Abstract
To understand the differences in immune responses between early feathering (EF) and late feathering (LF) chickens after infection with avian leukosis virus, subgroup J (ALV-J), we monitored the levels of prolactin, growth hormone and the immunoglobulins IgG and IgM in the serum of LF and EF chickens for 8 weeks. Moreover, we analysed the expression of immune-related genes in the spleen and the expression of PRLR, SPEF2 and dPRLR in the immune organs and DF-1 cells by qRT–PCR. The results showed that ALV-J infection affected the expression of prolactin, growth hormone, IgG and IgM in the serum. Regardless of whether LF and EF chickens were infected with ALV-J, the serum levels of the two hormones and two immunoglobulins in EF chickens were higher than those in LF chickens (P < 0.05). However, the expression of immune-related genes in the spleen of positive LF chickens was higher than that in the spleen of positive EF chickens. In the four immune organs, PRLR and SPEF2 expression was also higher in LF chickens than in EF chickens. Furthermore, the dPRLR expression of positive LF chickens was higher than that of negative LF chickens. After infection with ALV-J, the expression of PRLR in DF-1 cells significantly increased. In addition, overexpression of PRLR or dPRLR in DF-1 cells promoted replication of ALV-J. These results suggested that the susceptibility of LF chickens to ALV-J might be induced by dPRLR.
Similar content being viewed by others
Introduction
Avian leukosis virus (ALV) is an oncogenic retrovirus that causes immunosuppression and neoplastic diseases [1]. According to the host range, the interference of viral envelopes and the pattern of cross-neutralization, ALVs can be divided into 10 subgroups (from A to J) [2]. ALV-J was first detected in the late 1980s and subsequently spread worldwide [3, 4]. To date, ALV-J has been found to infect many egg-type stocks and local Chinese breeds, resulting in significant economic losses in the poultry industry [4,5,6,7].
Sex identification of chicks can be based on the difference in rates of feather growth, which can be divided into an early feathering (EF) type and a late feathering (LF) type. Both the EF and LF types contain the prolactin receptor (PRLR) and sperm flagellar 2 (SPEF2) genes in their genetic structure. However, LF chickens have one more fusion gene, dSPEF2/dPRLR, and endogenous retroviruses 21 (ev21) compared with EF chickens [8,9,10]. Breeders use the different feather growth rates for sex determination and have found that EF and LF chickens respond differently to ALV-J infection. Some White Leghorn breeders have reported reduced production and slightly higher mortality in female progeny from dams carrying the ev21 gene [11]. Breeders have had difficulties in eradicating ALV from most pure LF lines compared to EF chicken lines [12]. The existence of ev21 increases the susceptibility of chickens to ALV infection and affects production performance and tumorigenesis [13,14,15].
However, a recent study revealed that some LF chickens lack the ev21 gene and some EF chickens harbour the ev21 gene [16]. Furthermore, dPRLR encodes a new prolactin (PRL) functional receptor that is widely expressed in all chicken tissues, and the pattern of spatiotemporal expression is likely to match that of the original PRLR gene. Importantly, PRLR and dPRLR were shown to be functionally coupled to the intracellular JAK/STAT signalling pathway in vitro [17]. PRL functions by first binding to its receptors and activating the JAK/STAT signalling pathway [18]. Growth hormone (GH) and PRL have similar structures and functions. Their receptors and signal transduction pathways are fundamentally the same [19]. After infection with ALV-J, it is not clear whether the levels of the two hormones PRL and GH and the two immunoglobulins IgG and IgM in the serum and the expression of some immune-related genes in the spleen differ between LF and EF chickens. On the other hand, both the PRLR and dPRLR genes are present in LF chickens. Whether these two genes have any effect on the immune responses of LF chickens infected with ALV-J remains unknown.
To understand the difference in immune responses between EF and LF chickens after infection with ALV-J, we detected the levels of PRL, GH, IgG, and IgM in the serum of positive and negative individuals of these two types of chickens for eight consecutive weeks. Furthermore, we analysed the expression of some immune-related genes in the spleen to illustrate the different immune responses of EF and LF chickens after infection with ALV-J.
Materials and methods
Ethics statement
All animal experiments in this study were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of South China Agriculture University (No: SCAU 2018c008) and in accordance with the Animal Protection Law of the People’s Republic of China.
