Abstract
Enterococcus cecorum (EC) is one of the most relevant bacterial pathogens in modern broiler chicken production from an economic and animal welfare perspective. Although EC pathogenesis is generally well described, predisposing factors are still unknown. This study aimed to understand the effect of heat stress on the caecal microbiota, intestinal integrity, and EC pathogenesis. A total of 373 1-day-old commercial broiler chicks were randomly assigned to four groups: (1) noninoculated, thermoneutral conditions (TN); (2) noninoculated, heat stress conditions (HS); (3) EC-inoculated, thermoneutral conditions (TN + EC); and (4) EC-inoculated, heat stress conditions (HS + EC). Birds were monitored daily for clinical signs. Necropsy of 20 broilers per group was performed at 7, 14, 21, and 42 days post-hatch (dph). A trend towards enhanced and more pronounced clinical disease was observed in the EC-inoculated, heat-stressed group. EC detection rates in extraintestinal tissues via culture were higher in the HS + EC group (~19%) than in the TN + EC group (~11%). Significantly more birds were colonized by EC at 7 dph in the HS + EC group (100%) than in the TN + EC group (65%, p < 0.05). The caecal microbiota in the two EC-inoculated groups was significantly more diverse than that in the TN group (p < 0.05) at 14 dph, which may indicate an effect of EC infection. An influence of heat stress on mRNA expression of tight junction proteins in the caecum was detected at 7 dph, where all six investigated tight junction proteins were expressed at significantly lower levels in the heat stressed groups compared to the thermoneutral groups. These observations suggest that heat stress may predispose broilers to EC-associated disease and increase the severity thereof. Furthermore, heat stress may impair intestinal integrity and promote EC translocation.
Similar content being viewed by others
Introduction
Enterococcus cecorum (EC) is one of the most common bacterial pathogens in meat-type chickens worldwide [1,2,3,4,5,6,7]. EC-associated disease is characterized by progressive lameness or symmetrical paresis of the legs in the second half of the production cycle [1, 3]. Prior to this chronic stage, affected birds can be either asymptomatic or show nonspecific symptoms of septicemia [8]. Antibiotic therapy is only promising if it is started in time during the septic phase. Severely affected birds need to be culled in both stages of the disease [1, 3, 5, 8]. Despite the resulting economic losses and animal welfare issues, there is still a lack of knowledge regarding the pathogenesis of EC-associated disease, such as virulence factors of EC and predisposing factors for the disease [9, 10].
Pathogenic EC strains colonize the intestines of broiler chickens after oral infection within the first week of life [11]. In the second to third week of life, EC translocates to the bloodstream. Affected birds suffer from septicemia, and EC can be detected in several extraintestinal organs, including the heart, liver, or spleen [8, 11]. Subsequently, EC reaches osseous predilection sites at the free thoracic vertebra and the femoral heads via the bloodstream, where it may colonize osteochondrosis dissecans (OCD) lesions. These osteochondrotic lesions usually develop due to strong mechanical stress at the weight-bearing vertebral and coxofemoral articulations. The resulting formation of necrotic abscesses is the cause of lameness and paresis [11, 12]. Thus far, it is unknown which virulence factors enable EC to translocate from the gut to other tissues and how the caecal microbiota composition and intestinal barrier function might affect this translocation process and vice versa [9]. However, several predisposing factors in EC pathogenesis have been discussed. A recent study indicated that inadequate ventilation of the barn during the first days of life, shortened dark periods, and inadequate cleaning and disinfection between cycles increase the risk of EC-associated disease occurrence [13]. The impact of housing temperature on EC infection has not been studied. Nonetheless, it has been shown that heat stress might be a predisposing factor for intestinal colonization by pathogenic bacteria such as Clostridium perfringens [14], Salmonella Enteritidis [15], and Escherichia coli [16]. Heat stress is an environmental factor that can exert a decisive impact on chicken health in general and on the intestinal microbiota and intestinal integrity in particular [17].
The chicken intestinal microbiota interacts predominately with dietary compounds, the intestinal mucosa, and the immune system of the host. Consequently, it is of vital importance for chicken health [18,19,20]. The composition of the chicken caecal microbiota varies depending on diet, age, and housing [21,22,23]. Seasonal and environmental factors can substantially influence the development of the microbial community in chickens [24, 25]. Marked changes in the gut microbial composition have been found in heat-stressed broilers with decreased abundances of beneficial bacteria (e.g., Lactobacillus spp. and Bifidobacterium spp.) and increased abundances of coliforms and Clostridium spp. [26]. It has been speculated that heat stress might lead to dysbiosis and, in turn, to increased intestinal permeability and metabolic dysfunction [26,27,28].
The impact of high ambient temperatures on intestinal integrity in chickens has been the focus of several studies in recent years. Heat stress can increase intestinal permeability through a complex process of physiological adaptations [29]. Initially, increasing the peripheral blood flow and reducing intestinal blood supply, heat stress leads to hypoxia, an overproduction of reactive oxygen species (oxidative stress), and cell damage in intestinal tissues [17, 30]. Moreover, the expression of heat shock factors is upregulated, thus leading to an increased production of heat shock proteins, which are crucial for the regulation of protein homeostasis and can be considered a marker of tissue injury [29, 31]. This oxidative stress response is often associated with the disruption and dysfunction of the intercellular junctional complex [29]. Desmosomes, adherens junctions, and tight junctions form the intercellular junctional complex and are hence an important part of the intestinal barrier [32]. Tight junctions are transcellular and paracellular proteins that allow substances to transfer passively across the epithelium following a concentration gradient [33]. Heat stress compromises tight junctions, leading to a condition called “leaky gut”, which in turn facilitates translocation of bacteria from the intestinal tract [15, 34].
Since not only heat stress but also enteric pathogens can disrupt the intestinal barrier [34], it is highly important to investigate the interactions between high ambient temperatures, the caecal microbiota composition, intestinal integrity, and EC infection. Accordingly, this study aimed to understand the impact of EC infection with and without heat stress on gut health during the first 3 weeks of life. Therefore, the objectives of the study were (1) to investigate the course and severity of clinical disease and EC-associated gross lesions, (2) to analyse the development of the caecal microbiota, and (3) to investigate the effects of EC infection and heat stress on intestinal integrity.
Materials and methods
Animals and housing
A total of 373 1-day-old Ross 308 broiler chicks (BWE-Brüterei Weser-Ems GmbH & Co. KG, Visbek, Germany) were used in this experiment. Upon arrival, birds were randomly assigned to one of the four study groups and housed under controlled environmental conditions in floor pens at the animal facility of the Clinic for Poultry, University of Veterinary Medicine Hannover, Foundation, Hannover, Germany. Birds had ad libitum access to water and feed. A standard feeding protocol consisting of starter [1–10 days post-hatch (dph)], grower (11–35 dph) and finisher diet (36–42 dph) was applied throughout the trial (Deuka, Deutsche Tiernahrung Cremer GmbH & Co. KG, Duesseldorf, Germany). Birds were exposed to two different ambient temperature profiles. Table 1 shows the thermoneutral and heat stress conditions used throughout the study. The thermoneutral temperature profile was in line with recommendations from Aviagen EPI (Arendonk, Belgium) [35]. The building management system was used to manage and measure temperature settings. At least twice a day, the system was checked for correct functioning. On the day of placement, birds were provided with 24 h of light, and infrared lamps were set up in the pens, which were removed on the third day. Afterwards, the light program was set to 15 h light from 07:30 to 22:30. The Animal Ethics Committee of the University of Veterinary Medicine Hannover, Foundation and the Lower Saxony State Office for Consumer Protection and Food Safety approved the study design (33.19-42505-04-19/3170).
