from Tga20, TgOv-VRQ, TgOv-ARQ, TgBov, TgMet129 and TgPo mice as substrates (Figure 5).
Red deer CWD and cattle BSE-C prions passaged into TgBov mice after PMCA amplification. Duplicates for each combination of inoculum/substrate were analyzed. The first round used inoculum diluted at 10–2. Several substrates were used as indicated under each panel: TgBov, TgOv-ARQ, TgOv-VRQ, Tga20, TgPo, TgMet129. A Schematic representation of the PMCA results. B Representative PrPres profile of the PMCA amplified prions after 9 rounds blotted with Sha31 mAb. Cattle BSE-C and red deer CWD prions show the same PrPres profile. Protein standards are indicated as “M” (40, 30 and 20 kDa).
At the completion of round 1, clear differences were seen between the amplification properties of the two TgBov-adapted strains. Both TgBov-adapted cattle BSE-C prions and red deer CWD prions were readily amplified in TgBov substrate as expected (Figure 5A) showing the same PrPres profile (Figure 5B). However, they behaved differently when analyzed in other substrates. While cattle BSE-C prions were easily amplified in TgOv-ARQ substrate from round one, red deer CWD prions required at least five rounds of PMCA to amplify (Figure 5A). By contrast, red deer CWD prions were amplified in Tga20 slightly better than cattle BSE-C prions (round 1 vs round 2) (Figure 5A). Amplification in TgOv-VRQ substrate required several PMCA rounds for both inocula to be achieved (round 3 for cattle BSE-C prions and round 4 for red deer CWD prions). In all substrates that allowed the propagation of both TgBov-adapted inocula, the PrPres signature of cattle BSE-C and red deer CWD prions were similar (Figure 5B).
Most interestingly, while cattle BSE-C prions were amplified with TgPo and TgMet129 substrates (round 7), red deer CWD prions were not amplified even after nine PMCA rounds in the TgPo and TgMet129 substrates (Figure 5A). These results go in line with the different behavior of red deer CWD prions and BSE-C prions from different origins when propagation was assessed with the bovine substrate (perfused cow brain) (Additional file 1).
Red deer CWD thermostability assay
We and others have previously reported that prion resistance against heat treatment, or thermostability, is a valuable tool for prion strain typing [42]. By PMCA and bioassay studies, murine BSE-C infectivity and templating activity proved resistant to 98 °C treatment for 2 h [42]. Thus, the same heat treatment was applied to cattle BSE-C and red deer CWD prions after adaptation to TgBov and their templating activity in TgBov substrate was assayed before and after heat treatment (Figure 6).
As expected, cattle BSE-C prions passaged in TgBov were thermoresistant. The templating activity demonstrated by PMCA was not affected by the heat treatment, being detectable up to 10–8 dilution in both, heat treated and untreated samples (Figure 6A). It is worth mentioning that the level of non-amplified inoculum signal (frozen) was observed to be minimal in both the Cattle BSE and red deer CWD samples, displaying only a faint signal at a 10–1 dilution, with no further signal observed beyond this dilution (data not shown). The PrPres signature obtained after the thermostability assay was identical to its non-heated control counterpart (Figure 6B). By contrast, red deer CWD prions passaged in TgBov proved to be thermolabile since its templating activity was fully prevented by heat treatment (Figure 6A). The non-heated control prions could be amplified until 10–4 dilution showing a PrPres signature identical to the one of cattle BSE-C prions passaged in TgBov as previously reported (Figure 6B). When comparing the templating activity of both non-heated TgBov-adapted inocula, their seeding potency is different by four orders of magnitude. Such difference is not consistent with the two inocula containing the same prion strain. This observation, together with the distinct transmission properties in a panel of transgenic models, are strong evidences that BSE-C and red deer CWD prions have different strain properties.