Cells and antibodies
The chicken embryonic fibroblast cell line DF-1 was obtained from ATCC (Manassas, USA) and maintained in DMEM supplemented with 10% foetal bovine serum (FBS) and 0.1% penicillin/streptomycin at 37 °C in an atmosphere of 5% CO2. JE9, a specific mouse monoclonal antibody for the ALV-J envelope protein, was kindly provided by Prof. Aijian Qin (Yangzhou University). Goat anti-mouse IgG labelled with FITC was purchased from Bioss (China), while an ALV antigen-capture enzyme-linked immunosorbent assay kit was purchased from IDEXX (USA).
Animals
LF and EF yellow chickens (140 days) were sourced from Chinese chicken farms in Guangdong Province, China. Virus isolation and identification were performed with DF-1 cells as we described previously [20]. The virus identification primer sequences are listed in Additional file 3. The virus identification and ALV-J viremia results are shown in Additional files 1, 4.
We used molecular identification methods to identify the feathering genotypes of samples. Using primers designed by Tixier-Boichard et al. [21], we amplified the ev21 gene in our DNA samples. The expected PCR product of EF chickens was only a 515-bp band, while for LF chickens, there were two bands of 390 bp and 515 bp. We designed a pair of primers for dSPEF2/dPRLR gene amplification. A 1434-bp target fragment should be obtained in LF chickens but not in EF chickens. The primers are listed in Additional file 3. The feathering genotype detection results for LF and EF chickens are shown in Additional file 2.
Based on the results for virus isolation and identification and feathering genotype detection, 6 ALV-J-positive LF chickens, 6 ALV-J-negative LF chickens, 6 ALV-J-positive EF chickens and 6 ALV-J-negative EF chickens were selected. All animals were from the same farm. These selected chickens were raised separately, with a consistent feeding protocol among all individuals.
Sample collection
Once each week for 8 weeks, we aseptically collected 2 mL of anticoagulated blood from each individual. The plasma was then separated by centrifugation at 2000 rpm and 4 ℃ for 15 min and stored at −80 ℃. All the samples were collected at the same time: 10:00 am every Monday of each week. Eight weeks later, the spleen, bone marrow, thymus, and caecal tonsils of each chicken were collected and stored at −80 ℃. The plasma samples were analysed with a p27 test for each collection, and the cell supernatant p27 test results are shown in Additional file 4.
Determination of the levels of PRL, GH, IgG, and IgM in the serum by ELISA
The levels of PRL in the serum were measured using enzyme-linked immunosorbent assay (ELISA) kits for PRL (CLOUD-CLONE, Wuhan, China) following the manufacturer’s protocol. The levels of GH, IgG, and IgM were detected in the serum with specific ELISA kits purchased from Shanghai Enzyme-Linked Biotechnology Co., Ltd., (Shanghai, China) following the manufacturer’s protocol.
Cell infection and gene overexpression
DF-1 cells were infected with ALV-J as previously described [22]. After an incubation for 24 h or 48 h in culture, we collected cells and extracted RNA and then measured PRLR and SPEF2 expression by qRT–PCR. The laboratory ALV-J strain SCAU-HN06 was kindly provided by Prof. Weisheng Cao (South China Agricultural University, Guangzhou, China).
According to the PRLR sequence (NM_204854.1) in the NCBI database, Wuhan Genecreate Industrial Co., Ltd., was commissioned to construct pcDNA3.1-PRLR and pcDNA3.1-dPRLR plasmids. Then, we followed the method described by Li et al. [22] for cell transfection and infection. After an incubation for 24 h or 48 h in culture, we collected cells, extracted RNA, and then measured ALV-J viral gene expression by quantitative real-time polymerase chain reaction (qRT–PCR).
RNA isolation and cDNA synthesis
Total RNA was extracted from tissues with RNAiso reagent (Takara, Japan) according to the manufacturer’s protocol. The integrity and quantity of RNA were assessed using 1% agarose gel electrophoresis and spectrophotometry (ND-2000, USA), respectively. cDNA was synthesized using MonScript™ RTIII All-in-One Mix (with dsDNse) (Monad Co., Ltd., Guangzhou, China) following the manufacturer’s protocol. The synthesized cDNA was stored at −20 ℃ until subsequent analysis using qRT–PCR.