Experimental setup
At the day of arrival, the EC-negative status of 10 randomly selected and humanely sacrificed birds was confirmed by bacteriological examination of yolk sac and caecal samples via culture and EC-specific real-time PCR. The remaining 363 birds were randomly divided into four groups, and the EC challenge was performed. The four groups were treated as follows: noninoculated chickens were raised under thermoneutral conditions (TN), noninoculated chickens were raised under heat stress conditions (HS), EC-inoculated chickens were raised under thermoneutral conditions (TN + EC), and EC-inoculated chickens were raised under heat stress conditions (HS + EC; see Table 1). The day-old chicks in the two EC-inoculated groups were inoculated orally with 0.5 mL of an EC suspension (2 × 106 colony-forming units per millilitre (CFU/mL)), whereas the two noninoculated groups received physiological saline orally. Birds were checked at least daily for clinical signs of EC-associated disease, such as apathy, ruffled feathers, and lameness. Animals showing severe signs of apathy or lameness were euthanized, and necropsy was performed (irregular necropsies). Regular necropsies of 20 broilers per group were performed at 7, 14, 21, and 42 dph. The birds were weighed, pathologic lesions were documented, and samples were taken as follows: Amies medium swabs (Hain Lifesciences GmbH, Nehren, Germany) were taken from the heart, liver, and spleen at all necropsies. Additionally, Amies medium swabs were taken from the free thoracic vertebra and the femoral heads after cutting the respective osseous sampling site vertically to expose the bone marrow at 21 and 42 dph. Dry swabs (Applimed SA, Châtel-St-Denis, Switzerland) were taken from the jejunum (2 cm proximal to Meckel’s diverticulum), ileum (2 cm proximal to the ileocecal junction), and caecum (Corpus caeci) and stored at −20 °C. At the same intestinal sampling sites, 1 cm of the intestine was taken, digesta was removed, and the sample was placed in 1.5 mL Eppendorf tubes (Sarstedt AG & Co. KG, Nuembrecht, Germany) containing 0.5 mL RNAlater® (Merck KGaA, Darmstadt, Germany) and stored at −80 °C until further use. Caecal samples, including digesta, were taken for caecal microbiota analysis and stored at −20 °C.
Challenge isolate and preparation of the inoculum
The challenge isolate EC 14/086/4/A was used and prepared for inoculation as previously published [10]. The inoculation dose was set to 2 × 106 colony-forming units per mL (CFU/mL), which was confirmed by determining the total bacterial count.
EC detection via bacterial isolation and real-time PCR
Microbiological examination was performed as described previously [36]. Briefly, swabs taken from extraintestinal tissues at necropsy were cultured on Columbia colistin-nalidixic acid (CNA) agar (Oxoid GmbH, Wesel, Germany) for 24 h at 37 °C under microaerophilic conditions. Colonies of an EC-typical morphology (small, grey, mucoid colonies with slight alpha-haemolysis) were subcultured and identified as EC via oxidase and catalase testing, Gram staining, and, in case of doubt, by 16S rRNA partial gene sequencing (Microsynth AG, Lindau, Germany [37,38,39]). DNA was isolated from swabs taken from the jejunum, ileum, and caecum (InnuPrep DNA Mini Kit, Analytik Jena AG, Jena, Germany), and EC-specific real-time PCR was performed exactly as published previously [36].
Characterization of caecal microbiota
The analysis of the caecal microbiota composition was performed by sequencing the V3-V4 hypervariable region of 16S rRNA genes as described previously [24]. Sequencing results were analysed and classified with RDP Seqmatch using QIIME 2 software [40] with an operational taxonomic unit (OTU) discrimination level set to 97%.
Electrophysiological measurements
To study the intestinal integrity of the birds in the third week of life, Ussing chamber experiments were performed at 14–18 dph. Each day, four animals (one per group, final sample size n = 5 per group at 18 dph) were sacrificed, and the intestines were removed immediately, transferred to ice-cold physiological saline solution to cool down, and then placed in ice-cold carbogen aerated (95% O2, 5% CO2; pH 7.45–7.47) transport medium (serosal buffer, Additional file 1). Samples from three intestinal segments were prepared immediately after 15 min of transport. Jejunal samples were taken 5 cm proximal to Meckel’s diverticulum, ileal samples were taken 1 cm proximal to the ileocecal junction, and caecal samples were taken at the corpus caeci. The respective intestinal segments were placed on an ice-cold glass surface, opened longitudinally, and rinsed with cold physiological saline solution to remove all digesta. Afterwards, the serosal and muscular layers were stripped off, and the mucosa was mounted in the Ussing chamber, exposing an area of 1.00 cm2 to the two chamber halves. Each chamber half was filled with 10 mL of a buffer solution at a temperature of 37 °C and a pH of 7.4 under continuous aeration with carbogen to maintain physiological conditions. The composition of the buffer solution differed between the serosal and mucosal sides (Additional file 1). All buffers contained indomethacin (10–5 M) to inhibit endogenous prostaglandin production. Each segment was analysed in technical duplicate. The experiments were performed under short-circuited conditions, and the potential differences, tissue conductance (Gt) and short circuit currents (Isc) were continuously recorded by a computer-controlled voltage clamp device (Mussler Scientific Instruments, Aachen, Germany). After a 30-min equilibration period, 10 mM glucose (Merck KGaA, Darmstadt, Germany) and 10 mM mannitol (Sigma Aldrich Inc., St. Louis, MO, USA) were added to the mucosal and serosal sides, respectively, initiating sodium-dependent glucose transport. Moreover, 10–5 M carbachol (Sigma Aldrich Inc.) and 5 × 10–6 M forskolin (Sigma Aldrich Inc.) were added to the serosal side to initiate chloride secretion with 30 min of recovery after each addition. The experiment was terminated 30 min after the final addition of 10–4 M ouabain (Sigma Aldrich Inc.) to the serosal side to test for tissue viability [41].
mRNA expression of tight junction proteins
mRNA expression of the tight junction proteins claudin-1 (CLDN1), claudin-3 (CLDN3), claudin-5 (CLDN5), claudin-7 (CLDN7), tricellulin (MD2), and zonula occludens-1 (ZO1) in the jejunum and caecum was analysed using an RT‒qPCR assay. RNA was isolated from the intestinal tissue using the Analytik Jena RNA Mini Kit 2.0 (Analytik Jena AG) in accordance with the manufacturer’s instructions, and RNA was stored at −80 °C until further use. Primers and probes were designed exactly as described by von Buchholz et al. [42], and RT‒qPCR was performed with some minor modifications. The Luna® Universal Probe One-Step RT‒qPCR Kit (New England Biolabs GmbH, Frankfurt, Germany) and QuantStudio 3 Real-Time-PCR-System (Thermo Fisher Scientific Inc., Wilmington, NC, USA) were used for RT‒qPCR, and the setup was adapted to the given conditions. Each sample was tested in duplicate for the expression of target genes (CLDN 1, CLDN5, and ZO1 as Multiplex 1; CLDN3, CLDN7, and MD2 as Multiplex 2) and a reference gene (RPL13). Primers and probes for the reference gene were chosen according to Mitra et al. [43]. Additionally, each sample was tested for genomic DNA contamination by running in duplicate without reverse transcriptase for the reference gene. Each run also included two wells with no template control. Ct values were normalized against the reference gene [44].