Discussion
Some of the original red deer CWD isolate features (WB PrPres pattern, type of PrPSc deposition by IHC), as well as its transmission properties in TgBov suggested the presence of BSE-C prions in the red deer. To fully investigate the possible origin of this case, in vivo and in vitro strain typing approaches were applied to red deer CWD prions. The study of prion transmission properties in a collection of rodent models expressing the prion protein from different species is the best method for definitive strain typing. In vitro approaches such as PMCA allow faster results than the bioassay and usually provide a faithful mimic of the bioassay. In this work, thanks to the collaboration of different laboratories, we combined these approaches to compile the deepest possible investigation of the strain properties of red deer CWD prions in comparison to BSE-C prions. The results allow concluding solidly and reliably that the first red deer case of CWD detected in Norway was not caused by infection with the BSE-C prion agent. The obtained results suggest that the similarities, even after transmission in TgBov, were due to a phenotypic convergence phenomenon rather than strain identity. Phenotypic convergence can be described as the independent evolution of similar phenotypes [46]. This phenomenon is not unusual in the prion field, especially for experimental transmissions. For instance, when transmitted in TgOv-VRQ, both BSE-C and atypical BSE-L can phenotypically converge [36]. Indeed, the red deer CWD case is the first phenotypic mimic of BSE-C ever detected in nature. Previous suspects of natural BSE-C mimics, vCJD and BSE-C cases in goats, were eventually identified as true BSE-C prions by strain typing [24, 47, 48]. Another natural prion disease of small ruminants, CH1641, was initially found to have PrPres properties reminding C-BSE [49] and was later discriminated from BSE on both, molecular and biological grounds [50,51,52]. This red deer case represents a similar situation, as PrPres resemblance with C-BSE did not imply strain similarity and was indeed accompanied by obvious differences in the biological properties.
Even if they are not BSE-C, red deer CWD prions proved to be easily transmissible in vivo in TgBov, like cattle BSE-C isolates. This is of great concern because the TgBov mice models are efficiently used to predict cattle susceptibility to prion agents. Therefore, these results might suggest rather easy transmissibility of red deer CWD prions to cattle. Such a possibility will be of special importance for areas in which free-ranging cervids may be in contact with farmed species like cattle, sheep, goats or pigs [53]. Interestingly, in vitro amplification in bovine PrP context (cattle brain as substrate) was not successful. Similar results were observed in another work assessing European CWD potential to cross several species barriers [54]. This interesting discrepancy between in vitro PMCA and in vivo bioassays suggests that some in vivo cofactor key for crossing this species barrier is lacking in the in vitro system. Other possible explanation could be that the cattle brain may not have a high enough PrP expression level to support the propagation of the red deer CWD prion strain (successful in TgBov mice due to their high bovine PrP expression levels), that is probably less efficient in propagating than BSE-C prions. The in vivo bioassay in human PrP transgenic mice shows that there is a high transmission barrier for red deer CWD prions in humans, this work being the first assessment of red deer CWD zoonotic potential. At least red deer CWD prions transmit less efficiently than BSE-C prions to both TgMet129 and TgVal129 mice, suggesting that they have a lower zoonotic potential than BSE-C prions. Similar results have been obtained for other European CWD isolates (reindeer and moose) by performing bioassay in Met129 Tg35 and Val129 Tg152 human PrP transgenic mouse lines [55]. However, second passages might be needed to fully clarify European CWD zoonotic potential including isolates from all involved cervid species. In addition, the ability to cross species barriers can be modified after adaptation to a new species. This is of significant importance in the case of the human species barrier and prion zoonotic potential. For instance, BSE-C prions increase their virulence towards human PrP transgenic mice when primarily transmitted in sheep, goats and transgenic mice expressing sheep or goat PrP [44]. In addition, atypical BSE prions change their zoonotic abilities once adapted to transgenic mice expressing sheep PrP, producing prion agents that resemble sporadic CJD [36]. Thus, the possible adaptation of red deer CWD prions to other species, cattle being the most probable one as suggested by our results, should be monitored in order to be detected and abrogated as soon as possible.
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its Additional files) and will be available to those who request them.
