Abstract
An alphaherpesvirus carries dozens of viral proteins in the envelope, tegument and capsid structure, and each protein plays an indispensable role in virus adsorption, invasion, uncoating and release. After infecting the host, a virus eliminates unfavourable factors via multiple mechanisms to escape or suppress the attack of the host immune system. Post-translational modification of proteins, especially phosphorylation, regulates changes in protein conformation and biological activity through a series of complex mechanisms. Many viruses have evolved mechanisms to leverage host phosphorylation systems to regulate viral protein activity and establish a suitable cellular environment for efficient viral replication and virulence. In this paper, viral protein kinases and the regulation of viral protein function mediated via the phosphorylation of alphaherpesvirus proteins are described. In addition, this paper provides new ideas for further research into the role played by the post-translational modification of viral proteins in the virus life cycle, which will be helpful for understanding the mechanisms of viral infection of a host and may lead to new directions of antiviral treatment.
Similar content being viewed by others
1 Introduction
Herpesviruses constitute a family of DNA double-stranded viruses with a tegument structure. Based on the different biological characteristics and clinical pathogenic characteristics of viruses, the International Committee on the Taxonomy of Viruses (ICTV) categorizes Herpesviridae into three subfamilies: Alphaherpesvirinae, Betaherpesvirinae and Gammaherpesvirinae [1]. For all herpesviruses, a complete virion consists of four parts: a core containing a double-stranded DNA genome, a capsid, an envelope, and a tegument [2]. Alphaherpesvirinae subfamily includes herpes simplex virus type-1/2 (HSV-1/2), duck plague virus (DPV) [3,4,5,6], pseudorabies virus (PRV), varicella-zoster virus (VZV), equine herpesvirus (EHV), bovine herpesvirus (BoHV) and Marek’s disease virus (MDV) [7,8,9,10,11].
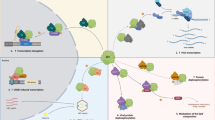
Protein post-translational modifications, which involve enzyme-mediated covalent addition of functional groups to proteins during or after synthesis, greatly increase the complexity of protein biological functions and result in order-of-magnitude changes between the types of proteins encoded in the genome and their biological functions. Therefore, post-translational modifications greatly expand the coding flexibility of living systems [12, 13]. Post-translational modifications include glycosylation [14, 15], phosphorylation, ubiquitination, methylation, arginylation and acetylation in eukaryotic cells [16,17,18,19,20,21,22,23]. Phosphorylation, in particular, is an important biological process controlled by a series of complex mechanisms to obtain a functional conformation and to regulate organisms exposed to external environmental stimuli and hormone signalling. After infecting host cells, viruses regulate the activity and stability of viral proteins and their interactions with other proteins through phosphorylation and dephosphorylation at various stages of the virus life cycle, thereby participating in a series of viral metabolic activities, such as viral replication and proliferation, as well as the assembly of virus particles (Figure 1). Most phosphorylated proteins carry multiple phosphorylation sites, but this does not mean that all potential phosphorylation sites in a protein molecule can be modified by phosphorylation. Of course, in some cases, phosphorylation at specific sites of certain proteins exerts a great impact on their function. For example, phosphorylation at specific sites in protein kinases regulates their activity [24]. In alphaherpesviruses, many viral protein functions are regulated via phosphorylation. For example, viral protein kinases such as HSV Us3 and UL13 can regulate other protein functions and their own catalytic activity via phosphorylation and autophosphorylation [25, 26]. In particular, the HSV transcriptional regulatory protein ICP4 regulates the underlying genome in sensory neurons through phosphorylation [27]; the HSV glycoprotein B (gB) affects viral replication and pathogenicity in mice through phosphorylation [28, 29]; the HSV-1 tegument protein pUL12 regulates its own nuclease activity through phosphorylation [30, 31]; VP8 of BoHV-1 regulates viral replication and assembly through phosphorylation [32, 33]; and phosphorylation of HSV-1, PRV and VZV pUL51 affects viral replication and pathogenicity [34,35,36]. In this paper, the effects of alphaherpesvirus protein kinases and the regulatory function of phosphorylation on the function of proteins are summarized to provide new ideas and directions for the future study of alphaherpesviruses.
Summary of the major functions of viral protein kinases and phosphorylated proteins in alphaherpesvirus. PKA protein kinase A, PKC protein kinase C, CK2 Casein kinase 2, PMLNBs promyelocytic leukaemia protein nuclear bodies, HCF-1 host cell factor-1, Oct-1 octamer-binding transcription factor-1, IE immediate early gene.
2 Alphaherpesvirus protein kinases
The reversible phosphorylation of proteins mediated by protein kinases and phosphatases is one of the most extensively studied post-translational modifications and one of the most common and effective ways in which viruses regulate their protein function [37,38,39]. In contrast to most viruses, herpesviruses encode specific protein kinases [40], and these protein kinases regulate viral gene expression, nucleocapsid nucleation, viral genome replication, host cell apoptosis, the intracellular transport of viral and cell membrane proteins, and axon transport of capsids to facilitate viral inhibition of the host immune response and achieve effective viral replication [28, 40,41,42,43,44,45,46,47,48,49,50,51]. In addition, viral protein kinases regulate not only the phosphorylation of viral proteins to regulate their function but also by autophosphorylation, which regulates their catalytic activity [52, 53] (Figure 2). Alphaherpesvirus protein kinases can be classified into the Us3 kinase and its homologues and the UL13 kinase and its homologues.
2.1 Us3 protein kinase and its homologues
Us3 is an early gene encoding a tegument protein that functions similar to protein kinase A (PKA), which is a serine/threonine protein kinase, and is conserved in all alphaherpesviruses but not in other Herpesviridae subfamilies. To date, deletion of Us3 homologue genes in HSV-1, HSV-2, VZV, PRV, MDV-1, BoHV-1/5, or DPV disrupts cell-type-dependent viral replication under cell culture conditions [54,55,56]. HSV-1 (among the most common of the representative alphaherpesviruses) carries the Us3 kinase, a regulator of viral replication and pathogenicity. Us3 functions related to viral replication and pathogenicity are becoming increasingly clear. For example, Us3 blocks apoptosis induced by viral and cellular proteins [49, 54, 57, 58], regulates the nuclear egress of the nucleocapsid [47, 48, 59, 60], controls the morphology or microtubule network of infected cells [25, 54, 61,62,63], escapes the host antiviral response [64,65,66,67], promotes gene expression through histone deacetylation [68,69,70] and regulates the intracellular transport of viral and cellular proteins in infected cells [56, 71, 72]. Thus, Us3 is a multifunctional protein that plays various roles in the viral life cycle through the phosphorylation of many viral substrates (Table 1). Moreover, Us3 undergoes self-specific autophosphorylation at Ser147, and Us3 phosphorylated at Ser147 shows higher kinase activity than unphosphorylated Us3 [25]. Although only a small amount of Us3 is autophosphorylated in virus-infected cells, this kinase is required for the proper viral localization and cytopathic induction. In addition, the autophosphorylation of Us3 at Ser147 promotes the development of herpes stromal keratitis (HSK) disease and viral replication in mice [73]. These results indicate that Us3 protein kinase activity is regulated by autophosphorylation at Ser147, mediating viral replication and pathogenicity to a certain extent. The ORF66 protein kinase, a homologue of HSV-1/2 Us3, is a VZV protein kinase originally identified based on its genomic location and homology to other HSV kinases and the presence of classical structural motifs common to all serine/threonine sequences. As mentioned above, protein kinases often maintain kinase function specificity via autophosphorylation; therefore, it is not surprising that ORF66 can be autophosphorylated.
2.2 UL13 protein kinase and its homologues
Similar to the Us3 protein kinase, the UL13 protein is a serine/threonine protein kinase located in the tegument structure [74]. HSV-1/2, DPV, MDV, PRV UL13 and VZV ORF47 are homologous [75], and the HSV-1 and HSV-2 UL13 homologues show the most similar amino acid sequence homology, which is 85% identical [76]. The widespread presence of UL13 also suggests that UL13 plays an important role in the life cycle of alphaherpesviruses.
Overall, UL13 exhibits three functions. First, UL13 promotes the disintegration of virions via the phosphorylation of other tegument proteins in the early stage of infection [77] and regulates the expression of viral genes through its kinase activity, such as by regulating ICP22 activity [43], stabilizing and regulating ICP0 activity [78, 79], and regulating VP11/12 activity [80]. Second, UL13 can inhibit interferon signalling pathway activation [81,82,83] and the transcription of host genes via the phosphorylation of cytokines [84]. Finally, UL13 can affect the horizontal transmission of a virus [85] (Table 2). Both HSV-1 UL13 and HSV-2 UL13 have been reported to be autophosphorylated in vitro [78, 86, 87]. Ser-118 and Ser-121 of HSV-2 UL13 were replaced with alanine residues, and both of the mutations significantly reduced the autophosphorylation of UL13. However, only the mutant in which Ser-121 was replaced with alanine significantly reduced the ability of HSV-2 UL13 to phosphorylate exogenous substrates. Similarly, Koyanagi et al. [26] found that phosphorylation of HSV-2 UL13 Ser-18 regulated its function in infected cells and that this regulatory effect was critical for HSV-2 replication and pathogenesis in vivo. These results indicate that Ser-18, Ser-118 and Ser-121 are the sites of UL13 autophosphorylation or are important to the recognition motif and that the autophosphorylation of HSV-2 UL13 affects the ability of this kinase to phosphorylate exogenous substrates, which is not strictly dependent on autophosphorylation [87]. ORF47, another serine/threonine kinase in VZV, is a homologue of the HSV UL13 protein kinase and an important regulator of the pathogenesis of VZV. Similar to UL13, ORF47 is autophosphorylated, but autophosphorylation is not required for its catalytic activity [88, 89].