Statistical analysis
Statistical analysis of data was performed using the SAS Enterprise Guide (Version 7.15, SAS Institute Inc., Cary, USA), and graphs were created with GraphPad Prism (Version 9.2, GraphPad Software, LLC, San Diego, USA). Descriptive statistics and Fisher’s exact test were used to analyse clinical signs and pathology. EC detection at the different intestinal and extraintestinal sampling sites via culture or real-time PCR was compared between groups using Fisher’s exact test. To determine within-sample diversity (alpha diversity), the diversity estimators Chao1, Shannon, and Simpson index were calculated in QIIME 2 [40]. The alpha diversity indices were further compared between groups using the Kruskal‒Wallis test and Mann‒Whitney U test. Different distance metrics implemented in QIIME 2 were used to visualize beta diversity in principal coordinate analysis (PCoA). The results from electrophysiological measurements (Gt and Isc) in the Ussing chamber and mRNA expression data of tight junction proteins were compared between groups per intestinal segment using the Kruskal‒Wallis test and the Mann‒Whitney U test. The Benjamini‒Hochberg correction method for multiple testing was used to adjust p values where applicable [45], and differences were considered significant at p ≤ 0.05.
Results
Clinical signs and gross lesions
No clinical signs or gross lesions of EC-associated disease were observed in the two noninoculated groups (Table 2). From 10 dph onwards, nonspecific symptoms, including ruffled feathers and apathy, were observed in 19.1% and 17.2% of chickens in the TN + EC and HS + EC groups, respectively. Lameness was seen less often than apathy in both EC-inoculated groups without any significant difference between them (p > 0.05, Table 2). Pericarditis was found significantly more often in the HS + EC group (17.2%) than in the TN + EC group (5.7%, p < 0.05). Splenomegaly was found to a similar extent in both EC-inoculated groups (Table 2). Spinal abscesses and osteomyelitis of the femoral heads were found less frequently than lameness in both EC-inoculated groups (Table 2). In some birds, the cause of lameness remained unclear, as examination of additional joints was not performed.
In the TN, HS, and TN + EC groups, a number of birds died due to reasons other than EC infection. In the TN group, two birds died due to noninfectious reasons (ascites syndrome and intestinal torsion). In the HS group, four birds died due to noninfectious reasons (ascites syndrome, sudden death syndrome and umbilical hernia). In the TN + EC group, nine birds died due to reasons other than EC infection (weak chick, intestinal invagination, E. coli infection, sudden death syndrome and ascites syndrome). However, three birds had to be euthanized due to EC-associated disease (paresis) in the HS + EC group between 21 and 42 dph.
EC detection in extraintestinal tissues
In addition to the absence of pathologic lesions, EC was not detected at any extraintestinal sampling site in the two noninoculated groups. In total, 11.2% of the birds in the TN + EC group and 19.5% of the birds in the HS + EC group were EC-positive in either one or more of the examined tissues (Figure 1). The number of EC-positive birds detected from the liver, spleen, free thoracic vertebra, and femoral heads was overall higher in the HS + EC group than in the TN + EC group. However, no significant difference was found between the two EC-inoculated groups for any of the examined tissues (p > 0.05). EC was most frequently detected in the spleen, with 8.99% in the TN + EC group and 16.09% in the HS + EC group. Moreover, EC was detected more often than splenomegaly at necropsy (6.7% in the TN + EC group and 8.1% in the HS + EC group). Although pericarditis was seen significantly more often in the HS + EC group (17.2%) than in the TN + EC group (5.6%, p < 0.05), EC was isolated to a similar extent from both groups (6.7% from TN + EC and 5.8% from HS + EC). When comparing gross lesions in the FTV and the femoral heads with EC detection rates, more birds were found to be EC-positive than to have gross lesions. At the FTV, 5.0% in the TN + EC group and 2.4% in the HS + EC group had a spinal abscess, whereas 5.0% in the TN + EC group and 14.3% in the HS + EC group were found to be EC-positive at the FTV. Lesions at the femoral heads were found in none of the birds in the TN + EC group and in 4.8% of the birds in the HS + EC group, but slightly more birds were found to be EC-positive on culture in both groups (2.5% in group TN + EC and 14.3% in group HS + EC). The free thoracic vertebra and the femoral heads were only examined between 21 and 42 dph, since EC is not expected to colonize the osseous predilection sites before the fourth week of life. At 21 dph, EC was not detected at the FTV or the femoral heads in the TN + EC group. However, in the HS + EC group, three birds (15%) were EC-positive at the FTV, and four birds (20%) were EC-positive at the femoral heads at 21 dph, but no gross lesions were found. At 42 dph, one (6.3%) and four (20%) birds were EC-positive at the FTV in the TN + EC and HS + EC groups, respectively. A spinal abscess was detected only in the EC-positive bird in the EC + TN group.
EC detection on culture in extraintestinal tissues. Groupwise comparisons were performed per tissue by Fisher’s exact test and post hoc Benjamini‒Hochberg adjustment (α = 0.05). Different superscript letters indicate significant differences between groups per tissue. p ≤ 0.05, n = 86 [noninoculated, thermoneutral (TN)], 88 [noninoculated, heat stress (HS)], 89 [EC-inoculated, thermoneutral (TN + EC)], and 87 [EC-inoculated, heat stress (HS + EC)] for heart, liver, spleen, and in total, n = 40 (TN, HS, and TN + EC), 42 (HS + EC) for free thoracic vertebra (FTV) and femoral heads (FH). FTV and FH were sampled only at 21 and 42 dph.
EC detection in the intestine
EC was detected via real-time PCR in all three intestinal segments at all sampling days in the TN + EC and HS + EC groups (Figure 2). In general, EC detection rates were highest in the caecum. At 7 dph, 100% of the birds in the HS + EC group were EC-positive in the caecum, which was significantly more than in the TN + EC group, in which 65% of the birds were colonized by EC (p < 0.05). At 14 and 21 dph, EC detection rates in the caecum were similar in both groups. At the end of the study, 60% of the birds in the HS + EC group were still EC-positive, whereas only 10% of the birds in the TN + EC group were EC-positive (p < 0.05). In the TN group, EC was detected in fewer than 20% of the birds in all three intestinal segments at 7, 14, and 21 dph. Interestingly, all birds in this group were EC-positive in the caecum, 75% in the ileum, and 20% in the jejunum at 42 dph. In the HS group, EC was detected in the ileum of one bird at 7 and 14 dph and not at all at 21 and 42 dph in all three intestinal segments.
EC detection along the intestine. A Jejunum, B Ileum, C Caecum. Groupwise comparisons were performed per tissue by Fisher’s exact test and post hoc Benjamini‒Hochberg adjustment (α = 0.05). Different superscript letters indicate significant differences between groups per sampling day. p ≤ 0.05, n = 20.
Characterization of the caecal microbiota
A total of 5 869 288 reads were obtained from 153 caecal samples. The mean coverage was 38,361 reads (range 20 030–89 883). These reads were assigned to 1298 operational taxonomic units (OTUs).