Quantitative real-time PCR
We previously performed RNA-seq (PRJNA552417) analysis of spleens from EF and LF chickens infected with ALV-J and identified some differentially expressed genes. Immune-related differentially expressed genes including TLR4, TLR7, MDA5, SOCS3, VIP, IL-10, IRF1, NFкB, TNFα, and IL-1β were selected, and the expression of each gene was detected in the spleen of LF and EF chickens. MonAmp™ SYBR® Green qPCR Mix (Monad Co., Ltd., Guangzhou, China) was used for qRT–PCR on an ABI 7500 Real-Time Detection instrument (Applied Biosystems, USA) according to the manufacturer’s protocol. Relative gene expression was measured by qRT–PCR for each sample, and the nuclear gene GAPDH was used as a control. The primers used for qRT–PCR are shown in Additional file 3.
Western blotting
Western blotting (WB) was performed as previously described [23]. The antibodies and their dilutions used for WB were as follows: the anti-ALV-J envelope protein monoclonal antibody JE9 (kindly provided by Prof. Aijian Qin, Yangzhou University; 1:1000), a rabbit anti-beta-actin antibody (Bioss, China; 1:500) and a goat anti-rabbit IgG H&L/HRP antibody (Bioss, China; 1:500).
Statistical analyses
Statistical comparisons were performed using GraphPad Prism 5 (GraphPad Software Inc., USA). Data are presented as the mean ± one standard error of the mean (SEM). The statistical analyses were performed using one-factor analysis of variance, and statistical significance is represented by P values. P < 0.05 was considered statistically significant, and P value bands of statistical significance are denoted as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
The serum PRL and GH levels of LF chickens are lower than those of EF chickens, regardless of infection status
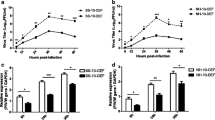
Comparing negative LF chickens with negative EF chickens, the PRL levels of the EF chickens were higher than those of the LF chickens except at the 5th, 7th and 8th weeks (Figure 1A). However, the PRL levels of positive EF chickens were higher than those of positive LF chickens in the 3rd week, while the 2nd and 8th weeks showed no difference, but were lower than those of positive LF chickens at the other times (Figure 1B). PRL and GH are similar in structure and function, and their receptors and signal transduction pathways are basically the same. Therefore, we also detected the serum GH content in LF and EF chickens. Intriguingly, regardless of the ALV-J infection status, the serum GH levels of LF chickens were always lower than those of EF chickens (Figures 1C, D). One explanation for these results is that PRL can bind to both PRLR and DPLR in LF chickens, which may lead to lower PRL levels in LF chickens than in EF chickens.
The PRL and GH levels in the plasma of sampled chickens. A PRL levels in the serum of negative LF and EF chickens. B PRL levels in the serum of positive LF and EF chickens. C GH levels in the serum of negative LF and EF chickens. D GH levels in the serum of positive LF and EF chickens. Positive LF, late feathering chickens infected with ALV-J; negative LF, late feathering chickens uninfected with ALV-J; positive EF early feathering chickens infected with ALV-J; negative EF early feathering chickens uninfected with ALV-J; n number of samples. The error bars represent one standard error of the mean (SEM) (*P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001).
The serum IgG and IgM levels of LF chickens are always lower than those of EF chickens
Immunoglobulin refers to an animal protein with antibody activity. IgM is the first antibody isotype secreted in immune responses, while IgG is the most abundant antibody in the serum. Immunoglobulins play an important role in the function of the immune system. PRL can promote lymphocyte mitosis in a dose-dependent manner [24] and stimulate the proliferation of chicken splenocytes and thymocytes. Notably, regardless of whether chickens were infected with ALV-J, the IgG (Figures 2A, B) and IgM (Figures 2C, D) levels of LF chickens were always significantly lower than those of EF chickens. This might have been caused by the lower PRL levels in LF chickens compared to those in EF chickens, which might fail to increase the levels of IgM and IgG in the serum.