References
Williams ES, Young S (1980) Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16:89–98
Sohn HJ, Kim JH, Choi KS, Nah JJ, Joo YS, Jean YH, Ahn SW, Kim OK, Kim DY, Balachandran A (2002) A case of chronic wasting disease in an elk imported to Korea from Canada. J Vet Med Sci 64:855–858
Benestad SL, Mitchell G, Simmons M, Ytrehus B, Vikøren T (2016) First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet Res 47:88
Pirisinu L, Tran L, Chiappini B, Vanni I, Di Bari MA, Vaccari G, Vikøren T, Madslien KI, Våge J, Spraker T, Mitchell G, Balachandran A, Baron T, Casalone C, Rolandsen CM, Røed KH, Agrimi U, Nonno R, Benestad SL (2018) Novel type of chronic wasting disease detected in moose (Alces alces), Norway. Emerg Infect Dis 24:2210–2218
Nonno R, Di Bari MA, Pirisinu L, D’Agostino C, Vanni I, Chiappini B, Marcon S, Riccardi G, Tran L, Vikøren T, Våge J, Madslien K, Mitchell G, Telling GC, Benestad SL, Agrimi U (2020) Studies in bank voles reveal strain differences between chronic wasting disease prions from Norway and North America. Proc Natl Acad Sci U S A 117:31417–31426
Ågren EO, Sörén K, Gavier-Widén D, Benestad SL, Tran L, Wall K, Averhed G, Doose N, Våge J, Nöremark M (2021) First detection of chronic wasting disease in moose (Alces alces) in Sweden. J Wildl Dis 57:461–463
Tranulis MA, Gavier-Widén D, Våge J, Nöremark M, Korpenfelt SL, Hautaniemi M, Pirisinu L, Nonno R, Benestad SL (2021) Chronic wasting disease in Europe: new strains on the horizon. Acta Vet Scand 63:48
Vikøren T, Våge J, Madslien KI, Røed KH, Rolandsen CM, Tran L, Hopp P, Veiberg V, Heum M, Moldal T, Neves CGD, Handeland K, Ytrehus B, Kolbjørnsen Ø, Wisløff H, Terland R, Saure B, Dessen KM, Svendsen SG, Nordvik BS, Benestad SL (2019) First detection of chronic wasting disease in a wild red deer (Cervus elaphus) in Europe. J Wildl Dis 55:970–972
Balachandran A, Harrington NP, Algire J, Soutyrine A, Spraker TR, Jeffrey M, González L, O’Rourke KI (2010) Experimental oral transmission of chronic wasting disease to red deer (Cervus elaphus elaphus): early detection and late-stage distribution of protease-resistant prion protein. Can Vet J 51:169–178
Dagleish MP, Martin S, Steele P, Finlayson J, Eaton SL, Sisó S, Stewart P, Fernández-Borges N, Hamilton S, Pang Y, Chianini F, Reid HW, Goldmann W, González L, Castilla J, Jeffrey M (2015) Susceptibility of European red deer (Cervus elaphus elaphus) to alimentary challenge with bovine spongiform encephalopathy. PLoS One 10:e0116094
Martin S, Jeffrey M, González L, Sisó S, Reid HW, Steele P, Dagleish MP, Stack MJ, Chaplin MJ, Balachandran A (2009) Immunohistochemical and biochemical characteristics of BSE and CWD in experimentally infected European red deer (Cervus elaphus elaphus). BMC Vet Res 5:26
Schwabenlander MD, Culhane MR, Hall SM, Goyal SM, Anderson PL, Carstensen M, Wells SJ, Slade WB, Armién AG (2013) A case of chronic wasting disease in a captive red deer (Cervus elaphus). J Vet Diagn Invest 25:573–576
Walther I, Staskevicius A, Soutyrine A, Mitchell G (2019) Diagnosis of chronic wasting disease in a herd of farmed red deer (Cervus elaphus) [abstract] Prion Edmonton
Lee YH, Sohn HJ, Kim MJ, Kim HJ, Lee WY, Yun EI, Tark DS, Cho IS, Balachandran A (2013) Strain characterization of the Korean CWD cases in 2001 and 2004. J Vet Med Sci 75:95–98
Béringue V, Herzog L, Reine F, Le Dur A, Casalone C, Vilotte JL, Laude H (2008) Transmission of atypical bovine prions to mice transgenic for human prion protein. Emerg Infect Dis 14:1898–18901
Bessen RA, Marsh RF (1992) Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J Gen Virol 73:329–334