Alpha diversity and beta diversity
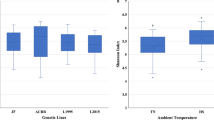
Regarding the whole trial, there was an increase in species richness (Chao1 estimator) up to 42 dph. At 14 and 21 dph, species richness was significantly higher in the HS + EC group than in the other three groups (p < 0.05, Figure 3). At 42 dph, no significant difference in species richness was found between the four groups (p > 0.05).
Alpha diversity estimators. A Chao1 estimator, B Shannon index, C Gini-Simpson index. Boxes represent the 25th to 75th percentiles, and whiskers extend to the smallest and largest values. Groupwise comparisons were performed per diversity index by the Kruskal‒Wallis test, Mann‒Whitney U test, and post hoc Benjamini‒Hochberg adjustment (α = 0.05). Different superscript letters indicate significant differences between groups per sampling day. p ≤ 0.05, n = 20.
At 7 dph, no significant differences in species diversity (Shannon index) were found (p > 0.05, Figure 3). At 14 dph, the caecal microbiota in the two EC-inoculated groups was significantly more diverse than that in the TN group (p < 0.05). At 21 dph, species diversity was significantly higher in the HS + EC group than in the HS group (p < 0.05). At 42 dph, species diversity was significantly higher in the TN + EC group than in the HS group (p < 0.05).
Significant differences in species diversity represented by the Gini-Simpson index were found only at 42 dph, when species diversity was significantly higher in the TN + EC group than in the HS group (p < 0.05, Figure 3). At day 42, similar differences were expressed by the Shannon index and the Gini-Simpson index. In summary, species richness differed between the four groups throughout the study period, whereas the species were similarly evenly distributed in all four groups throughout the study period.
In principal coordinate analysis of different distance metrics, no distinct clusters were detected for any group at any time point (data not shown).
Relative abundances at the phylum and family levels
Throughout the study, Firmicutes was the most abundant phylum in all groups on all study days (>68%). Whereas Proteobacteria were most frequently observed at 7 dph (>14%) and 14 dph (>2%) in all groups, Bacteroidota became more abundant towards the end of the study (>24%) in all groups except for TN + EC (Additional file 2).
Significant differences in the relative abundances of different phyla were observed mainly at 42 dph (Table 3). The phylum Firmicutes was significantly more abundant in the TN + EC group than in the other three groups (p < 0.05). At the family level, this difference was mainly due to the higher abundances of the family Lachnospiraceae at 42 dph (Table 4). Conversely, the phylum Bacteroidota was significantly less abundant in the TN + EC group at 42 dph than in all other groups based on the absence of the family Rikenellaceae (p < 0.05, Tables 3, 4). The phylum Verrucomicrobiota became significantly more abundant in the HS + EC group than in all other groups at 14 and 21 dph (p < 0.05).
The most abundant family at 7, 14, and 21 dph was the family Lachnospiraceae, followed by Enterobacteriaceae (phylum Proteobacteria) at 7 dph and Ruminococcaceae (phylum Firmicutes) at 14 and 21 dph. At 42 dph, Rikenellaceae (phylum Bacteroidota) became highly abundant in all groups except for TN + EC. The family Enterococcaceae (phylum Firmicutes) was less abundant on all sampling days (<1%, Additional file 3 and Table 4).
Electrophysiological measurements
Basal tissue conductance (Gt) and basal short-circuit currents (Isc) were measured continuously during the experiment. The lowest basal Gt and Isc values were observed in the jejunum, and the highest values were observed in the caecum (Figure 4). No significant differences in basal Gt or Isc values between groups were found for each intestinal segment (p > 0.05). Adding glucose (mucosal), carbachol (serosal), forskolin (serosal), and ouabain (serosal) did not alter Gt significantly in any of the groups (p > 0.05, Figure 5). Additionally, no significant differences in ΔGt between the groups in any intestinal segment were found after adding the abovementioned substances (p > 0.05). Although changes in Isc values were observed after adding the substances, these were not statistically significant (p > 0.05, Figure 6). Furthermore, no significant differences in ΔIsc were found between groups (p > 0.05).
Basal tissue conductance (Gt) and short-circuit currents (Isc) in three intestinal segments. A Tissue conductance (Gt) in mS/cm2. B Short-circuit current (Isc) in µA/cm2. Data are presented as the mean ± standard error of the mean (SEM). No significant differences between groups per intestinal segment were detected by the Mann‒Whitney U test and post hoc Benjamini‒Hochberg adjustment (α = 0.05), p ≤ 0.05, n = 5.
Maximum changes in tissue conductance (ΔGt) after adding substances. Substances that were added included A glucose (mucosal), B carbachol (serosal), C forskolin (serosal), and D ouabain (serosal). Data are presented as the mean ± standard error of the mean (SEM). ΔGt values did not differ significantly between groups according to the Mann‒Whitney U test and post hoc Benjamini‒Hochberg adjustment (α = 0.05), p ≤ 0.05, n = 5.
Maximum changes in short-circuit currents (ΔIsc) after adding substances. Substances that were added included A glucose (mucosal), B carbachol (serosal), C forskolin (serosal), and D ouabain (serosal). Data are presented as the mean ± standard error of the mean (SEM). ΔIsc values did not differ significantly between groups according to the Mann‒Whitney U test and post hoc Benjamini‒Hochberg adjustment (α = 0.05), p ≤ 0.05, n = 5.
mRNA expression of tight junction proteins in different intestinal sections
No significant differences in jejunal tight junction protein mRNA expression levels between the different groups were observed at most time points (p > 0.05, Figure 7). However, at 7 dph, the mRNA encoding CLDN3, MD2, and ZO1 was expressed at significantly higher levels in the TN group than in the HS (CLDN3, MD2, and ZO1) and HS + EC (ZO1; p < 0.05) groups. At 42 dph, the mRNA expression levels of CLDN1, CLDN5, and ZO1 were significantly higher in the TN + EC group than in the noninoculated groups (CLDN1, CLDN5, and ZO1) and the HS + EC group (CLDN5 and ZO1; p < 0.05). Nonetheless, the mRNA expression of MD2 was significantly higher in the HS group than in all other groups at the same time point (p < 0.05).
mRNA expression levels of different tight junction proteins in the jejunum. Data are presented as the mean ± standard deviation. Groupwise comparisons were performed per tight junction protein by the Kruskal‒Wallis test, Mann‒Whitney U test, and post hoc Benjamini‒Hochberg adjustment (α = 0.05). Different superscript letters indicate significant differences between groups per sampling day. p ≤ 0.05, n = 20.
A clear pattern of significant differences in tight junction mRNA expression was observed in the caecum (Figure 8). At 7 dph, significantly higher normalized 40-Ct values were observed in the thermoneutral groups than in the heat-stressed groups for all six investigated tight junction proteins (p < 0.05). Further differences were observed for CLDN1 expression levels at 21 and 42 dph and for MD2 at 42 dph (p < 0.05; Figure 8). While CLDN1 expression was higher in the HS group than in both EC-inoculated groups at 21 dph (p < 0.05), it was significantly lower at 42 dph when comparing the HS group to the TN + EC and HS + EC groups (p > 0.05). MD2 expression was significantly higher in the TN group than in all other groups at 42 dph (p < 0.05).
mRNA expression levels of different tight junction proteins in the caecum. Data are presented as the mean ± standard deviation. Groupwise comparisons were performed per tight junction protein by the Kruskal‒Wallis test, Mann‒Whitney U test, and post hoc Benjamini‒Hochberg adjustment (α = 0.05). Different superscript letters indicate significant differences between groups per sampling day. p ≤ 0.05, n = 20.