The expression of most immune-related genes in the spleen of LF chickens is higher than that in the spleen of EF chickens
In negative chickens, the expression of the TLR4, TLR7, SOCS3, VIP, IL-10, IRF1, NFкB, TNFα, and IL-1β genes but not that of the MDA5 gene in EF chickens was lower than that in LF chickens. However, the differences in the expression of TLR7, IRF1, and NFкB between EF and LF chickens were not significant (P > 0.05) (Figure 3A). In positive chickens, the expression of these genes other than IRF1 in EF chickens was significantly lower than that in LF chickens (Figure 3B). This could be because PRL combines with PRLR and dPRLR to stimulate the expression of immune-related genes through the JAK/STAT signalling pathway. The LF chickens showed higher expression of most of the above immune-related genes because of the existence of dPRLR.
The expression of PRLR, SPEF2 and dPRLR in the immune organs of LF chickens is significantly higher than that in the immune organs of EF chickens
We analysed the expression of the PRLR, SPEF2 and dPRLR genes in the four immune organs, the spleen, bone marrow, thymus and caecal tonsils, by qRT–PCR. The results showed that the expression of the PRLR and SPEF2 genes in each immune organ was significantly higher in LF chickens than in EF chickens (P < 0.05). Compared with that in the immune organs of LF chickens, the PRLR and SPEF2 expression in the four immune organs of EF chickens was very low (Figures 4A, B). In the spleen and thymus, the PRLR expression in negative LF individuals was higher than that in positive LF individuals. In the bone marrow and caecal tonsils, the PRLR expression in positive LF individuals was higher than that in negative LF individuals (Figure 4A). The SPEF2 expression of positive LF individuals was higher than that of negative LF individuals for the spleen, bone marrow and caecal tonsils but not the thymus (Figure 4B). dPRLR expression in the spleen and bone marrow was higher in positive LF individuals than in negative LF individuals. However, there was no difference in dPRLR expression in the thymus or caecal tonsils between EF and LF chickens (Figure 4C).
Expression of the PRLR, SPEF2 and dPRLR genes in the four immune organs and DF-1 cells measured by qRT–PCR. A The expression of PRLR in the four immune organs of LF and EF chickens. B The expression of SPEF2 in the four immune organs of LF and EF chickens. C The expression of dPRLR in the four immune organs of LF chickens. D The expression of PRLR in DF-1 cells after infection with ALV-J. E The expression of SPEF2 in DF-1 cells after infection with ALV-J.
After infection with ALV-J, PRLR and SPEF2 expression in DF-1 cells was evaluated (Figure 4). The PRLR expression in the infection group was significantly higher than that in the control group at 24 h and 48 h (P < 0.05) (Figure 4D). The SPEF2 expression in the infection group was significantly higher than that in the control group at 24 h (P < 0.01) (Figure 4E).
Overexpression of PRLR or dPRLR promotes ALV-J replication
Next, we overexpressed the PRLR and dPRLR genes separately in vitro to investigate the role of each gene in ALV-J replication in chickens. The results showed that overexpression of PRLR (Figures 5A, B) or dPRLR (Figures 5C, D) promoted the expression of the ALV-J gp85 gene at 24 h and 48 h, indicating that PRLR and dPRLR significantly promoted ALV-J replication in chickens.
Overexpression of PRLR or dPRLR promoted ALV-J replication. The expression of the ALV-J gp85 gene in PRLR-overexpressing DF-1 cells after infection with ALV-J measured by qRT–PCR (A) and WB (B). The expression of the ALV-J gp85 gene in dPRLR-overexpressing DF-1 cells after infection with ALV-J measured by qRT–PCR (C) and WB (D).
Discussion
Ev21 has been used as a molecular marker for LF detection in chickens. It is believed that the harbouring of ev21 causes LF chicken susceptibility to ALV-J [13, 25, 26]. Beginning in the 1980s, researchers have reported reduced performance and slightly higher mortality in female progeny of dams carrying the ev21 gene [11, 12]. Harris et al. [13] reported that decreased egg productivity was associated with an increased incidence of ALV-J. Fadly and Smith [27] concluded that the status of ev21 may affect ALV-J infection and tumour development. Williams et al. [28] showed that harbouring ev21 influenced ALV-J viremia and antibody production in some White Leghorn chickens. After inoculation with ALV-J at hatching, 5% of chickens lacking ev21 were viremia tolerant compared with 54% of chickens harbouring ev21 [28]. The incidence of tumours in chickens harbouring ev21 (13.8%) was significantly higher (P < 0.01) than that in chickens lacking ev21 (2.6%) [29]. Collectively, these studies demonstrate that chickens harbouring ev21 are more susceptible to ALV-J infection than are chickens lacking ev21.