Lambert ZJ, Greenlee JJ, Cassmann ED, West Greenlee MH (2021) Differential accumulation of misfolded prion strains in natural hosts of prion diseases. Viruses 13:2453
Bessen RA, Marsh RF (1994) Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol 68:7859–7868
Telling GC, Parchi P, DeArmond SJ, Cortelli P, Motagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB (1996) Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079–2082
Huor A, Espinosa JC, Vidal E, Cassard H, Douet JY, Lugan S, Aron N, Marín-Moreno A, Lorenzo P, Aguilar-Calvo P, Badiola J, Bolea R, Pumarola M, Benestad SL, Orge L, Thackray AM, Bujdoso R, Torres JM, Andréoletti O (2019) The emergence of classical BSE from atypical/Nor98 scrapie. Proc Natl Acad Sci U S A 116:26853–26862
Marín B, Otero A, Lugan S, Espinosa JC, Marín-Moreno A, Vidal E, Hedman C, Romero A, Pumarola M, Badiola JJ, Torres JM, Andréoletti O, Bolea R (2021) Classical BSE prions emerge from asymptomatic pigs challenged with atypical/Nor98 scrapie. Sci Rep 11:17428
Betancor M, Marín B, Otero A, Hedman C, Romero A, Barrio T, Sevilla E, Douet JY, Huor A, Badiola JJ, Andréoletti O, Bolea R (2023) Detection of classical BSE prions in asymptomatic cows after inoculation with atypical/Nor98 scrapie. Vet Res 54:89
Konold T, Spiropoulos J, Hills J, Abdul H, Cawthraw S, Phelan L, McKenna A, Read L, Canoyra S, Marín-Moreno A, Torres JM (2023) Experimental transmission of ovine atypical scrapie to cattle. Vet Res 54:98
Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey LJ, Lantos P (1997) The same prion strain causes vCJD and BSE. Nature 389:448–450
Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ (1997) Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 389:498–501
The national CJD research & surveillance unit (NCJDRSU). The University of Edinburgh. http://www.cjd.ed.ac.uk/surveillance/data-and-reports. Accessed 17 Jan 2024
Torres JM, Espinosa JC, Aguilar-Calvo P, Herva ME, Relaño-Ginés A, Villa-Diaz A, Morales M, Parra B, Alamillo E, Brun A, Castilla J, Molina S, Hawkins SA, Andréoletti O (2014) Elements modulating the prion species barrier and its passage consequences. PLoS One 9:e89722
Espinosa JC, Nonno R, Di Bari M, Aguilar-Calvo P, Pirisinu L, Fernández-Borges N, Vanni I, Vaccari G, Marín-Moreno A, Frassanito P, Lorenzo P, Agrimi U, Torres JM (2016) PrPC governs susceptibility to prion strains in bank vole, while other host factors modulate strain features. J Virol 90:10660–10669
Sola D, Tran L, Våge J, Madslien K, Vuong TT, Korpenfelt SL, Ågren EO, Averhed G, Nöremark M, Sörén K, Isaksson M, Acín C, Badiola JJ, Gavier-Widén D, Benestad SL (2023) Heterogeneity of pathological prion protein accumulation in the brain of moose (Alces alces) from Norway, Sweden and Finland with chronic wasting disease. Vet Res 54:74
Yull HM, Ritchie DL, Langeveld JP, van Zijderveld FG, Bruce ME, Ironside JW, Head MW (2006) Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am J Pathol 168:151–157
Harmeyer S, Pfaff E, Groschup MH (1998) Synthetic peptide vaccines yield monoclonal antibodies to cellular and pathological prion proteins of ruminants. J Gen Virol 79:937–945
Spraker TR, O’Rourke KI, Balachandran A, Zink RR, Cummings BA, Miller MW, Powers BE (2002) Validation of monoclonal antibody F99/97.6.1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J Vet Diagn Invest 14:3–7
Andréoletti O, Berthon P, Levavasseur E, Marc D, Lantier F, Monks E, Elsen JM, Schelcher F (2002) Phenotyping of protein-prion (PrPsc)-accumulating cells in lymphoid and neural tissues of naturally scrapie-affected sheep by double-labeling immunohistochemistry. J Histochem Cytochem 50:1357–1370
Fraser H, Dickinson AG (1968) The sequential development of the brain lesion of scrapie in three strains of mice. J Comp Pathol 78:301–311