Discussion
Despite the economic importance of EC infection in broiler production, EC pathogenesis is not fully understood. To date, the role of the caecal microbiota, intestinal integrity, and predisposing factors such as heat stress during EC pathogenesis remain largely unknown. This study aimed to understand the interaction between EC infection and gut health with and without underlying heat stress conditions.
Heat stress conditions enhance and accelerate EC-associated disease
The onset of clinical signs was observed at 10 dph in both EC-inoculated groups, but more severe clinical signs were found in the HS + EC group, leading to euthanasia of three of 87 birds prior to the last sampling day. Additionally, EC was detected at the osseous predilection sites earlier in the HS + EC group than in the TN + EC group. Although significant differences in EC detection rates via culture were lacking, the percentage of EC-positive birds was higher in the HS + EC group (19.5%) than in the TN + EC group (11.2%) in total and in all extraintestinal organs except for the heart. Accordingly, heat stress might not only slightly enhance the number of affected birds but also accelerate and exacerbate the course of the disease in these animals. The nonspecific nature of the EC-associated disease and our study design focusing on the early infection phase complicate the interpretation of the results to some extent. EC detection rates in both inoculated groups were lower than in previous studies where more than 23% of the birds were EC-positive in either one or more of the examined extraintestinal tissues (heart, liver, spleen, FTV, femoral heads) [10, 36]. One of the reasons for this discrepancy may be the termination of the experiment for 75% of the birds before 21 dph, leaving only 20 birds per group that could fully develop chronic lesions of the EC-associated disease. EC was isolated from osseous predilection sites in some birds in this study, although no macroscopic lesions were detected. It may be suggested that emerging lesions could have been detected by histological examination, since previous studies reported that EC can be detected before gross lesions develop [10, 36]. Consequently, this may explain the differences observed between clinical signs, gross lesions, and EC detection rates in the present study.
Intestinal EC detection rates were highest in the caecum, which is in accordance with previously reported EC colonization patterns [46]. Heat stress may be the reason for the higher caecal EC detection rates in the HS + EC group at 7 dph. Furthermore, the lower caecal EC detection rate in the TN + EC group may have reduced the chance of translocation. This might be an explanation for the lower rates of clinical signs, gross lesions, and EC detection in extraintestinal tissues during the septic phase. Intestinal colonization is thought to be a crucial step in EC pathogenesis [11]. It may be assumed that heat stress deteriorated colonization resistance against the pathogenic EC strain and promoted translocation to the bloodstream, resulting in an increased incidence of the disease.
The effect of heat stress on other bacterial diseases of chickens has been the focus of several studies in recent years. Tsiouris et al. [14] found that heat stress might be a predisposing factor for necrotic enteritis. They observed a relative, but not significant, trend towards more severe lesions and an increased incidence of necrotic enteritis due to cyclic acute heat stress. The lack of significant effects was assumed to be caused by the use of a cyclic heat stress protocol, which might have provided recovery periods for the birds [14]. In contrast, a study conducted several years prior to that study indicated no impact of heat stress on necrotic enteritis in broilers [47]. However, since infection and heat stress protocols differed between the studies, they are difficult to compare [14]. In E. coli infection, heat stress has been shown to increase the inflammatory response in comparison to thermoneutral conditions [16]. Conversely, another study indicated that cyclic acute heat stress affected the immune system but not resistance against E. coli infection [48]. Cyclic acute heat stress may also promote translocation of Salmonella spp. and endotoxins due to increased intestinal permeability [15, 49]. Based on these different studies and the present data, it can be concluded that heat stress is a predisposing factor for enteric pathogens, including EC.
Heat stress may adversely affect immunity, productivity, and animal welfare [50, 51]. Existing studies on heat stress effects in broilers mostly focus on performance during the second half of the production cycle, since average daily weight gain is highest and effects are most prominent during that period [52,53,54,55]. Accordingly, the comparability of the present study with other studies is limited. The first 3 weeks of life are supposed to be crucial in EC pathogenesis [11], but even the egg incubation period could be important for disease resistance against EC. Heat stress during the egg incubation period may negatively affect bone development [56]. However, increased egg incubation temperature around hatching does not affect the incidence of OCD in the FTV, which is considered to be another predisposing factor for EC-associated disease [57].
Stress and immunosuppression have previously been shown to be influencing factors during pathogenesis of the so-called bacterial chondronecrosis and osteomyelitis (BCO) in broilers [58]. Among other bacteria, EC is considered a causative agent of BCO [59]. In the respective study, repeated dexamethasone injections were used to mimic immunosuppression, and the impact of repeated episodes of heat stress on BCO lesions was investigated. While the present investigation exposed birds to chronic heat stress in the first 3 weeks of life, Wideman et al. used a different model consisting of 3 days of heat stress per week from 21 to 42 dph [58]. This type of heat stress accelerated and exacerbated BCO lesions at the femoral and tibial heads at least within the fourth and fifth weeks of life. Afterwards, no differences could be observed, suggesting acclimation to heat stress conditions [58]. These adaptation processes might also explain the lack of significant effects of permanent heat stress in the present study. In a study investigating adaptation to repeated episodes of heat stress, it was shown that acclimation is a key factor in managing and surviving subsequent acute heat stress phases [60].
Finally, EC is thought to be an opportunistic pathogen with several factors predisposing broilers to EC-associated disease [61]. Heat stress alone does not significantly increase the incidence of the associated disease but may still contribute to EC pathogenesis in the field.
Commensal EC strains from an unknown source tend to colonize noninoculated birds
Interestingly, the TN group was completely colonized by EC at the end of the study. It may be suggested that birds in this group were colonized by a commensal EC strain from an unknown source. Although the experiment was performed under strict hygiene conditions, it is possible that this strain was introduced into the isolation unit via biotic or abiotic factors. Similar observations were made in two previous studies, but we still lack identification of the respective strain [10, 36]. Isolation of EC strains from caecal contents can be challenging due to overgrowth by other bacteria, such as enterococci, streptococci, and E. coli [62]. There is no selective medium for EC available, and although highly specific, our real-time PCR assay does not allow us to distinguish different EC strains [46, 63]. To better understand the colonization patterns of different EC strains, culture-independent methods that allow differentiation of EC strains should be implemented in future studies. This approach might further help to distinguish commensal and pathogenic strains that can appear within experiments even under controlled environmental conditions [10].