2.3 Synergistic effect of the protein kinases Us3 and UL13
In all Herpesviridae, protein kinases encoded by viruses can be classified into two main categories, namely, conserved herpesvirus protein kinases (CHPKs) are conserved in the α-, β-, and γ-Herpesviridae subfamilies [90], and the remaining protein kinases are found only in the α-Herpesviridae subfamily [55]. UL13 is a CHPK, but Us3 is an α-subfamily kinase. It has been reported that Us3 kinase deletion did not affect viral DNA accumulation or virion-release levels but resulted in nucleocapsid aggregation, which was strictly cell type dependent [59, 91,92,93]. Knocking out UL13 resulted in a significant reduction in extracellular virions (almost a 30-fold decrease at a low MOI). This effect was greatly enhanced when both Us3 and UL13 kinases were knocked out [94], suggesting that UL13 may functionally complement the kinase activity of Us3 and that HSV-1 protein kinases promote viral replication in a cooperative manner comparable to CHPKs in β- and γ-herpesviruses during viral replication. In addition, Akihisa [95] demonstrated that UL13 specifically and directly phosphorylated the Us3 peptide encoded by a codon sequence from position 405 to 481 in vitro, and UL13-mediated phosphorylation of Us3 was not necessary for optimal kinase activity of Us3 in infected cells. In conclusion, UL13 functionally supplements Us3 kinase activity in vivo, but this effect is not induced by phosphorylation.
3 Alphaherpesvirus phosphorylated proteins
3.1 Phosphorylation of the serine-rich region of ICP4
ICP4 is an immediate early gene of HSV that encodes the main transcriptional regulatory protein of this virus [96, 97], which is necessary for the early and late transcription of viral genes and the inhibition of its own expression and that of other immediate early genes of the virus [98,99,100]. In infected cells, ICP4 has been shown to be phosphorylated at serine and threonine residues [101, 102], and the serine-rich region (residues 142–210) of ICP4 has been identified as the target of phosphorylation, with multiple serine residues and one threonine residue phosphorylated [103]. Previous reports have led to a hypothesis suggesting that after viral infection, ICP4 is first phosphorylated by PKA, PKC, or another kinase at its serine-rich region, and then, phosphorylated ICP4 stimulates ICP4-related or shows inherent kinase activity, which triggers the phosphorylation in the remainder of the ICP4 molecule in either a cis or trans manner. Thus, ICP4 may be continuously phosphorylated at multiple phosphorylation sites [103, 104]. In addition, the serine-rich region of ICP4 can induce ICP4 phosphorylation and viral replication, but this region is not absolutely necessary for this effect; therefore, certain ICP4 phosphorylation mechanisms function independent of its serine-rich region [103]. Kramer et al. [27] found that ICP4 is expressed and phosphorylated at low levels during latent infection, which may affect the latent state by inhibiting its activity. Phosphorylation of ICP4 may affect its ability to bind DNA and inhibit transcription. Thus, phosphorylation of ICP4 regulates underlying gene expression in sensory neurons via a signal transduction pathway.
3.2 Phosphorylation of the three major phosphorylation regions (11 potential phosphorylation sites) in ICP0
ICP0 is a 110 kDa phosphorylated nuclear protein that transactivates the early and late genes of HSV-1 as well as many cellular and other viral genes [105, 106]. In addition, ICP0 is a SUMO-targeted ubiquitin ligase (STUbL). Its significant function is induction of promyelocytic leukaemia protein nuclear body (PML NB) degradation through the ubiquitin protease pathway [107]. To date, two kinases have been confirmed to affect the phosphorylation of ICP0: Cdk1 phosphorylates the second exon (residues from 20 to 241) of ICP0 in vitro [108], Cdk2 phosphorylates the SUMO-binding motif SLS4 in ICP0, which increases the affinity of ICP0 for ubiquitinated proteins and enhances the STUbL activity of ICP0. In addition, ICP0 can neutralize the host antiviral response via phosphorylation of SLS4 mediated by Cdk2 to affect the degradation of PML NBs [109]. Davido et al. showed that ICP0 carries at least three phosphorylation-prone regions (11 potential phosphorylation sites): Phos.1 (Ser-224, Thr-226, Thr-232 and Thr-232), Phos.2 (Ser-365, Ser-367 and Ser-371) and Phos.3 (Ser-508, Ser-514, Ser-517 and Thr-518) [110]. Mutations in these three phosphorylation regions affect subcellular and subnuclear localization of ICP0 (Phos.3), ND10-disrupting activity in certain cells (Phos.2 and Phos.3), and transactivation activity in Vero cells (Phos.1 and Phos.3). Among these mutants, Phos.1 exerts the greatest influence on ICP0-induced transactivation and viral replication [110]. Thus, phosphorylation of ICP0 affects not only its subcellular localization but also its transactivation activity and antiviral response to host cells.
3.3 Phosphorylation of gB at Thr-887
In the herpesvirus family, gB is a glycoprotein that is highly conserved in the envelope, which allows the virus to adsorb onto a host cell plasma membrane, mediates the fusion of the virus envelope and cell membrane, allows viral penetration and intercellular diffusion, and causes viral cyclic replication [111,112,113]. HSV-1 gB is a substrate of Us3, and its phosphorylation site, Thr-887, is located near an endocytic motif. After replacing the residue at this site with alanine, the endocytosis of gB was significantly increased, thus downregulating the cell surface expression of gB in infected cells. However, the wild-type phenotype was restored after a phosphorylation mutation of this site was simulated [28, 29]. gB on the cell surface is an important target for inducing antibody-dependent cytotoxicity and an effective inducer of the immune responses in vivo [114,115,116]. The lysis of HSV-1-infected cells by natural killer cells is related to the amount of gB on the cell surface [114]. Therefore, phosphorylation of gB by Us3 reduces the amount of gB molecules on the cell surface exposed to the immune system, which may make it difficult for the infected cells to be recognized and attacked by the immune system in vivo. Consistent with this supposition, blocking the phosphorylation of gB at Thr-887 significantly reduced the development of HSK disease and viral replication in mice with ocular infection. These results suggest that phosphorylation of HSV-1 gB at Thr-887 plays an important role in viral replication and pathogenicity in mice by regulating the expression of gB on the cell surface and nucleation of the nucleocapsid.
3.4 Phosphorylation of pUL12 at Thr-371, Thr-474 and Ser-604
The HSV-1 UL12 gene encodes an alkaline nuclease with 5′–3′ exonuclease activity and is homologous to nucleases of other viruses in the Herpesviridae family [117, 118]. The exonuclease activity of pUL12 is necessary for HSV-1 to generate infectious viral particles that spread between cells, and the phosphorylation of pUL12 regulates nuclease activity and plays an important role in viral replication and pathogenesis [30, 31, 119]. HSV-1 pUL12 has been reported to carry three phosphorylation sites: Tyr-371, Thr-474 and Ser-604. Among these sites, Tyr-371 is highly conserved in pUL12 homologues in all herpesvirus subfamilies, and the regulation of phosphorylation at this site in the pUL12 protein has been investigated via mutation of Tyr-371. The results of mutation showed that the phosphorylation of Tyr-371 remained unaffected, of the expression of pUL12 stable and pUL12 exonuclease activity in HSV-1-infected cells proceeded in a cell-dependent manner; this effect was most obvious in HEL cells [31]. In addition, phosphorylation of Tyr-371 was required for the efficient replication of HSV-1 in cell culture, and its effect on viral replication varied with cell type and MOI. Similarly, intracranial injection of an pUL12 Tyr-371 mutant virus led to much lower neurotoxicity in mice than that the injection of a mutant virus that was pseudophosphorylated at this site [31]. These results suggest that pUL12 mainly promotes HSV-1 replication, intercellular diffusion and neurotoxicity in mice through the phosphorylation of Tyr371.