In this study, we found that the levels of GH, IgG, and IgM in LF chickens (harbouring ev21) were significantly lower than those in EF chickens (lacking ev21), regardless of the ALV-J infection status. In LF chickens, the serum PRL levels were generally lower than those in EF chickens. The results further confirmed the difference in ALV-J infection between LF and EF chickens. However, the expression of most immune-related genes in the LF chicken spleen was higher than that in the EF chicken spleen. Therefore, we speculated that the dPRLR gene might regulate the immune response in LF chickens.
Some LF chickens lack the ev21 gene, and some EF chickens harbour the ev21 gene [16]. dPRLR is likely to encode a novel functional receptor for PRL and is widely expressed in all chicken tissues, and its spatiotemporal expression pattern is likely to match that of the PRLR gene [17]. Similar to the PRLR gene, dPRLR can significantly increase the level of STAT5 phosphorylation after activation [17]. In LF chickens, the serum PRL levels were generally lower than those in EF chickens, but the expression of most immune-related genes including TLR4, TLR7, MDA5, SOCS3, VIP, IL-10, IRF1, NFкB, TNFα, and IL-1β in the spleen was significantly higher in LF chickens than in EF chickens. PRL and GH are similar in structure and function, and their receptors and signal transduction pathways are basically the same. GH can also regulate the immune response through the JAK/STAT signalling pathway. When PRL levels decrease, the body may compensate by increasing GH levels. PRL can promote lymphocyte mitosis [24, 30]. Thus, the serum IgG and IgM levels of EF chickens were observed to be higher than those of LF chickens. This may be because there is one more dPRLR gene in LF chickens than in EF chickens.
We found that the expression of PRLR and SPEF2 in the four immune organs in LF chickens was significantly higher than that in the corresponding immune organs in EF chickens. The expression of dPRLR in the spleen and bone marrow in positive LF individuals was higher than that in negative LF individuals, and the expression of PRLR in DF-1 cells was significantly increased after infection with ALV-J. Like PRLR, dPRLR significantly increases the level of STAT5 phosphorylation after activation [17]. The JAK/STAT signalling pathway is a signal transduction pathway stimulated by a variety of cytokines and is involved in multiple immune signalling pathways [31, 32]. These results indicate that PRLR and dPRLR play an important role in ALV-J infection. Okamura et al. [33] showed that the transcription of the dSPEF2 gene possibly represses the expression of dPRLR mRNA and alters the alternative splicing bias in the 5′ UTR of PRL receptor mRNAs to increase translational efficiency. The immune functions of the SPEF2 and dSPEF2 genes are still unclear.
Receptors for growth factors, cytokines or hormones are clearly potential viral receptors, as they are already adapted to bind to specific circulating ligands and subsequently promote viral entry and survival by receptor-mediated endocytosis [34]. The receptors known to be used by viruses include insulin-like growth factor 1 receptor (IGF1R), platelet-derived growth factor receptor (PDGFR) and epidermal growth factor receptor (EGFR) [35, 36]. Recently, Griffiths et al. [37] found that IGF1R is an entry receptor for respiratory syncytial virus (RSV). RSV glycoprotein F binds to IGF1R, triggering activation of protein kinase C zeta, which recruits nucleolin from the nuclei of cells to the cell surface, where it probably facilitates RSV entry into the cell [37]. To infect a host cell, a virus must first bind to receptors on the host cell surface. PRLR and dPRLR clearly have potential as viral receptors. They are widely distributed in many tissues. Our present results demonstrated that overexpression of PRLR or dPRLR promoted the expression of the ALV-J gp85 gene. Therefore, we speculated that PRLR and dPRLR are receptors for ALV-J. The presence of the dPRLR gene may be the reason why LF chickens are susceptible to ALV-J.
In conclusion, after infection with ALV-J, the levels of PRL, GH, IgG, and IgM in the serum and the expression of some immune-related genes in the spleen were different between LF and EF chickens. The reason why LF chickens are susceptible to ALV-J is probably due to the presence of the dPRLR gene.