EC infection and heat stress have a limited effect on caecal microbiota
The caecum harbours the most complex and diverse microbiota within the intestinal tract of chickens due to the availability of nutrients such as polysaccharides and longer transit times in this compartment. Interactions within the microbiota and between the microbiota and the host are highly complex and may be influenced by various factors [18, 19]. Accordingly, the differences in alpha diversity as well as caecal composition at the phylum and family levels may be explained by chance rather than by EC infection and temperature conditions. During the initial infection phase at 7 dph, species diversity was not altered by heat stress or EC infection. Minor differences in species richness and relative abundances at the phylum and family levels were mainly observed between the two thermoneutral groups, indicating a possible effect of EC infection at that time point. Differences at later time points, such as higher richness at 14 and 21 dph in the HS + EC group than in the other groups, might be associated with heat stress, since it has been reported that species richness is higher in birds hatched during the summer months [25]. However, there is also an increased risk of pathogenic and commensal bacteria being introduced by insects during summer, as reported for Campylobacter spp. [64]. If heat stress was the only factor influencing species richness, a similarly high species richness as observed in the HS + EC group would have been expected for the HS group. Moreover, lower species diversity was observed in the HS group than in the TN + EC group at 42 dph. Since similar temperature conditions in all four groups were reached a week earlier at 35 dph, it is unlikely that this difference was caused by heat stress. Instead, other factors may have influenced species diversity at that time point, especially since birds were housed in separate isolation units. Although housing conditions, except for temperature, were similar in each group, different development of caecal microbiota is possible. It has been described previously that the caecal microbiota composition may be affected by various factors and varies greatly even between highly comparable experimental setups [19]. In recent studies, enterococci were shown to form a minority subpopulation within the caecal microbiota in the first weeks of life [36, 65]. Furthermore, it was reported that EC can induce disease in experimentally infected chickens despite its low abundance in the caecum [36]. This is in accordance with the findings of the present study, which suggest that EC infection had no direct impact on the caecal microbial composition. In addition, the genus Enterococcus generally seems to be less influenced by interventions such as antibiotic treatment [36], administration of probiotic Bacillus strains, palm oil, and water soluble esterified butyrins [65], or synbiotic administration under heat stress conditions [66]. Based on our observations, heat stress also elicits no clear effects on the family Enterococcaceae. Finally, in our study, there was no clear evidence that either EC infection or ambient temperature conditions had a decisive impact on the caecal microbiota.
Heat stress and EC infection may impair intestinal integrity
To investigate the influence of heat stress and EC infection on intestinal integrity, Ussing chamber experiments were performed in the third week of this study. Only slight changes in tissue conductance without significant differences between groups were observed, which implies that intestinal permeability was not altered by heat stress or enterococcal infection in the third week of life. Although the added substances triggered their receptors and the associated ion transport mechanisms, the observed changes in the Isc values were not significant compared to the basal values, and no significant differences between groups were detected. An influence of heat stress or enterococcal infection on intestinal integrity and active transport mechanisms in the third week post-hatch may thus be ruled out. However, data obtained in these Ussing chamber experiments are limited due to the small sample size (n = 5) and the restriction of the time point to the third week of the study. Small sample sizes in Ussing chamber experiments generally limit the outcome and interpretation of results [67]. Since heat stress was applied in the first week of the trial and an adaptation of the birds to the high ambient temperatures cannot be excluded, the third week as a time point for the Ussing chamber experiment may simply be too late. Additionally, EC is expected to escape the intestine soon after infection in the first to second week of life [11]. Earlier investigations of intestinal integrity via Ussing chamber experiments were considered but not performed because of the small size of the intestine. For that reason, the mRNA expression of tight junction proteins was investigated at all sampling days, and a greater sample size was chosen.
Significant differences indicating an effect of heat stress and EC infection on the mRNA expression of tight junction proteins were observed mainly in the caecum at 7 dph. All six investigated tight junction proteins were expressed at significantly lower levels in the heat-stressed groups than in the thermoneutral groups. Heat stress has been shown to influence tight junction integrity in several studies in modern broiler lines [55, 68, 69]. However, adaptation to heat stress exposure is reached within 7 days, which may explain the absence of similar significant differences in tight junction mRNA expression after the first sampling day at 7 dph [60]. Interestingly, as discussed above, EC was shown to be mainly present in the caecum, with higher detection rates observed in the HS + EC group than in the TN + EC group at 7 dph. In relation to mRNA expression data, it may be suggested that the caecum is not only the most favourable location of colonization but also the location of translocation to the bloodstream, which is one of the crucial steps in EC pathogenesis [11]. Intestinal integrity impairment by heat stress may promote this translocation.
Differences observed for expression levels of CLDN1 at 14 and 21 dph and CLDN1, CLDN5, MD2, and ZO1 at 42 dph in the jejunum and caecum may only partly be explained by EC infection. An effect of heat stress at these time points seems to be unlikely. Similar to the caecal microbiota, tight junction protein expression may be influenced by several factors in addition to heat stress and infection. Delayed feeding post-hatch [70], probiotic supplementation [71, 72], and different dietary compounds [73, 74] have been shown to influence tight junction mRNA expression. Moreover, it was shown in previous studies that EC pathogenesis is not necessarily associated with histologically detectable intestinal damage [9, 11]. Thus, it is still possible that EC does not affect intestinal integrity at all. Finally, based on the results discussed here, further experiments on EC pathogenesis should focus on the first 2 weeks post-infection to identify possible effects of EC on intestinal integrity and translocation mechanisms of EC.
In conclusion, heat stress seemed to accelerate EC translocation and make birds susceptible to more severe disease development in the present study. Heat stress may predispose broilers to EC-associated disease in interaction with other, as yet unknown, environmental and host factors. No clear effects of heat stress and EC infection on the caecal microbiota were found in this study. Nevertheless, EC may be assumed to be a key species in affected birds despite being less abundant in the caecal microbiota. The first 2 weeks of life seem to be the critical time period for EC translocation to the bloodstream. Disruptive effects of heat stress on tight junction integrity in the first week of life may have contributed to increased extraintestinal EC detection rates in the present study. Our research contributes to understanding the unknown role of intestinal integrity and caecal microbiota in EC infection. Possible interactions of EC with the intestinal barrier should be further investigated to better understand the pathogenesis of EC-associated disease.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional files.
Change history
11 February 2023
Missing Open Access funding information has been added in the Funding Note.