3.5 Phosphorylation of the nucleus-egressing complex UL31/UL34
In recent years, studies on HSV-1, HSV-2, PRV, EHV-1 and DPV have shown that the UL31 protein plays a positive role in the formation of the primary envelope of the viral nucleocapsid and the synthesis, assembly and release of viral DNA and that it regulates the DNA damage response of virus-infected cells [120,121,122,123,124,125]. The HSV-1 UL31 protein is an alkaline phosphorylated nuclear protein with a hydrophilic amino terminal and nuclear localization signal sequence [126]. HSV-1/2 UL34 is a membrane-anchoring protein that can also function as a nucleoplasmic protein. It affects the redistribution of the endoplasmic reticulum (ER) around the nuclear membrane and is necessary for the effective localization of ER-associated regulators related to the nuclear egress of a nucleocapsid to the nuclear membrane [127, 128]. UL31 and UL34 proteins interact with each other in the nuclear membrane to form a nuclear egress complex (NEC), enhancing the stability of each other. Removal of one protein leads to the mislocalization of the other protein, and NEC plays an important role in the formation of the primary envelope of a virus [129,130,131]. Serine phosphorylation of HSV-1 UL31 by US3 has been reported to be essential for the function of the UL31 protein, which is involved in regulating the proper localization of UL31/UL34, the formation of the primary envelope, and the fusion of the nascent virion envelope with the outer nuclear membrane [47, 48, 92, 95]. After the deletion of Us3, UL31 and UL34 accumulated abnormally in the nuclear membrane, presenting as distributed puncta and leading to nuclear invagination with multiple primary envelope virions. However, in the absence of Us3 protein kinase activity, a pseudophosphorylated UL31 mutant of UL31 restored the wild-type localization of UL31 and UL34, suggesting that Us3 regulates the localization of UL31 and UL34 to the nuclear membrane through phosphorylation of UL31 [62, 132,133,134]. Nevertheless, phosphorylation of UL34 Thr-195 and Ser-198 by US3 exerted little effect on viral replication, localization of UL34 and UL31, or their nuclear export [48]; therefore, although UL34 was phosphorylated, the significance of its phosphorylation was unclear. In addition, phosphorylation of PRV UL31, a protein homologous to HSV-1 UL31, may play an important role during PRV infection, possibly by regulating the proper localization of the virus or other uncharacterized functions; for example, it may play regulatory roles in NEC localization and nucleocapsid expulsion from a cell [135].
3.6 Phosphorylation of pUL47 at Ser-77, Ser-88 and Thr-685
VP8, a 96 kDa phosphorylated protein encoded by the UL47 gene, is one of the most abundant tegument proteins in BoHV-1 and is critical for viral replication. Casein kinase 2 (CK2) and Us3 mediate BoHV-1 post-translational phosphorylation [33]. By mutating the VP8 protein of BoHV-1, Zhang et al. [33] found that the effect of VP8 on virions mainly occurred in two stages: one stage before or during the expulsion of the capsid, during which no phosphorylation is needed, and the other stage is in the Golgi apparatus, where phosphorylation of VP8 is needed. This finding indicates that phosphorylation of VP8 plays a role in DNA encapsulation and secondary envelopment of the virus, while nuclear localization of VP8 is not affected by phosphorylation. In contrast, NLS-mediated nuclear localization of VP13/14 (a homologue of VP8) in HSV requires Us3 phosphorylation of VP13/14 [136], which temporally occurs between cell penetration and viral protein synthesis [77]. Kato et al. conducted biological analysis of the phosphorylation sites of pUL47 and found that the phosphorylation sites of pUL47 (Ser-77, Ser-88 and Thr-685) were all very close to the nuclear localization signal (NLS) sequence and nuclear export signal (NES) sequence. It has been reported that the sites near the phosphorylated protein NLS sequences are common regulatory sites that mediate the transport of NLS-containing proteins into the nucleus. Therefore, it can be speculated that the phosphorylation of pUL47 regulates its nuclear localization in cells. Consistent with this supposition, a Ser-77 mutation in pUL47 affected the nuclear localization of pUL47 in cells, and after pseudophosphorylation of this mutated site, pUL47 showed wild-type nuclear localization [71]. These results suggest that phosphorylation of pUL47 promotes nuclear exportation of the virus by regulating its nuclear localization in infected cells.
3.7 Phosphorylation of VP16 at Ser-355 and Ser-375
VP16, a phosphorylated protein encoded by the UL48 gene, is a transcriptional activator of immediate early genes. In alphaherpesviruses, homologous VP16 proteins carry transactivation domains (TADs) and DNA-binding domains (DBDs), which are called core domains. The DBD of the VP16 protein is conserved in multiple alphaherpeviruses, but neither the location nor sequence of the TAD is conserved [137]. In the case of HSV, once a virus infects a host cell, VP16 forms a transcription regulatory complex with two cellular proteins, octamer-binding transcription factor 1 (Oct-1) and host cell factor-1 (HCF-1), to activate the transcription of immediate early genes [138,139,140,141]. The phosphorylation of VP16 mainly occurs at a serine residue, with threonine phosphorylation rare, and tyrosine phosphorylation negligible. VP16 can be phosphorylated by one or more cellular kinases, and the main phosphorylation sites are located at C-terminal serine residues downstream of amino acid 370 [142, 143]. Phosphorylation of HSV VP16 at Ser-355 and Ser-375 regulates the interaction of VP16 with HCF-1 and Oct-1, which is required for the Oct-1-HCF-1-VP16 complex to bind to an immediate early gene promoter [144]. In summary, phosphorylation of VP16 switches on the VP16 transcriptional activation function and regulates the earliest stage of HSV entry into the lytic cycle and in vivo latent reactivation.
3.8 Phosphorylation of the tegument protein VP22
VP22 is a 300-amino acid structural protein encoded by the UL49 gene in herpesviruses. It is one of the most abundant tegument proteins, with more than 2000 copies per virion [145]. VP22 can interact with a variety of tegument proteins and glycoproteins to regulate virion assembly, mediate the transport of capsid proteins from the outer membrane of the nucleus to the Golgi apparatus, and promote the formation of mature virions [146]. In addition, the VP22 proteins of HSV-1, HSV-2, VZV and BoHV-1 can be post-translationally modified via phosphorylation. Moreover, during HSV-1/2 infection, the tegument protein VP22 exists in both phosphorylated and nonphosphorylated forms, and only the VP22 with low phosphorylation levels is assembled into an virion. However, phosphorylation is not required for efficient assembly into the tegument [147,148,149,150,151,152], suggesting that the phosphorylation levels of VP22 regulate the assembly of virus particles. Similarly, a tyrosine phosphorylation site in BoHV-1 VP22 is critical for the effective assembly of VP22 into a virion [149, 150, 153]. Many viruses encode their own kinases; therefore, the kinases critical for VP22 phosphorylation vary among herpesviruses. For example, CK2 and UL13 are protein kinases critical for phosphorylating VP22 in HSV-1-infected cells. In VZV, ORF47 is the protein kinase critical for the phosphorylation of VP22, while in BoHV-1, Us3 is the protein kinase critical for phosphorylation of VP22 [32, 150, 151, 153].
3.9 Phosphorylation of pUL51 at Ser-184
The HSV-1 pUL51 protein is conserved in the α-, β- and γ-herpesvirus subfamilies. It is phosphorylated by protein kinases and plays an important role in viral secondary envelopment and cell-to-cell spread, as well as in the effective replication of the virus in cells [34,35,36, 154,155,156,157,158]. Akihisa et al. identified a functional phosphorylation site in pUL51, thereby elucidating the regulatory mechanism of pUL51 in HSV-1 infected cells [34]. They identified five phosphorylation sites of pUL51 via mass spectrometry and found that mutations in the phosphorylation site Ser-184 induced abnormal accumulation of primary enveloped virions in the nuclear membrane space and accumulation of secondary enveloped virions in the cytoplasm. Moreover, Ser-184 phosphorylation significantly affected the virulence of HSV-1. These results indicate that phosphorylation of pUL51 is crucial for morphological changes and the stable local adhesion of HSV-1-infected cells, as well as for the replication and pathogenicity of the HSV-1 virus itself.
3.10 Phosphorylation of IE62 at Ser686 and Ser722
IE62 is a nuclear transcriptional regulatory protein of VZV that drives VZV transcription through interactions with transcriptional activators and components of the mediator complex [159, 160]. It was reported that IE62 was distributed in the nucleus at the early stage of VZV infection before the expression of the ORF66 protein kinase. With increasing expression of the ORF66 protein kinase, IE62 begins to accumulate in the cytoplasm. However, in VZV-infected cells without ORF66 kinase, IE62 no longer appears in the cytoplasm. The kinase activity of ORF66 is necessary for the localization of IE62 in cells. In addition, ORF66 directly phosphorylates the Ser-686 and Ser-722 sites of IE62 in cells and in vitro [161, 162]. Moreover, Ser-686 is highly conserved in all IE62 homologues of herpesviruses, and this site is closely associated with a nuclear input signal, again suggesting that the cellular localization of viral proteins may be regulated by their phosphorylation. In addition to OFR66, IE62 is phosphorylated by the ORF47 kinase and CK2 [163]. The VZV protein phosphorylated by ORF47 is a homologue of HSV ICP22, IE63, which is a major transcript found in the human ganglion and latent animal models of VZV infection [164, 165].