References
Payne LN, Nair V (2012) The long view: 40 years of avian leukosis research. Avian Pathol 41:11–19
Payne LN (1998) Retrovirus-induced disease in poultry. Poult Sci 77:1204–1212
Payne LN, Brown SR, Bumstead N, Howes K, Frazier JA, Thouless ME (1991) A novel subgroup of exogenous avian leukosis virus in chickens. J Gen Virol 72:801–807
Zavala G, Cheng S, Jackwood MW (2007) Molecular epidemiology of avian leukosis virus subgroup J and evolutionary history of its 3′ untranslated region. Avian Dis 51:942–953
Xu B, Dong W, Yu C, He Z, Lv Y, Sun Y, Feng X, Li N, Lee LF, Li M (2004) Occurrence of avian leukosis virus subgroup J in commercial layer flocks in China. Avian Pathol 33:13–17
Sun S, Cui Z (2007) Epidemiological and pathological studies of subgroup J avian leukosis virus infections in Chinese local “yellow” chickens. Avian Pathol 36:221–226
Gao Y, Qin L, Pan W, Wang Y, Qi X, Gao H, Wang X (2010) Avian leukosis virus subgroup J in layer chickens, China. Emerg Infect Dis 16:1637–1638
Elferink MG, Vallée AA, Jungerius AP, Crooijmans RP, Groenen MA (2008) Partial duplication of the PRLR and SPEF2 genes at the late feathering locus in chicken. BMC Genom 9:391
Zhang X, Wang H, Zhang L, Wang Q, Du X, Ge L, Zhou R, Li L, Li X (2018) Analysis of a genetic factors contributing to feathering phenotype in chickens. Poult Sci 97:3405–3413
Luo C, Shen X, Rao Y, Xu H, Tang J, Sun L, Nie Q, Zhang X (2012) Differences of Z chromosome and genomic expression between early- and late-feathering chickens. Mol Biol Rep 39:6283–6288
Lowe PC, Garwood VA (1981) Independent effects of K and k+ alleles and maternal origin on mortality and performance of crossbred chickens. Poult Sci 6:1123–1126
Havenstein GB, Toelle VD, Towner RH, Emsley A (1989) Effects of genetic strain, slow versus rapid-feathering maternal genotype, and cage density on the performance of single comb white leghorns. Poult Sci 68:596–607
Harris DL, Garwood VA, Lowe PC, Hester PY, Crittenden LB, Fadly AM (1984) Influence of sex-linked feathering phenotypes of parents and progeny upon lymphoid leukosis virus infection status and egg production. Poult Sci 63:401–413
Smith EJ, Fadly AM (1994) Male-mediated venereal transmission of endogenous avian leukosis virus. Poult Sci 73:488–494
Fadly AM, Smith EJ (1997) Role of contact and genetic transmission of endogenous virus-21 in the susceptibility of chickens to avian leukosis virus infection and tumors. Poult Sci 76:968–973
Takenouchi A, Toshishige M, Ito N, Tsudzuki M (2018) Endogenous viral gene ev21 is not responsible for the expression of late feathering in chickens. Poult Sci 97:403–411
Bu G, Huang G, Fu H, Li J, Huang S, Wang Y (2013) Characterization of the novel duplicated PRLR gene at the late-feathering K locus in lohmann chickens. J Mol Endocrinol 51:261–276
Wilkanowska A, Mazurowski A, Mroczkowski S, Kokoszynski D (2014) Prolactin (PRL) and prolactin receptor (PRLR) genes and their role in poultry production traits. Folia Biol 62:1–8
Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA (1998) Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Res 3:225–268
Zhang Q, Mo G, Xie T, Zhang Z, Fu H, Wei P, Zhang X (2021) Phylogenetic analysis of ALV-J associated with immune responses in yellow chicken flocks in south China. Mediat Inflamm 2021:6665871
Tixier-Boichard MH, Benkel BF, Chambers JR, Gavora JS (1994) Screening chickens for endogenous virus ev21 viral element by the polymerase chain reaction. Poult Sci 73:1612–1616
Li Z, Luo Q, Xu H, Zheng M, Abdalla BA, Feng M, Cai B, Zhang X, Nie Q, Zhang X (2017) MiR-34b-5p suppresses melanoma differentiation-associated gene 5 (MDA5) signaling pathway to promote avian leukosis virus subgroup J (ALV-J)-infected cells proliferaction and ALV-J replication. Front Cell Infect Microbiol 7:17