References
Devriese LA, Cauwerts K, Hermans K, Wood AM (2002) Enterococcus cecorum septicemia as a cause of bone and joint lesions resulting in lameness in broiler chickens. Vlaams Diergeneeskd Tijdschr 71:219–221
Wood AM, MacKenzie G, McGiliveray NC, Brown L, Devriese LA, Baele M (2002) Isolation of Enterococcus cecorum from bone lesions in broiler chickens. Vet Rec 150:27
Herdt PD, Defoort P, Steelant JV, Swam H, Tanghe L, Goethem SV, Vanrobaeys M (2009) Enterococcus cecorum osteomyelitis and arthritis in broiler chickens. Vlaams Diergeneeskd Tijdschr 78:44–48
Stalker MJ, Brash ML, Weisz A, Ouckama RM, Slavic D (2010) Arthritis and osteomyelitis associated with Enterococcus cecorum infection in broiler and broiler breeder chickens in Ontario, Canada. J Vet Diagn Invest 22:643–645
Makrai L, Nemes C, Simon A, Ivanics E, Dudas Z, Fodor L, Glavits R (2011) Association of Enterococcus cecorum with vertebral osteomyelitis and spondylolisthesis in broiler parent chicks. Acta Vet Hung 59:11–21
Aitchison H, Poolman P, Coetzer M, Griffiths C, Jacobs J, Meyer M, Bisschop S (2014) Enterococcal-related vertebral osteoarthritis in South African broiler breeders: a case report. J S Afr Vet Assoc 85:1077
Talebi A, Taifebagherlu J, Sharifi A, Delkhosh-Kasmaie F (2016) Spondylitis in broiler breeder farms in West-Azerbaijan province, Iran: clinical report. Vet Res Forum 7:353–355
Jung A, Rautenschlein S (2014) Comprehensive report of an Enterococcus cecorum infection in a broiler flock in Northern Germany. BMC Vet Res 10:311
Borst LB, McLamb KA, Suyemoto MM, Chen LR, Levy MG, Sarsour AH, Cordova HA, Barnes HJ, Oviedo-Rondon EO (2019) Coinfection with Eimeria spp. decreases bacteremia and spinal lesions caused by pathogenic Enterococcus cecorum. Anim Feed Sci Technol 250:59–68
Schreier J, Rautenschlein S, Jung A (2021) Different virulence levels of Enterococcus cecorum strains in experimentally infected meat-type chickens. PLoS One 16:e0259904
Borst LB, Suyemoto MM, Sarsour AH, Harris MC, Martin MP, Strickland JD, Oviedo EO, Barnes HJ (2017) Pathogenesis of enterococcal spondylitis caused by Enterococcus cecorum in broiler chickens. Vet Pathol 54:61–73
Wideman RF Jr (2016) Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult Sci 95:325–344
Remiot P, Panaget G, Chataigner E, Chevalier D (2019) Enterococcus cecorum in broilers: a survey in farm to identify risky zootechnical practices. In: 13èmes Journées de la Recherche Avicole et Palmipèdes à Foie Gras, Tours, March 2019, pp 116–120
Tsiouris V, Georgopoulou I, Batzios C, Pappaioannou N, Ducatelle R, Fortomaris P (2018) Heat stress as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol 47:616–624
Quinteiro-Filho WM, Gomes AV, Pinheiro ML, Ribeiro A, Ferraz-de-Paula V, Astolfi-Ferreira CS, Ferreira AJ, Palermo-Neto J (2012) Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol 41:421–427
Tang LP, Li WH, Liu YL, Lun JC, He YM (2021) Heat stress aggravates intestinal inflammation through TLR4-NF-kappaB signaling pathway in Ma chickens infected with Escherichia coli O157:H7. Poult Sci 100:101030
Rostagno MH (2020) Effects of heat stress on the gut health of poultry. J Anim Sci 98:skaa090
Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ (2014) Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One 9:e91941
Stanley D, Geier MS, Hughes RJ, Denman SE, Moore RJ (2013) Highly variable microbiota development in the chicken gastrointestinal tract. PLoS One 8:e84290
Zenner C, Hitch TCA, Riedel T, Wortmann E, Tiede S, Buhl EM, Abt B, Neuhaus K, Velge P, Overmann J, Kaspers B, Clavel T (2021) Early-life immune system maturation in chickens using a synthetic community of cultured gut bacteria. mSystems 6:01300–20
Torok VA, Hughes RJ, Mikkelsen LL, Perez-Maldonado R, Balding K, MacAlpine R, Percy NJ, Ophel-Keller K (2011) Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl Environ Microbiol 77:5868–5878
Richards P, Fothergill J, Bernardeau M, Wigley P (2019) Development of the caecal microbiota in three broiler breeds. Front Vet Sci 6:201
Torok VA, Hughes RJ, Ophel-Keller K, Ali M, Macalpine R (2009) Influence of different litter materials on cecal microbiota colonization in broiler chickens. Poult Sci 88:2474–2481
Seidlerova Z, Kubasova T, Faldynova M, Crhanova M, Karasova D, Babak V, Rychlik I (2020) Environmental impact on differential composition of gut microbiota in indoor chickens in commercial production and outdoor, backyard chickens. Microorganisms 8:767
Oakley BB, Vasconcelos EJR, Diniz P, Calloway KN, Richardson E, Meinersmann RJ, Cox NA, Berrang ME (2018) The cecal microbiome of commercial broiler chickens varies significantly by season. Poult Sci 97:3635–3644
Song J, Xiao K, Ke YL, Jiao LF, Hu CH, Diao QY, Shi B, Zou XT (2014) Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult Sci 93:581–588
Shi D, Bai L, Qu Q, Zhou S, Yang M, Guo S, Li Q, Liu C (2019) Impact of gut microbiota structure in heat-stressed broilers. Poult Sci 98:2405–2413
Zhu L, Liao R, Wu N, Zhu G, Yang C (2019) Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl Microbiol Biotechnol 103:461–472
Varasteh S, Braber S, Akbari P, Garssen J, Fink-Gremmels J (2015) Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One 10:e0138975
Mishra B, Jha R (2019) Oxidative stress in the poultry gut: potential challenges and interventions. Front Vet Sci 6:60
Gu XH, Hao Y, Wang XL (2012) Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult Sci 91:790–799
Tellez G Jr, Tellez-Isaias G, Dridi S (2017) Heat stress and gut health in broilers: role of tight junction proteins. Adv Food Techn Nutr Sci 3:e1–e4
Hu YJ, Wang YD, Tan FQ, Yang WX (2013) Regulation of paracellular permeability: factors and mechanisms. Mol Biol Rep 40:6123–6142
Awad WA, Hess C, Hess M (2017) Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins (Basel) 9:60
Ross 308 Broiler Management Handbook. https://en.aviagen.com/brands/ross/products/ross-308. Accessed 15 Sept 2022
Schreier J, Karasova D, Crhanova M, Rychlik I, Rautenschlein S, Jung A (2022) Influence of lincomycin-spectinomycin treatment on the outcome of Enterococcus cecorum infection and on the cecal microbiota in broilers. Gut Pathog 14:3
Mignard S, Flandrois JP (2006) 16S rRNA sequencing in routine bacterial identification: a 30-month experiment. J Microbiol Methods 67:574–581
Patel JB (2001) 16S rRNA gene sequencing for bacterial pathogen identification in the clinical laboratory. Mol Diagn 6:313–321
Wilson KH, Blitchington RB, Greene RC (1990) Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J Clin Microbiol 28:1942–1946
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857
Rzeznitzeck J, Breves G, Rychlik I, Hoerr FJ, von Altrock A, Rath A, Rautenschlein S (2022) The effect of Campylobacter jejuni and Campylobacter coli colonization on the gut morphology, functional integrity, and microbiota composition of female turkeys. Gut Pathog 14:33
von Buchholz JS, Bilic I, Aschenbach JR, Hess M, Mitra T, Awad WA (2021) Establishment of a novel probe-based RT-qPCR approach for detection and quantification of tight junctions reveals age-related changes in the gut barriers of broiler chickens. PLoS One 16:e0248165
Mitra T, Bilic I, Hess M, Liebhart D (2016) The 60S ribosomal protein L13 is the most preferable reference gene to investigate gene expression in selected organs from turkeys and chickens, in context of different infection models. Vet Res 47:105
Chrzastek K, Borowska D, Kaiser P, Vervelde L (2014) Class B CpG ODN stimulation upregulates expression of TLR21 and IFN-gamma in chicken Harderian gland cells. Vet Immunol Immunopathol 160:293–299