In addition to the aforementioned proteins, many other viral proteins can be phosphorylated, which regulates their functions. For example, UL7 is phosphorylated by HSV-2 Us3. Although its phosphorylation exerted no effect on viral replication in cell culture, it was necessary for effective replication and pathogenicity of HSV-2 in vivo [166]; ICP1/2, also known as VP1/2, is a 270 kDa structural protein located in the HSV-1 tegument that is prone to serine phosphorylation, but the role played by phosphorylated VP1/2 has not been clarified [167]. The virion host shutoff (VHS) protein encoded by HSV UL41 specifically degrades mRNA, inducing the abrogation of host gene expression. Phosphorylation of VHS by Us3 and UL13 regulates the degradation of host mRNAs and the abrogation of host protein synthesis, contributing to the effective replication of the virus and regulating the protein kinase R (PKR)-mediated immune response [168,169,170,171]; VZV gE is phosphorylated by serine/threonine protein kinases and CK2 in mammalian cells [172, 173], while Us8A is colocalized with gE in HSV-1 and is phosphorylated in infected cells [174, 175]. Us8A Ser-61 is a target of Us3, and Us3 likely regulates the nerve invasion ability of the virus by regulating phosphorylation at Ser-61, particularly enhancing the replication ability of the virus in the trigeminal ganglion, further suggesting that phosphorylation of Us8A Ser-61 can effectively regulate viral replication [176, 177]. In addition, HSV VP11/12 is not only phosphorylated by viral protein kinases Us3/UL13 or cell protein kinases on serine/threonine residues but also phosphorylated on tyrosine residues. This tyrosine phosphorylation modification may help regulate the expression of viral genes in lymphocytes and alter their interactions with other tegument proteins or progeny viruses during assembly [77, 178,179,180].
4 Multiple interactions between phosphorylated alphaherpesvirus proteins
4.1 Interaction among VHS, VP22, VP16, and pUL47
VHS is an endoribonuclease that degrades cell and viral mRNA. The degradation of cellular mRNA by VHS promotes the synthesis of viral proteins by increasing the availability of cellular translation mechanisms, while the degradation of viral mRNA helps regulate the sequence expression of different viral genes [181,182,183,184]. In the course of viral infection, many viral proteins cooperate in the regulation of VHS activity to optimize the proliferation of viruses in host cells. For example, VP22 can promote the accumulation and translation efficiency of viral mRNA in the late stage of infection and can increase mRNA abundance by protecting mRNA from degradation through its RNA-binding activity [185]. The absence of VP22 leads to a significant reduction in translation efficiency, but this effect does not depend on mRNA abundance, and secondary mutations in VHS compensate for translation defects, suggesting that in HSV-1 infection, VP22 plays a role in regulating the translation inhibitory activity of VHS [186]. In addition, as a part of the VP22-VP16-VHS complex, the VHS protein can interact with VP16-VP22 complex during the initiation of translation but cannot interact with VP22 alone; therefore, VP22 and VP16 jointly regulate the activity of VHS [187, 188]. Finally, pUL47 has been reported to deliver a portion of VHS to the nucleus. In transfected cells, NES-mutated VHS degraded stable mRNA, but not in infected cells, suggesting that pUL47 largely blocks VHS degradation of viral mRNA [189]. In summary, the VP22, VP16 and pUL47 proteins regulate intracellular VHS levels, regulate viral gene cascade activation mechanisms and maintain viral mRNA degradation activity.
4.2 Interaction among pUL47, UL31, UL34, and Us3
It has previously been reported that pUL47 can form a complex with Us3 in HSV-1-infected cells, and pUL47 can colocalize with UL31 and UL34 to the nuclear membrane regardless of whether Us3 shows catalytic activity [71], increasing the likelihood that pUL47 interacts with UL31, UL34 and Us3 on the nuclear membrane. Similarly, Liu et al. [190] found that pUL47, UL31, UL34 and Us3 interacted on the nuclear membrane in a immunoprecipitation experiment. After the kinase activity of Us3 was eliminated, pUL47, UL31 and UL34 were all abnormally located in the nuclear membrane, presenting as puncta [190]. These results indicate that in HSV-1-infected cells, the localization of pUL47, UL31 and UL34 on the nuclear membrane is regulated by Us3 kinase activity, and the four proteins can jointly localize to the nuclear membrane to form higher-order complexes. In addition, loss of pUL47 resulted in aggregation of the capsid in the nucleus and a significant decrease in the number of primary envelope virions in the nuclear space. Thus, pUL47 promotes the formation of the HSV-1 primary envelope via its regulatory function mediated by its interactions with the UL31/UL34 complex and Us3, key regulators of HSV-1 nuclear egress.
4.3 Interaction among gE,VP22, gM, andICP0
Elliott et al. [191] found that HSV VP22 interacted with the immediate early protein ICP0, which not only affected ICP0 expression, subcellular localization and its participation in viral assembly but also inhibited the transactivation ability of ICP0 [192]. The absence of VP22 led to cell type-specific replication defects in MDBK cells and delays in viral protein synthesis, especially the production of the immediate early protein ICP0. Further studies have shown that this effect of VP22 on ICP0 is caused by ICP0 phosphorylation [152, 191]. A gE-VP22-gM-ICP0 multicomponent glycoprotein-tegument complex has been identified in HSV-1-infected cells. The complex centres on VP22, and its C-terminal binds to gE and gM, while its N-terminal recruits ICP0 to the complex [193]. VP22-ICP0 can form, but gE and gM are both needed for the multicomponent complex to form effectively and reside stably in a cell, suggesting that gE and gM may stabilize the binding of ICP0 through VP22 [193]. Interestingly, the glycoprotein-binding domain of VP22 is conserved in all alphaherpesviruses [194], suggesting that the gE-VP22-gM(-ICP0) complex may be critical for the replication of alphaherpesviruses.
5 Conclusion
After a virus infects host cells, a number of mechanisms are activated to induce or inhibit the cascade of intracellular signalling pathway activation, through phosphorylation and dephosphorylation to regulate the activity and stability of viral proteins and their interactions with intracellular proteins; their regulatory effects of viral replication, virus proliferation and virus particle assembly; and a series of metabolic activities. For example, major structural components of the HSV-1 tegument are phosphorylated when the virus enters an infected cell, and this phosphorylation mediates the dissociation of the tegument from the capsid in vivo under the action of VP13/14 and VP22 [77]. In addition, some viral proteins, such as VP1/2, VP13/14, VP16 and VP22, are phosphorylated during virion assembly and dephosphorylated after virion maturation, suggesting that phosphorylation and dephosphorylation are mechanisms that regulate HSV-1 dissociation and assembly. In addition to regulating the performance and metabolism of the viruses themselves, the evolution of phosphorylation has driven phenotypic diversity among species, further influencing their adaptation during evolution [166]. From this perspective, the effect of viral protein phosphorylation on antiviral drugs effectivity is very helpful for treatment development, but not all of the viral protein phosphorylation events during physiological viral activities show a significant regulatory effect; therefore, further study of viruses in host cell protein phosphorylation and their corresponding regulatory functions will lead to a better understanding of the viral infection mechanisms and identification of effective targets for virus prevention and control.
References
Gatherer D, Depledge DP, Hartley CA, Szpara ML, Vaz PK, Benkő M, Brandt CR, Bryant NA, Dastjerdi A, Doszpoly A, Gompels UA, Inoue N, Jarosinski KW, Kaul R, Lacoste V, Norberg P, Origgi FC, Orton RJ, Pellett PE, Schmid DS, Spatz SJ, Stewart JP, Trimpert J, Waltzek TB, Davison AJ (2021) ICTV virus taxonomy profile: herpesviridae. J Gen Virol 102:001673
Boštíková V, Salavec M, Smetana J, Sleha R, Coufalová M, Spliňo M, Boštík P (2014) Infections caused by human alpha herpes viruses. Epidemiol Mikrobiol Imunol 63:206–213 (in Czech)
Shi Y, Cheng A, Wang M (2012) Characterization of codon usage bias in UL21gene from Duck enteritis virus. Aasri Procedia 1:112–123
Hua C, Cheng A, Wang M, Guo Y, Xie W, Lou K (2009) Complete nucleotide sequence of the duck plague virus gE gene. Arch Virol 154:163–165