Féraudet C, Morel N, Simon S, Volland H, Frobert Y, Créminon C, Vilette D, Lehmann S, Grassi J (2005) Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J Biol Chem 280:11247–11258
Marín-Moreno A, Huor A, Espinosa JC, Douet JY, Aguilar-Calvo P, Aron N, Píquer J, Lugan S, Lorenzo P, Tillier C, Cassard H, Andréoletti O, Torres JM (2020) Radical change in zoonotic abilities of atypical BSE prion strains as evidenced by crossing of sheep species barrier in transgenic mice. Emerg Infect Dis 26:1130–1139
Pirisinu L, Marcon S, Di Bari MA, D’Agostino C, Agrimi U, Nonno R (2013) Biochemical characterization of prion strains in bank voles. Pathogens 2:446–456
Demart S, Fournier JG, Creminon C, Frobert Y, Lamoury F, Marce D, Lasmézas C, Dormont D, Grassi J, Deslys JP (1999) New insight into abnormal prion protein using monoclonal antibodies. Biochem Biophys Res Commun 265:652–657
Le Dur A, Béringue V, Andréoletti O, Reine F, Laï TL, Baron T, Bratberg B, Vilotte JL, Sarradin S, Benestad SL, Laude H (2005) A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc Natl Acad Sci U S A 102:16031–16036
Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C (1996) Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J 115:1255–1264
Espinosa JC, Marín-Moreno A, Aguilar-Calvo P, Benestad SL, Andréoletti O, Torres JM (2021) Porcine prion protein as a paradigm of limited susceptibility to prion strain propagation. J Infect Dis 223:1103–1112
Marín-Moreno A, Aguilar-Calvo P, Moudjou M, Espinosa JC, Béringue V, Torres JM (2019) Thermostability as a highly dependent prion strain feature. Sci Rep 9:11396
Gough KC, Bishop K, Maddison BC (2014) Highly sensitive detection of small ruminant bovine spongiform encephalopathy within transmissible spongiform encephalopathy mixes by serial protein misfolding cyclic amplification. J Clin Microbiol 52:3863–3868
Padilla D, Béringue V, Espinosa JC, Andréoletti O, Jaumain E, Reine F, Herzog L, Gutierrez-Adan A, Pintado B, Laude H, Torres JM (2011) Sheep and goat BSE propagate more efficiently than cattle BSE in human PrP transgenic mice. PLoS Pathog 7:e1001319
Fernández-Borges N, Espinosa JC, Marín-Moreno A, Aguilar-Calvo P, Asante EA, Kitamoto T, Mohri S, Andréoletti O, Torres JM (2017) Protective effect of Val129-PrP against bovine spongiform encephalopathy but not variant Creutzfeldt-Jakob disease. Emerg Infect Dis 23:1522–1530
Rosenblum EB, Parent CE, Brandt EE (2014) The molecular basis of phenotypic convergence. Annu Rev Ecol Evol Syst 45:203–226
Spiropoulos J, Lockey R, Sallis RE, Terry LA, Thorne L, Holder TM, Beck KE, Simmons MM (2011) Isolation of prion with BSE properties from farmed goat. Emerg Infect Dis 17:2253–2261
Eloit M, Adjou K, Coulpier M, Fontaine JJ, Hamel R, Lilin T, Messiaen S, Andréoletti O, Baron T, Bencsik A, Biacabe AG, Béringue V, Laude H, Le Dur A, Vilotte JL, Comoy E, Deslys JP, Grassi J, Simon S, Lantier F, Sarradin P (2005) BSE agent signatures in a goat. Vet Rec 156:523–524
Hope J, Wood SC, Birkett CR, Chong A, Bruce ME, Cairns D, Goldmann W, Hunter N, Bostock CJ (2000) Molecular analysis of ovine prion protein identifies similarities between BSE and an experimental isolate of natural scrapie, CH1641. J Gen Virol 81:851
Stack MJ, Chaplin MJ, Clark J (2002) Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep-passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol 104:279–286
Pirisinu L, Migliore S, Di Bari MA, Esposito E, Baron T, D’Agostino C, De Grossi L, Vaccari G, Agrimi U, Nonno R (2011) Molecular discrimination of sheep bovine spongiform encephalopathy from scrapie. Emerg Infect Dis 17:695–698