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Statist Soc B 57:289–300
Jung A, Petersen H, Teske L, Rautenschlein S (2017) Colonization patterns of Enterococcus cecorum in two different broiler production cycles detected with a newly developed quantitative real-time PCR. BMC Microbiol 17:106
Calefi AS, Honda BT, Costola-de-Souza C, de Siqueira A, Namazu LB, Quinteiro-Filho WM, Fonseca JG, Aloia TP, Piantino-Ferreira AJ, Palermo-Neto J (2014) Effects of long-term heat stress in an experimental model of avian necrotic enteritis. Poult Sci 93:1344–1353
Norup LR, Jensen KH, Jørgensen E, Sørensen P, Juul-Madsen HR (2008) Effect of mild heat stress and mild infection pressure on immune responses to an E. coli infection in chickens. Animal 2:265–274
Alhenaky A, Abdelqader A, Abuajamieh M, Al-Fataftah AR (2017) The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J Therm Biol 70:9–14
Bartlett J, Smith M (2003) Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult Sci 82:1580–1588
Johnson JS (2018) Heat stress: impact on livestock well-being and productivity and mitigation strategies to alleviate the negative effects. Anim Prod Sci 58:1404–1413
Deeb N, Cahaner A (2002) Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult Sci 81:293–301
Garriga C, Hunter RR, Amat C, Planas JM, Mitchell MA, Moreto M (2006) Heat stress increases apical glucose transport in the chicken jejunum. Am J Physiol Regul Integr Comp Physiol 290:195–201
Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sakai M, Sa LR, Ferreira AJ, Palermo-Neto J (2010) Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult Sci 89:1905–1914
Tabler TW, Greene ES, Orlowski SK, Hiltz JZ, Anthony NB, Dridi S (2020) Intestinal barrier integrity in heat-stressed modern broilers and their ancestor wild jungle fowl. Front Vet Sci 7:249
Sgavioli S, Domingues C, Santos E, de Quadros T, Borges L, Garcia R, Louzada M, Boleli I (2016) Effect of in-ovo ascorbic acid injection on the bone development of broiler chickens submitted to heat stress during incubation and rearing. Braz J Poult Sci 18:153–162
Chen LR, Suyemoto MM, Sarsour AH, Cordova HA, Oviedo-Rondon EO, Barnes HJ, Borst LB (2018) Prevalence and severity of osteochondrosis of the free thoracic vertebra in three modern broiler strains and the Athens Canadian Random Bred control broiler. Avian Pathol 47:152–160
Wideman RF Jr, Pevzner I (2012) Dexamethasone triggers lameness associated with necrosis of the proximal tibial head and proximal femoral head in broilers. Poult Sci 91:2464–2474
Ekesi NS, Hasan A, Parveen A, Shwani A, Rhoads DD (2021) Embryo lethality assay as a tool for assessing virulence of isolates from bacterial chondronecrosis with osteomyelitis in broilers. Poult Sci 100:101455
Abdelqader A, Al-Fataftah AR (2014) Thermal acclimation of broiler birds by intermittent heat exposure. J Therm Biol 39:1–5
Jung A, Chen LR, Suyemoto MM, Barnes HJ, Borst LB (2018) A review of Enterococcus cecorum infection in poultry. Avian Dis 62:261–271
Logue CM, Andreasen CB, Borst LB, Eriksson H, Hampson DJ, Sanchez S, Fulton RM (2020) Other bacterial diseases. In: Swayne DE, Boulianne M, Logue CM, McDougald LR, Venugopal N, Suarez DL (eds) Diseases of poultry, vol I. Wiley, New York
Suyemoto MM, Barnes HJ, Borst LB (2017) Culture methods impact recovery of antibiotic-resistant enterococci including Enterococcus cecorum from pre- and postharvest chicken. Lett Appl Microbiol 64:210–216
Bahrndorff S, Rangstrup-Christensen L, Nordentoft S, Hald B (2013) Foodborne disease prevention and broiler chickens with reduced Campylobacter infection. Emerg Infect Dis 19:425–430
Hankel J, Bodmann B, Todte M, Galvez E, Strowig T, Radko D, Antakli A, Visscher C (2021) Comparison of chicken cecal microbiota after metaphylactic treatment or following administration of feed additives in a broiler farm with enterococcal spondylitis history. Pathogens 10:1068
Mohammed AA, Jiang S, Jacobs JA, Cheng HW (2019) Effect of a synbiotic supplement on cecal microbial ecology, antioxidant status, and immune response of broiler chickens reared under heat stress. Poult Sci 98:4408–4415
Clarke LL (2009) A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 296:1151–1166
Tellez G (2020) Effects of a noni-supplemented diet on intestinal tight junction proteins and stress biomarkers in heat-stressed broiler chickens. Bachelor Thesis. University of Arkansas
Santos RR, Awati A, Roubos-van den Hil PJ, van Kempen T, Tersteeg-Zijderveld MHG, Koolmees PA, Smits C, Fink-Gremmels J (2019) Effects of a feed additive blend on broilers challenged with heat stress. Avian Pathol 48:582–601
Proszkowiec-Weglarz M, Schreier LL, Kahl S, Miska KB, Russell B, Elsasser TH (2020) Effect of delayed feeding post-hatch on expression of tight junction- and gut barrier-related genes in the small intestine of broiler chickens during neonatal development. Poult Sci 99:4714–4729
Park I, Lee Y, Goo D, Zimmerman NP, Smith AH, Rehberger T, Lillehoj HS (2020) The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poult Sci 99:725–733
Stefanello C, Rosa DP, Dalmoro YK, Segatto AL, Vieira MS, Moraes ML, Santin E (2019) Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front Vet Sci 6:491
Cheng YF, Chen YP, Chen R, Su Y, Zhang RQ, He QF, Wang K, Wen C, Zhou YM (2019) Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult Sci 98:4767–4776
Macelline SP, Wickramasuriya SS, Cho HM, Kim E, Shin TK, Hong JS, Kim JC, Pluske JR, Choi HJ, Hong YG, Heo JM (2020) Broilers fed a low protein diet supplemented with synthetic amino acids maintained growth performance and retained intestinal integrity while reducing nitrogen excretion when raised under poor sanitary conditions. Poult Sci 99:949–958
Acknowledgements
The authors wish to thank Marion Burmester, Ilka Baumann, and Susanne Nagel for their excellent technical support and Melanie Bode, Sonja Bernhardt, and Amanda Silberborth for their help in conducting the animal experiment.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the QS Fachgesellschaft Geflügel GmbH, Bonn, Germany. The Open Access publication was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 491094227 “Open Access Publication Funding” and the University of Veterinary Medicine Hannover, Foundation.
Author information
Authors and Affiliations
Contributions
AJ, JS, and SR planned and designed the experiments. JS conducted the animal experiments, performed laboratory work and statistical analysis, and drafted the first manuscript. DK and MC performed the sequencing of the caecal microbiota. Sequencing output was analysed by IR. GB helped with the analysis and interpretation of the Ussing chamber experiments. AJ, IR, GB, and SR reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Animal Ethics Committee of the University of Veterinary Medicine, Foundation, Hannover and the Lower Saxony State Office for Consumer Protection and Food Safety approved the study design (33.19-42505-04-19/3170).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Composition of serosal and mucosal buffer solutions used for the Using chamber experiments.
Additional file 2.
Relative abundance (%) of caecal microbiota at the phylum level.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schreier, J., Rychlik, I., Karasova, D. et al. Influence of heat stress on intestinal integrity and the caecal microbiota during Enterococcus cecorum infection in broilers. Vet Res 53, 110 (2022). https://doi.org/10.1186/s13567-022-01132-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-022-01132-y