Guo Y, Cheng A, Wang M, Zhou Y (2009) Purification of anatid herpesvirus 1 particles by tangential-flow ultrafiltration and sucrose gradient ultracentrifugation. J Virol Methods 161:1–6
Yang L, Wang M, Cheng A, Yang Q, Wu Y, Huang J, Tian B, Jia R, Liu M, Zhu D, Chen S, Zhao X, Zhang S, Ou X, Mao S, Gao Q, Sun D, Yu Y, Zhang L (2022) UL11 protein is a key participant of the duck plague virus in its life cycle. Front Microbiol 12:792361
Kobty M (2015) Herpes simplex virus: beyond the basics. Neonatal Netw 34:279–283
Smith GA (2017) Assembly and egress of an alphaherpesvirus clockwork. Adv Anat Embryol Cell Biol 223:171–193
You Y, Cheng AC, Wang MS, Jia RY, Sun KF, Yang Q, Wu Y, Zhu D, Chen S, Liu MF, Zhao XX, Chen XY (2017) The suppression of apoptosis by α-herpesvirus. Cell Death Dis 8:e2749
He T, Wang M, Cao X, Cheng A, Wu Y, Yang Q, Liu M, Zhu D, Jia R, Chen S, Sun K, Zhao X, Chen X (2018) Molecular characterization of duck enteritis virus UL41 protein. Virol J 15:12
Lefkowitz EJ, Dempsey DM, Hendrickson RC, Orton RJ, Siddell SG, Smith DB (2018) Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res 46:D708–D717
Chen L, Kashina A (2021) Post-translational modifications of the protein termini. Front Cell Dev Biol 9:719590
Marino G, Eckhard U, Overall CM (2015) Protein termini and their modifications revealed by positional proteomics. ACS Chem Biol 10:1754–1764
Varki A (2016) Biological roles of glycans. Glycobiology 27:cww086
Reily C, Stewart TJ, Renfrow MB, Novak J (2019) Glycosylation in health and disease. Nat Rev Nephrol 15:346–366
Prabakaran S, Lippens G, Steen H, Gunawardena J (2012) Post-translational modification: nature’s escape from genetic imprisonment and the basis for dynamic information encoding. Wiley Interdiscip Rev Syst Biol Med 4:565–583
Liu Z, Li K, Liu X, Yu Y, Wang L, Kong Y, Chen M (2022) Production of microhomogeneous glycopeptide by a mutated NGT according FuncLib with unique sugar as substrate. Enzyme Microb Technol 154:109949
Addicks GC, Zhang H, Ryu D, Vasam G, Green AE, Marshall PL, Patel S, Kang BE, Kim D, Katsyuba E, Williams EG, Renaud JM, Auwerx J, Menzies KJ (2022) GCN5 maintains muscle integrity by acetylating YY1 to promote dystrophin expression. J Cell Biol 221:e202104022
Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, Gevaert K (2009) Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc Natl Acad Sci USA 10:8157–8162
Chen T, Muratore TL, Schaner-Tooley CE, Shabanowitz J, Hunt DF, Macara IG (2007) N-terminal alpha-methylation of RCC1 is necessary for stable chromatin association and normal mitosis. Nat Cell Biol 9:596–603
Kaji A, Kaji H, Novelli GD (1963) A soluble amino acid incorporating system. Biochem Biophys Res Commun 10:406–409
Kashina AS (2015) Protein arginylation: over 50 years of discovery. Methods Mol Biol 1337:1–11
Breitschopf K, Bengal E, Ziv T (1998) A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J 17:5964–5973
Lienhard GE (2008) Non-functional phosphorylations? Trends Biochem Sci 33:351–352
Kato A, Tanaka M, Yamamoto M, Asai R, Sata T, Nishiyama Y, Kawaguchi Y (2008) Identification of a physiological phosphorylation site of the herpes simplex virus 1-encoded protein kinase Us3 which regulates its optimal catalytic activity in vitro and influences its function in infected cells. J Virol 82:6172–6189
Koyanagi N, Kato A, Takeshima K (2018) Regulation of herpes simplex virus 2 protein kinase UL13 by phosphorylation and its role in viral pathogenesis. J Virol 92:e00807-18
Koyanagi N, Kato A, Takeshima K, Maruzuru Y, Kozuka-Hata H, Oyama M, Arii J, Kawaguchi Y (1995) Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J Virol 69:1389–1399
Kato A, Arii J, Shiratori I, Akashi H, Arase H, Kawaguchi Y (2009) Herpes simplex virus 1 protein kinase Us3 phosphorylates viral envelope glycoprotein B and regulates its expression on the cell surface. J Virol 83:250–261
Imai T, Arii J, Minowa A, Kakimoto A, Koyanagi N, Kato A, Kawaguchi Y (2011) Role of the herpes simplex virus 1 Us3 kinase phosphorylation site and endocytosis motifs in the intracellular transport and neurovirulence of envelope glycoprotein B. J Virol 85:5003–5015
Grady LM, Szczepaniak R, Murelli RP, Masaoka T, Le Grice SFJ, Wright DL, Weller SK (2017) The exonuclease activity of herpes simplex virus 1 UL12 is required for production of viral DNA that can be packaged to produce infectious virus. J Virol 91:e01380-17
Fujii H, Kato A, Mugitani M, Kashima Y, Oyama M, Kozuka-Hata H, Arii J, Kawaguchi Y (2014) The UL12 protein of herpes simplex virus 1 is regulated by tyrosine phosphorylation. J Virol 88:10624–10634
Labiuk SL, Lobanov V, Lawman Z, Snider M, van Babiuk LA, Hurk S (2010) Bovine herpesvirus-1 US3 protein kinase: critical residues and involvement in the phosphorylation of VP22. J Gen Virol 91:1117–1126
Zhang K, Brownlie R, van Snider M, Hurk S (2016) Phosphorylation of bovine herpesvirus 1 VP8 plays a role in viral DNA encapsidation and is essential for its cytoplasmic localization and optimal virion incorporation. J Virol 90:4427–4440
Kato A, Oda S, Watanabe M, Oyama M, Kozuka-Hata H, Koyanagi N, Maruzuru Y, Arii J, Kawaguchi Y (2018) Roles of the phosphorylation of herpes simplex virus 1 UL51 at a specific site in viral replication and pathogenicity. J Virol 92(18):e01035-18
Loret S, Guay G, Lippe R (2008) Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J Virol 82:8605–8618
Bechtel JT, Winant RC, Ganem D (2005) Host and viral proteins in the virion of Kaposi’s sarcoma-associated herpesvirus. J Virol 79:4952–4964
Sugiyama N, Ishihama Y (2016) Large-scale profiling of protein kinases for cellular signaling studies by mass spectrometry and other techniques. J Pharm Biomed Anal 130:264–272
Heierhorst J, Pearson R, Horne J (2000) Protein serine/threonine kinases. Annu Rev Biochem 56:567–613
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298:1912–1934
Jacob T, Van den Broeke C, Favoreel HW (2011) Viral serine/threonine protein kinases. J Virol 85:1158
Mcgeoch DJ, Davison AJ (1986) Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res 14:1765–1777
Smith RF, Smith TF (1989) Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein–Barr virus. J Virol 63:450–455
Purves FC, Ogle WO, Roizman B (1993) Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc Natl Acad Sci USA 90:6701–6705
Wolf DG, Courcelle CT, Prichard MN, Mocarski ES (2001) Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc Natl Acad Sci USA 98:1895–1900
Gershburg E, Raffa S, Torrisi MR, Pagano JS (2007) Epstein–Barr virus-encoded protein kinase (BGLF4) is involved in production of infectious virus. J Virol 81:5407–5412
Krosky PM, Baek MC, Coen DM (2003) The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J Virol 77:905–914
Reynolds AE, Wills EG, Roller RJ, Ryckman BJ, Baines JD (2002) Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J Virol 76:8939–8952
Ryckman BJ, Roller RJ (2004) Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J Virol 78:399–412
Leopardi R, Van Sant C, Roizman B (1997) The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA 94:7891–7896
Perkins D, Pereira EF, Gober M, Yarowsky PJ, Aurelian L (2002) The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J Virol 76:1435–1449
Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR (2007) Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol 81:9034–9049
Johnson LN, Noble ME, Owen DJ (1996) Active and inactive protein kinases: structural basis for regulation. Cell 85:149–158
Beenstock J, Mooshayef N, Engelberg D (2016) How do protein kinases take a selfie (autophosphorylate)? Trends Biochem Sci 41:938–953
Munger J, Roizman B (2001) The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc Natl Acad Sci USA 98:10410–10415
Deruelle MJ, Favoreel HW (2011) Keep it in the subfamily: the conserved alphaherpesvirus US3 protein kinase. J Gen Virol 92:18–30
Deng L, Wang M, Cheng A, Yang Q, Wu Y, Jia R, Chen S, Zhu D, Liu M, Zhao X, Zhang S, Huang J, Ou X, Mao S, Zhang L, Liu Y, Yu Y, Tian B, Pan L, Rehman MU, Chen X (2020) The pivotal roles of US3 protein in cell-to-cell spread and virion nuclear egress of duck plague virus. Sci Rep 10:7181
Wang X, Patenode C, Roizman B (2011) US3 protein kinase of HSV-1 cycles between the cytoplasm and nucleus and interacts with programmed cell death protein 4 (PDCD4) to block apoptosis. Proc Natl Acad Sci USA 108:14632–14636
Benetti L, Munger J, Roizman B (2003) The herpes simplex virus 1 US3 protein kinase blocks caspase-dependent double cleavage and activation of the proapoptotic protein BAD. J Virol 77:6567–6573
Mou F, Forest T, Baines JD (2007) US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J Virol 81:6459–6470