Taema MM, Maddison BC, Thorne L, Bishop K, Owen J, Hunter N, Baker CA, Terry LA, Gough KC (2012) Differentiating ovine BSE from CH1641 scrapie by serial protein misfolding cyclic amplification. Mol Biotechnol 51:233–239
Escobar LE, Pritzkow S, Winter SN, Grear DA, Kirchgessner MS, Dominguez-Villegas E, Machado G, Townsend Peterson A, Soto C (2020) The ecology of chronic wasting disease in wildlife. Biol Rev Camb Philos Soc 95:393–408
Pritzkow S, Gorski D, Ramirez F, Telling GC, Benestad SL, Soto C (2022) North American and Norwegian chronic wasting disease prions exhibit different potential for interspecies transmission and zoonotic risk. J Infect Dis 225:542–551
Wadsworth JDF, Joiner S, Linehan JM, Jack K, Al-Doujaily H, Costa H, Ingold T, Taema M, Zhang F, Sandberg MK, Brandner S, Tran L, Vikøren T, Våge J, Madslien K, Ytrehus B, Benestad SL, Asante EA, Collinge J (2021) Humanised transgenic mice are resistant to chronic wasting disease prions from Norwegian reindeer and moose. J Infect Dis 226:933–937
Cartoni C, Schininà ME, Maras B, Nonno R, Vaccari G, Di Baria MA, Conte M, Liu QG, Lu M, Cardone F, Windl O, Pocchiari M, Agrimi U (2005) Identification of the pathological prion protein allotypes in scrapie-infected heterozygous bank voles (Clethrionomys glareolus) by high-performance liquid chromatography-mass spectrometry. J Chromatogr A 1081:122–126
Aguilar-Calvo P, Espinosa JC, Pintado B, Gutiérrez-Adán A, Alamillo E, Miranda A, Prieto I, Bossers A, Andréoletti O, Torres JM (2014) Role of the goat K222-PrPC polymorphic variant in prion infection resistance. J Virol 88:2670–2676
Castilla J, Gutiérrez Adán A, Brun A, Pintado B, Ramírez MA, Parra B, Doyle D, Rogers M, Salguero FJ, Sánchez C, Sánchez-Vizcaíno JM, Torres JM (2003) Early detection of PrPres in BSE-infected bovine PrP transgenic mice. Arch Virol 148:677–691
Castilla J, Gutiérrez-Adán A, Brun A, Doyle D, Pintado B, Ramírez MA, Salguero FJ, Parra B, Segundo FD, Sánchez-Vizcaíno JM, Rogers M, Torres JM (2004) Subclinical bovine spongiform encephalopathy infection in transgenic mice expressing porcine prion protein. J Neurosci 24:5063–5069
Cassard H, Torres JM, Lacroux C, Douet JY, Benestad SL, Lantier F, Lugan S, Lantier I, Costes P, Aron N, Reine F, Herzog L, Espinosa JC, Béringue V, Andréoletti O (2014) Evidence for zoonotic potential of ovine scrapie prions. Nat Commun 5:5821
Otero A, Barrio T, Eraña H, Charco JM, Betancor M, Díaz-Domínguez CM, Marín B, Andréoletti O, Torres JM, Kong Q, Badiola JJ, Bolea R, Castilla J (2022) Glycans are not necessary to maintain the pathobiological features of bovine spongiform encephalopathy. PLoS Pathog 18:e1010900
Acknowledgements
The authors wish to thank the Section for Pathology at the NVI for testing TSE samples in the frame of the CWD surveillance programme, funded by the Norwegian Food Safety Authority.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The work was partially financed by funding from ICRAD, an ERA-NET co-funded under European Union’s Horizon 2020 research and innovation programme (https://ec.europa.eu/programmes/horizon2020/en), under Grant Agreement n° 862605, the internal CWD project 12081 at the Norwegian Veterinary institute, the French research project “Assessing and controlling the risks associated with the emergence of Chronic Wasting Disease in Europe”- EU-CWD, ANR-20-CE35-0015, the Spanish research projects PID2019-105837RB-I00 financed by MCIU/AEI/10.13039/501100011033, PCI2020-120680-2 financed by MCIN/ AEI/10.13039/501100011033 and PID2021-122201OB-C21, and by the EU “NextGenerationEU”/PRTR and the Italian research project “Assessment of the zoonotic potential of emerging prion diseases of animals” (project code ISS20-97e1d82bda5a) granted by Istituto Superiore di Sanità.