Johnson DC, Baines JD (2011) Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol 9:382–394
Ogg PD, McDonell PJ, Ryckman BJ, Knudson CM, Roller RJ (2004) The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 319:212–224
Reynolds AE, Ryckman BJ, Baines JD, Zhou Y, Liang L, Roller RJ (2001) U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J Virol 75:8803–8817
Naghavi MH, Gundersen GG, Walsh D (2013) Plus-end tracking proteins, CLASPs, and a viral Akt mimic regulate herpesvirus-induced stable microtubule formation and virus spread. Proc Natl Acad Sci USA 110:18268–18273
Sloan DD, Zahariadis G, Posavad CM, Pate NT, Kussick SJ, Jerome KR (2003) CTL are inactivated by herpes simplex virus-infected cells expressing a viral protein kinase. J Immunol 171:6733–6741
Sen J, Liu X, Roller (2013) Herpes simplex virus US3 tegument protein inhibits Toll-like receptor 2 signaling at or before TRAF6 ubiquitination. Virology 439:65–73
Wang S, Wang K, Lin R, Zheng C (2013) Herpes simplex virus 1 serine/threonine kinase US3 hyperphosphorylates IRF3 and inhibits beta interferon production. J Virol 87:12814–12827
Rao P, Pham HT, Kulkarni A, Yang Y, Liu X, Knipe DM, Cresswell P, Yuan W (2011) Herpes simplex virus 1 glycoprotein B and US3 collaborate to inhibit CD1d antigen presentation and NKT cell function. J Virol 85:8093–8104
Walters MS, Kinchington PR, Banfield BW, Silverstein S (2010) Hyperphosphorylation of histone deacetylase 2 by alphaherpesvirus US3 kinases. J Virol 84:9666–9676
Poon AP, Liang Y, Roizman B (2003) Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J Virol 77:12671–12678
Poon AP, Gu H, Roizman B (2006) ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc Natl Acad Sci USA 103:9993–9998
Kato A, Liu Z, Minowa A, Imai T, Tanaka M, Sugimoto K, Nishiyama Y, Arii J, Kawaguchi Y (2011) Herpes simplex virus 1 protein kinase Us3 and major tegument protein UL47 reciprocally regulate their subcellular localization in infected cells. J Virol 85:9599–9613
Kato A, Tsuda S, Liu Z, Kozuka-Hata H, Oyama M, Kawaguchi Y (2014) Herpes simplex virus 1 protein kinase Us3 phosphorylates viral dUTPase and regulates its catalytic activity in infected cells. J Virol 88:655–666
Sagou K, Imai T, Sagara H, Uema M, Kawaguchi Y (2009) Regulation of the catalytic activity of herpes simplex virus 1 protein kinase Us3 by autophosphorylation and its role in pathogenesis. J Virol 83:5773–5783
Coulter LJ, Moss HW, Lang J, McGeoch DJ (1993) A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupte. J Gen Virol 74:387–395
Yang L, Hu X, Cheng A, Wang M, Jia R, Yang Q, Wu Y, Chen S, Liu M, Zhu D, Ou X, Wen X, Mao S, Sun D, Zhang S, Zhao X, Huang J, Gao Q, Liu Y, Yu Y, Zhang L, Tian B, Pan L, Chen X (2021) Two nuclear localization signals regulate intracellular localization of the duck enteritis virus UL13 protein. Poult Sci 100:26–38
Daikoku T, Shibata S, Goshima F, Oshima S, Tsurumi T, Yamada H, Yamashita Y, Nishiyama Y (1997) Purification and characterization of the protein kinase encoded by the UL13 gene of herpes simplex virus type 2. Virology 235:82–93
Morrison EE, Wang YF, Meredith DM (1998) Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J Virol 72:7108–7114
Ogle WO, Ng TI, Carter KL, Roizman B (1997) The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology 235:406–413
Zhu Z, Du T, Zhou G, Roizman B (2014) The stability of herpes simplex virus 1 ICP0 early after infection is defined by the RING finger and the UL13 protein kinase. J Virol 88:5437–5443
Eaton HE, Saffran HA, Wu FW, Quach K, Smiley JR (2014) Herpes simplex virus protein kinases US3 and UL13 modulate VP11/12 phosphorylation, virion packaging, and phosphatidylinositol 3-kinase/Akt signaling activity. J Virol 88:7379–7388
Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Miura S, Jimbow K, Fujii N (2004) Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J Virol 78:6282–6286
Shibaki T, Suzutani T, Yoshida I, Ogasawara M, Azuma M (2001) Participation of type I interferon in the decreased virulence of the UL13 gene-deleted mutant of herpes simplex virus type 1. J Interferon Cytokine Res 21:279–285
Bo Z, Miao Y, Xi R, Zhong Q, Bao C, Chen H, Sun L, Qian Y, Jung YS, Dai J (2020) PRV UL13 inhibits cGAS-STING-mediated IFN-β production by phosphorylating IRF3. Vet Res 51:118
Jenkins HL, Spencer CA (2001) RNA polymerase II holoenzyme modifications accompany transcription reprogramming in herpes simplex virus type 1-infected cells. J Virol 75:9872–9884
Jarosinski J, Margulis KW, Kamil NG, Spatz JP, Nair SJ, Osterrieder VK N (2007) Horizontal transmission of Marek’s disease virus requires US2, the UL13 protein kinase, and gC. J Virol 81:10575–10587
Cunningham C, Davison AJ, Dolan A, Frame MC, McGeoch DJ, Meredith DM, Moss HW, Orr AC (1992) The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J Gen Virol 73:303–311
Cano-Monreal GL, Tavis JE, Morrison LA (2008) Substrate specificity of the herpes simplex virus type 2 UL13 protein kinase. Virology 374:1–10
Kenyon TK, Lynch J, Hay J, Ruyechan W, Grose C (2001) Varicella-zoster virus ORF47 protein serine kinase: characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J Virol 75:8854–8858
Besser J, Sommer MH, Zerboni L, Bagowski CP, Ito H, Moffat J, Ku CC, Arvin AM (2003) Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse. J Virol 77:5964–5974
Jacob T, Van den Broeke C, Favoreel HW (2011) Viral serine/threonine protein kinases. J Virol 85:1158–1173
Mou F, Wills EG, Park R, Baines JD (2008) Effects of lamin A/C, lamin B1, and viral US3 kinase activity on viral infectivity, virion egress, and the targeting of herpes simplex virus U(L)34-encoded protein to the inner nuclear membrane. J Virol 82:8094–8104
Mou F, Wills E, Baines JD (2009) Phosphorylation of the U(L)31 protein of herpes simplex virus 1 by the U(S)3-encoded kinase regulates localization of the nuclear envelopment complex and egress of nucleocapsids. J Virol 83:5181–5191
Park R, Baines JD (2006) Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J Virol 80:494–504
Gershburg S, Geltz J, Peterson KE, Halford WP, Gershburg E (2015) The UL13 and US3 protein kinases of herpes simplex virus 1 cooperate to promote the assembly and release of mature, infectious virions. PLoS One 10:e0131420
Kato A, Yamamoto M, Ohno T, Tanaka M, Sata T, Nishiyama Y, Kawaguchi Y (2006) Herpes simplex virus 1-encoded protein kinase UL13 phosphorylates viral Us3 protein kinase and regulates nuclear localization of viral envelopment factors UL34 and UL31. J Virol 80:1476–1486
Rivas T, Goodrich JA, Kugel JF (2021) The herpes simplex virus 1 protein ICP4 acts as both an activator and a repressor of host genome transcription during infection. Mol Cell Biol 41:e0017121
Sampath P, Deluca NA (2008) Binding of ICP4, TATA-binding protein, and RNA polymerase II to herpes simplex virus type 1 immediate-early, early, and late promoters in virus-infected cells. J Virol 82:2339–2349
Deluca NA, Schaffer PA (1988) Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol 62:732–743
Wagner LM, Bayer A, Deluca NA (2013) Requirement of the N-terminal activation domain of herpes simplex virus ICP4 for viral gene expression. J Virol 87:1010–1018
Bruce JW, Wilcox KW (2002) Identification of a motif in the C terminus of herpes simplex virus regulatory protein ICP4 that contributes to activation of transcription. J Virol 76:195–207
Wettenhall RE, Morgan FJ (1984) Phosphorylation of hepatic ribosomal protein S6 on 80 and 40 S ribosomes. Primary structure of S6 in the region of the major phosphorylation sites for cAMP-dependent protein kinases. J Biol Chem 259:2084–2091
Wilcox KW, Kohn A, Sklyanskaya E, Roizman B (1980) Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol 33:167–182
Xia K, DeLuca NA, Knipe DM (1996) Analysis of phosphorylation sites of herpes simplex virus type 1 ICP4. J Virol 70:1061–1071
Xia K, Knipe DM, Deluca NA (1996) Role of protein kinase A and the serine-rich region of herpes simplex virus type 1 ICP4 in viral replication. J Virol 70:1050–1060
Cai WZ, Schaffer PA (1989) Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol 63:4579–4589
Everett RD (1985) Activation of cellular promoters during herpes virus infection of biochemically transformed cells. EMBO J 4:1973–1980
Hembram DSS, Negi H, Biswas P, Tripathi V, Bhushan L, Shet D, Kumar V, Das R (2020) The viral SUMO-targeted ubiquitin ligase ICP0 is phosphorylated and activated by host kinase Chk2. J Mol Biol 432:1952–1977
Advani SJ, Weichselbaum RR, Roizman B (2000) The role of cdc2 in the expression of herpes simplex virus genes. Proc Natl Acad Sci USA 97:10996–11001
Boutell C, Everett R, Hilliard J, Schaffer P, Orr A, Davido D (2008) Herpes simplex virus type 1 ICP0 phosphorylation mutants impair the E3 ubiquitin ligase activity of ICP0 in a cell type-dependent manner. J Virol 82:10647–10656
Davido DJ, von Zagorski WF, Lane WS, Schaffer PA (2005) Phosphorylation site mutations affect herpes simplex virus type 1 ICP0 function. J Virol 79:1232–1243
Wisner TW, Johnson DC (2004) Redistribution of cellular and herpes simplex virus proteins from the trans-golgi network to cell junctions without enveloped capsids. J Virol 78:11519–11535
Kohl S, Strynadka NC, Hodges RS, Pereira L (1990) Analysis of the role of antibody-dependent cellular cytotoxic antibody activity in murine neonatal herpes simplex virus infection with antibodies to synthetic peptides of glycoprotein D and monoclonal antibodies to glycoprotein B. J Clin Invest 86:273–278
Shelly SS, Cairns TM, Whitbeck JC, Lou H, Krummenacher C, Cohen GH, Eisenberg RJ (2012) The membrane-proximal region (MPR) of herpes simplex virus gB regulates association of the fusion loops with lipid membranes. mBio 3:e00429-12
Bishop GA, Glorioso JC, Schwartz SA (1983) Relationship between expression of herpes simplex virus glycoproteins and susceptibility of target cells to human natural killer activity. J Exp Med 157:1544–1561
Bishop GA, Marlin SD, Schwartz SA (1984) Human natural killer cell recognition of herpes simplex virus type 1 glycoproteins: specificity analysis with the use of monoclonal antibodies and antigenic variants. J Immunol 133:2206–2214
Sanchez-Pescador L, Paz P, Navarro D (1992) Epitopes of herpes simplex virus type 1 glycoprotein B that bind type-common neutralizing antibodies elicit type-specific antibody-dependent cellular cytotoxicity. J Infect Dis 166:623–627
Martinez R, Shao L, Bronstein JC, Weber PC, Weller SK (1996) The product of a 1.9-kb mRNA which overlaps the HSV-1 alkaline nuclease gene (UL12) cannot relieve the growth defects of a null mutant. Virology 215:152–164
Goldstein JN, Weller SK (1998) The exonuclease activity of HSV-1 UL12 is required for in vivo function. Virology 244:442–457
Fujii H, Mugitani M, Koyanagi N, Liu Z, Tsuda S, Arii J, Kato A, Kawaguchi Y (2014) Role of the nuclease activities encoded by herpes simplex virus 1 UL12 in viral replication and neurovirulence. J Virol 88:2359–2364
Cai M, Si J, Li X, Zeng Z, Li M (2016) Characterization of the nuclear import mechanisms of HSV-1 UL31. Biol Chem 397:555–561
Sherry MR, Hay TJM, Gulak MA, Nassiri A, Finnen RL, Banfield BW (2017) The herpesvirus nuclear egress complex component, UL31, can be recruited to sites of DNA damage through poly-ADP ribose binding. Sci Rep 7:1882
Li M, Jiang S, Mo C, Zeng Z, Li X, Chen C, Yang Y, Wang J, Huang J, Chen D, Peng T, Cai M (2015) Identification of molecular determinants for the nuclear import of pseudorabies virus UL31. Arch Biochem Biophys 587:12–17
Li M, Jiang S, Wang J, Mo C, Zeng Z, Yang Y, Chen C, Li X, Cui W, Huang J, Peng T, Cai M (2015) Characterization of the nuclear import and export signals of pseudorabies virus UL31. Arch Virol 160:2591–2594
Kim S, Ahn BC, O’Callaghan DJ, Kim SK (2012) The early UL31 gene of equine herpesvirus 1 encodes a single-stranded DNA-binding protein that has a nuclear localization signal sequence at the C-terminus. Virology 432:306–315
Xie W, Cheng A, Wang M, Chang H, Zhu D, Luo Q (2010) Molecular cloning and characterization of the UL31 gene from duck enteritis virus. Mol Biol Rep 37:1495–1503
Chang YE, Roizman B (1993) The product of the UL31 gene of herpes simplex virus 1 is a nuclear phosphoprotein which partitions with the nuclear matrix. J Virol 67:6348–6356
Shiba C, Daikoku T, Goshima F, Takakuwa H, Yamauchi Y, Koiwai O, Nishiyama Y (2000) The UL34 gene product of herpes simplex virus type 2 is a tail-anchored type II membrane protein that is significant for virus envelopment. J Gen Virol 81:2397–2405
Maeda F, Arii J, Hirohata Y, Maruzuru Y, Koyanagi N, Kato A, Kawaguchi Y (2017) Herpes simplex virus 1 UL34 protein regulates the global architecture of the endoplasmic reticulum in infected cells. J Virol 91:e00271-17
Roller RJ, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D (2000) Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. J Virol 74:117
Klupp BG, Granzow H, Mettenleiter TC (2000) Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J Virol 74:10063–10073
Fuchs W, Klupp BG, Granzow H, Osterrieder N, Mettenleiter TC (2002) The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J Virol 76:364–378
Kato A, Yamamoto M, Ohno T, Kodaira H, Nishiyama Y, Kawaguchi Y (2005) Identification of proteins phosphorylated directly by the Us3 protein kinase encoded by herpes simplex virus 1. J Virol 79:9325–9331
Roller RJ, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D (2000) Herpes simplex virus type 1 U(L)34 gene product is required for viral envelopment. J Virol 74:117–129
Roizman B (2001) Herpes simplex viruses and their replication. Virology 19:2231–2295
Li ML, Zhao ZY, Cui W, Mo CC, Wang JL, Cai MS (2014) Molecular cloning and characterization of the pseudorabies virus UL31 gene. Genet Mol Res 13:1832–1847
Zhang K, Afroz S, Brownlie R, van Snider M, Hurk S (2015) Regulation and function of phosphorylation on VP8, the major tegument protein of bovine herpesvirus 1. J Virol 89:4598–4611
Hall DB, Struhl K (2002) The VP16 activation domain interacts with multiple transcriptional components as determined by protein-protein cross-linking in vivo. J Biol Chem 277:46043–46050
Preston CM (2000) Repression of viral transcription during herpes simplex virus latency. J Gen Virol 81:1–19
Andersen B, Rosenfeld MG (2001) POU domain factors in the neuroendocrine system: lessons from developmental biology provide insights into human disease. Endocr Rev 22:2–35
Herr W, Cleary MA (1995) The POU domain: versatility in transcriptional regulation by a flexible two-in-one DNA-binding domain. Genes Dev 9:1679–1693
Lai JS, Herr W (1997) Interdigitated residues within a small region of VP16 interact with Oct-1, HCF, and DNA. Mol Cell Biol 17:3937–3946
O’Reilly D, Hanscombe O, O’Hare P (1997) A single serine residue at position 375 of VP16 is critical for complex assembly with Oct-1 and HCF and is a target of phosphorylation by casein kinase II. EMBO J 16:2420–2430
Ottosen S, Herrera FJ, Doroghazi JR, Hull A, Mittal S, Lane WS, Triezenberg SJ (2006) Phosphorylation of the VP16 transcriptional activator protein during herpes simplex virus infection and mutational analysis of putative phosphorylation sites. Virology 345:468–481
Sawtell NM, Triezenberg SJ, Thompson RL (2011) VP16 serine 375 is a critical determinant of herpes simplex virus exit from latency in vivo. J Neurovirol 17:546–551
Heine JW, Honess RW, Cassai E (1974) Proteins specified by herpes simplex virus XII. The virion polypeptides of type 1 strains. J Virol 14:640–651
Wu L, Cheng A, Wang M, Jia R, Yang Q, Wu Y, Zhu D, Zhao X, Chen S, Liu M, Zhang S, Ou X, Mao S, Gao Q, Sun D, Wen X, Liu Y, Yu Y, Zhang L, Tian B, Pan L, Chen X (2020) Alphaherpesvirus major tegument protein VP22: its precise function in the viral life cycle. Front Microbiol 11:1908
Elliott G, O’Hare P (1997) Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell 88:223–233
Pinard MF, Simard R, Bibor-Hardy V (1987) DNA-binding proteins of herpes simplex virus type 1-infected BHK cell nuclear matrices. J Gen Virol 68:727–735
Ren X, Harms JS, Splitter GA (2001) Tyrosine phosphorylation of bovine herpesvirus 1 tegument protein VP22 correlates with the incorporation of VP22 into virions. J Virol 75:9010–9017
Geiss BJ, Tavis JE, Metzger LM, Leib DA, Morrison LA (2001) Temporal regulation of herpes simplex virus type 2 VP22 expression and phosphorylation. J Virol 75:10721–10729
Elliott G, O’reilly D, O’hare P (1996) Phosphorylation of the herpes simplex virus type 1 tegument protein VP22. Virology 226:140–145
Potel C, Elliott G (2005) Phosphorylation of the herpes simplex virus tegument protein VP22 has no effect on incorporation of VP22 into the virus but is involved in optimal expression and virion packaging of ICP0. J Virol 79:14057–14068
Qie L, Marcellino D, Herold BC (1999) Herpes simplex virus entry is associated with tyrosine phosphorylation of cellular proteins. Virology 256:220–227
Schauflinger M, Fischer D, Schreiber A, Chevillotte M, Walther P, Mertens T, von Einem J (2011) The tegument protein UL71 of human cytomegalovirus is involved in late envelopment and affects multivesicular bodies. J Virol 85:3821–3832
Meissner CS, Suffner S, Schauflinger M, von Einem J, Bogner E (2012) A leucine zipper motif of a tegument protein triggers final envelopment of human cytomegalovirus. J Virol 86:3370–3382