Author information
Authors and Affiliations
Contributions
Conception and design of the work was done by JMT, SLB and OA. The manuscript was drafted by JMT, AMM and SLB and revised by all the authors. All authors contributed to data acquisition, analysis and interpretation. Necropsy of red deer case was performed by JV. Diagnosis of the red deer case was performed by SLB and LT. Immunohistochemistry was performed by SLB, LT and JS. Bioassays in transgenic mice were performed by AMM, JMT, JCE, TB, NFB, AH and OA. Bioassays in bank vole were performed by LP, MD, CD and RN. Thermostability assays were performed by AMM, SC and JMT. Discriminatory PMCAs were performed by AMM, HE, JC, BM, NFB, SC, NJG, JCE, KB, KG, OA and JMT. Discriminatory WB were performed by LP and RN. All authors agreed with each author’s individual contributions and ensured that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved and that the resolution was documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were performed in compliance with the European Directives 86/609/EEC and 2010/63/EU. Experiments developed in CISA-INIA-CSIC (Madrid, Spain) were approved by the Committee on the Ethics of Animal Experiments of the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria and the General Directorate of the Madrid Community Government (permit numbers: PROEX 291-19, PROEX 047.1-21). In France, the animal experiments that are part of this study were approved by the local ENVT ethics committee (permit number: APAFIS #32336-2021070714414470 v3). In Italy, the experimental protocol was approved and supervised by the Service for Biotechnology and Animal Welfare of the Istituto Superiore di Sanità (ISS) and authorized by the Italian Ministry of Health (decree number 1119/2015-PR).
Competing interests
The authors declare that they have no competing interests.
Additional information
Handling editor: Vincent Béringue.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. PMCA amplification in perfused bovine brain homogenate
. PrPres profile of different BSE-C adapted seeds (pig, human, cattle, red deer and sheep), CWD cases from different cervid species, including red deer, and non-infected deer samples in 10% perfused bovine brain homogenate. Amplified samples from round 3 were digested with 50 µg/mL of proteinase K (PK) and analyzed by WB using the Sha31 mAb. None of the presumable CWD samples or negative controls were able to be amplified. Protein standards are indicated as “M” (40, 30 and 20 kDa).
Additional file 2. Detection of BSE-C by sPMCA using alternative ovine-genotype substrate
. sPMCA was applied to a range of TSE isolates and negative controls from ovine, caprine, bovine and cervid origin, each sample was analyzed in duplicate reactions. A. PrPSc detected using Sha31 mAb. Negative controls (uninfected, n = 4), scrapie (n = 3), or CWD (n = 4) samples did not amplify above the background cut-off. Ovine scrapie 3 gave a low signal for PrPSc below the cut-off signal upon densitometry analysis. All BSE-C infected samples produced signal above the densitometry analysis cut-off (n = 4). The red deer CWD sample did not produce any PrPSc amplification. B. For samples that gave PrPSc bands detected by Sha31 mAb, re-analysis with antibody P4 gave very low or no signal, as expected for BSE-C PrPSc amplification. Sc and BSE-C blotting controls are brain homogenates from an ovine scrapie isolate and an ovine BSE-C isolate respectively. Protein standards are indicated by “M" (30 and 20 kDa). WTD, white tailed deer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marín-Moreno, A., Benestad, S.L., Barrio, T. et al. Classical BSE dismissed as the cause of CWD in Norwegian red deer despite strain similarities between both prion agents. Vet Res 55, 62 (2024). https://doi.org/10.1186/s13567-024-01320-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-024-01320-y