Selariu A, Cheng T, Tang Q, Silver B, Yang L, Liu C, Ye X, Markus A, Goldstein RS, Cruz-Cosme RS, Lin Y, Wen L, Qian H, Han J, Dulal K, Huang Y, Li Y, Xia N, Zhu H (2012) ORF7 of varicella-zoster virus is a neurotropic factor. J Virol 86:8614–8624
Jiang HF, Wang W, Jiang X, Zeng WB, Shen ZZ, Song YG, Yang H, Liu XJ, Dong X, Zhou J, Sun JY, Yu FL, Guo L, Cheng T, Rayner S, Zhao F, Zhu H, Luo MH (2017) ORF7 of varicella-zoster virus is required for viral cytoplasmic envelopment in differentiated neuronal cells. J Virol 91:e00127-17
Klupp BG, Granzow H, Klopfleisch R, Fuchs W, Kopp M, Lenk M, Mettenleiter TC (2005) Functional analysis of the pseudorabies virus UL51 protein. J Virol 79:3831–3840
Ruyechan WT, Peng H, Yang M (2003) Cellular factors and IE62 activation of VZV promoters. J Med Virol 70:S90–S94
Yang M, Hay J, Ruyechan WT (2008) Varicella-zoster virus IE62 protein utilizes the human mediator complex in promoter activation. J Virol 82:12154–12163
Erazo A, Kinchington PR (2010) Varicella-zoster virus open reading frame 66 protein kinase and its relationship to alphaherpesvirus US3 kinases. Curr Top Microbiol Immunol 342:79–98
Erazo A, Yee MB, Osterrieder N, Kinchington PR (2008) Varicella-zoster virus open reading frame 66 protein kinase is required for efficient viral growth in primary human corneal stromal fibroblast cells. J Virol 82:7653–7665
Lynch JM, Kenyon TK, Grose C, Hay J, Ruyechan WT (2002) Physical and functional interaction between the varicella zoster virus IE63 and IE62 proteins. Virology 302:71–82
Cohrs RJ, Gilden DH (2003) Varicella zoster virus transcription in latently-infected human ganglia. Anticancer Res 23:2063–2069
Sommer MH, Zagha E, Serrano OK (2001) Mutational analysis of the repeated open reading frames, ORFs 63 and 70 and ORFs 64 and 69, of varicella-zoster virus. J Virol 75:8224–8239
Shibazaki M, Kato A, Takeshima K, Ito J, Suganami M, Koyanagi N, Maruzuru Y, Sato K, Kawaguchi Y (2020) Phosphoregulation of a conserved herpesvirus tegument protein by a virally encoded protein kinase in viral pathogenicity and potential linkage between its evolution and viral phylogeny. J Virol 94:e01055-20
Mcnabb DS, Courtney RJ (1992) Characterization of the large tegument protein (ICP1/2) of herpes simplex virus type 1. Virology 190:221–232
Asai R, Ohno T, Kato A, Kawaguchi Y (2007) Identification of proteins directly phosphorylated by UL13 protein kinase from herpes simplex virus 1. Microbes Infect 9:1434–1438
Tanaka M, Nishiyama Y, Sata T (2005) The role of protein kinase activity expressed by the UL13 gene of herpes simplex virus 1: the activity is not essential for optimal expression of UL41 and ICP0. Virology 341:301–312
Read GS, Karr BM, Knight K (1993) Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J Virol 67:7149–7160
Pennisi R, Musarra-Pizzo M, Lei Z, Zhou GG, Sciortino MT (2020) VHS, US3 and UL13 viral tegument proteins are required for Herpes Simplex Virus-Induced modification of protein kinase R. Sci Rep 10:5580
Olson JK, Bishop GA, Grose C (1997) Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol 71:110–119
Grose C (1990) Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol 44:59–80
Georgopoulou U, Michaelidou A, Roizman B, Mavromara-Nazos P (1993) Identification of a new transcriptional unit that yields a gene product within the unique sequences of the short component of the herpes simplex virus 1 genome. J Virol 67:3961–3968
Georgopoulou U, Kakkanas A, Miriagou V, Michaelidou A, Mavromara P (1995) Characterization of the US8.5 protein of herpes simplex virus. Arch Virol 140:2227–2241
Kato A, Ando T, Oda S, Watanabe M, Koyanagi N, Arii J, Kawaguchi Y (2016) Roles of Us8A and its phosphorylation mediated by Us3 in herpes simplex virus 1 pathogenesis. J Virol 90:5622–5635
Koyanagi N, Imai T, Arii J, Kato A, Kawaguchi Y (2014) Role of herpes simplex virus 1 Us3 in viral neuroinvasiveness. Microbiol Immunol 58:31–37
Matsuzaki A, Yamauchi Y, Kato A, Goshima F, Kawaguchi Y, Yoshikawa T, Nishiyama Y (2005) US3 protein kinase of herpes simplex virus type 2 is required for the stability of the UL46-encoded tegument protein and its association with virus particles. J Gen Virol 86:1979–1985
Kato K, Daikoku T, Goshima F, Kume H, Yamaki K, Nishiyama Y (2000) Synthesis, subcellular localization and VP16 interaction of the herpes simplex virus type 2 UL46 gene product. Arch Virol 145:2149–2162
Zahariadis G, Wagner MJ, Doepker RC, Maciejko JM, Crider CM, Jerome KR, Smiley JR (2008) Cell-type-specific tyrosine phosphorylation of the herpes simplex virus tegument protein VP11/12 encoded by gene UL46. J Virol 82:6098–6108
Feng P, Everly DN Jr, Read GS (2005) mRNA decay during herpes simplex virus (HSV) infections: protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J Virol 79:9651–9664
Pheasant K, Möller-Levet CS, Jones J, Depledge D, Breuer J, Elliott G (2018) Nuclear-cytoplasmic compartmentalization of the herpes simplex virus 1 infected cell transcriptome is co-ordinated by the viral endoribonuclease vhs and cofactors to facilitate the translation of late proteins. PLoS Pathog 14:e1007331
Dauber B, Pelletier J, Smiley JR (2011) The herpes simplex virus 1 vhs protein enhances translation of viral true late mRNAs and virus production in a cell type-dependent manner. J Virol 85:5363–5373
He T, Wang M, Cheng A, Yang Q, Jia R, Wu Y, Huang J, Tian B, Liu M, Chen S, Zhao XX, Zhu D, Zhang S, Ou X, Mao S, Gao Q, Sun D (2021) DPV UL41 gene encoding protein induces host shutoff activity and affects viral replication. Vet Microbiol 255:108979
Sciortino MT, Taddeo B, Poon AP, Mastino A, Roizman B (2002) Of the three tegument proteins that package mRNA in herpes simplex virions, one (VP22) transports the mRNA to uninfected cells for expression prior to viral infection. Proc Natl Acad Sci USA 99:8318–8323
Mbong EF, Woodley L, Dunkerley E, Schrimpf JE, Morrison LA, Duffy C (2012) Deletion of the herpes simplex virus 1 UL49 gene results in mRNA and protein translation defects that are complemented by secondary mutations in UL41. J Virol 86:12351–12361
Smibert CA, Popova B, Xiao P, Capone JP, Smiley JR (1994) Herpes simplex virus VP16 forms a complex with the virion host shutoff protein vhs. J Virol 68:2339–2346
Taddeo B, Sciortino MT, Zhang W, Roizman B (2007) Interaction of herpes simplex virus RNase with VP16 and VP22 is required for the accumulation of the protein but not for accumulation of mRNA. Proc Natl Acad Sci USA 104:12163–12168
Shu M, Taddeo B, Roizman B (2013) The nuclear-cytoplasmic shuttling of virion host shutoff RNase is enabled by pUL47 and an embedded nuclear export signal and defines the sites of degradation of AU-rich and stable cellular mRNAs. J Virol 87:13569–13578
Liu Z, Kato A, Shindo K, Noda T, Sagara H, Kawaoka Y, Arii J, Kawaguchi Y (2014) Herpes simplex virus 1 UL47 interacts with viral nuclear egress factors UL31, UL34, and Us3 and regulates viral nuclear egress. J Virol 88:4657–4667
Elliott G, Hafezi W, Whiteley A, Bernard E (2005) Deletion of the herpes simplex virus VP22-encoding gene (UL49) alters the expression, localization, and virion incorporation of ICP0. J Virol 79:9735–9745
Yu X, Liu L, Wu L, Wang L, Dong C, Li W, Li Q (2010) Herpes simplex virus type 1 tegument protein VP22 is capable of modulating the transcription of viral TK and gC genes via interaction with viral ICP0. Biochimie 92:1024–1030
Maringer K, Stylianou J, Elliott G (2012) A network of protein interactions around the herpes simplex virus tegument protein VP22. J Virol 86:12971–12982
Mouzakitis G, McLauchlan J, Barreca C, Kueltzo L, O’Hare P (2005) Characterization of VP22 in herpes simplex virus-infected cells. J Virol 79:12185–12198
Acknowledgements
We thank the members of our laboratory for their guidance, assistance and support on this manuscript. They have provided us with many valuable suggestions and solved many problems during the writing process.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31872476), the China Agriculture Research System of MOF and MARA, and the Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (SCCXTD-2020-18).
Author information
Authors and Affiliations
Contributions
All authors listed contributed to the completion of the article. TZ and MW contributed to the design and wrote the article. MW, XO, SM, DS, SZ, QY, YW, RJ, DZ, SC, and ML provided ideas contributing to the conception of this article. XZ, JH, QG, and BT helped to create the tables and figures. AC modified the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, T., Wang, M., Cheng, A. et al. Regulation of alphaherpesvirus protein via post-translational phosphorylation. Vet Res 53, 93 (2022). https://doi.org/10.1186/s13567-022-01115-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-022-01115-z