Zhang J, Cai B, Ma M, Luo W, Zhang Z, Zhang X, Nie Q (2020) ALDH1A1 inhibits chicken preadipocytes’ proliferation and differentiation via the PPARγ pathway in vitro and in vivo. Int J Mol Sci 21:3150
Skwarlo-Sonta K (1990) Mitogenic effect of prolactin on chicken lymphocytes in vitro. Immunol Lett 24:171–177
Iraqi F, Smith EJ (1994) Determination of the zygosity of ev21-K in late-feathering male white leghorns using the polymerase chain reaction. Poult Sci 73:939–946
Nakamura A, Ishikawa A, Nagao K, Watanabe H, Uchida M, Kansaku N (2011) Characteristics of reversion to early feathering phenotype in the late feathering line of nagoya breed chickens. J Poult Sci 48:155–161
Fadly AM, Smith EJ (1991) Influence of maternal antibody on avian leukosis virus infection in white leghorn chickens harboring endogenous virus-21 (EV21). Avian Dis 35:454–489
Williams SM, Reed WM, Bacon LD, Fadly AM (2004) Response of white leghorn chickens of various genetic lines to infection with avian leukosis virus subgroup. J Avian Dis 48:61–67
Bacon LD, Smith EJ, Crittenden LB, Havenstein GB (1988) Association of the slow feathering (K) and an endogenous viral (ev21) gene on the Z chromosome of chickens. Poult Sci 67:191–197
Bhat G, Gupta SK, Maiti BR (1983) Influence of prolactin on mitotic activity of the bursa of Fabricius of the chick. Gen Comp Endocrinol 52:452–455
Rawlings JS, Rosler KM, Harrison DA (2004) The JAK/STAT signaling pathway. J Cell Sci 117:1281–1283
Harrison DA (2012) The JAK/STAT pathway. Cold Spring Harb Perspect Bio 4:11205–11205
Okamura A, Masumoto A, Takenouchi A, Kudo T, Aizawa S, Ogoshi M, Takahashi S, Tsudzuki M, Takeuchi S (2019) Changes in prolactin receptor homodimer availability may cause late feathering in chickens. Gen Comp Endocrinol 272:109–116
Wallis M (2021) Do some viruses use growth hormone, prolactin and their receptors to facilitate entry into cells? BioEssays 43:2000268
Staring J, Raaben M, Brummelkamp TR (2018) Viral escape from endosomes and host detection at a glance. J Cell Sci 131:jcs216259
Spriggsa CC, Harwood MC, Tsai B (2019) How non-enveloped viruses hijack host machineries to cause infection. Adv Virus Res 104:97–122
Griffiths CD, Bilawchuk LM, McDonough JE, Jamieson KC, Elawar F, Cen Y (2020) IGF1R is an entry receptor for respiratory syncytial virus. Nature 583:615–619
Acknowledgements
The ALV-J strain SCAU-HN06 was kindly provided by Prof. Weisheng Cao, South China Agricultural University. The ALV-J envelope protein-specific mouse monoclonal antibody JE9 was kindly provided by Prof. Aijian Qin, Yangzhou University.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31571269) and the China Agriculture Research System of MOF and MARA (No. CARS-41).
Author information
Authors and Affiliations
Contributions
GM designed the study, drafted the paper, carried out experiments, and analysed data. BH helped by providing useful discussion and language correction and contributed to performing some of the experiments. QZ, ZR, WL, JL, YS, ZM, and ZZ helped with performing some of the experiments. ZW and MS helped with valuable suggestions and comments. XZ participated in the design, manuscript writing, and final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
ALV-J isolation and identification. A PCR detection results for the cell DNA of a positive sample using an ALV-J-specific primer. B PCR detection results for the cell DNA of a negative sample using an ALV-J-specific primer. C IFA results for DF1 cells using the ALV-J-specific antibody JE9 (200x magnification). bp base pairs. The numbers on the left indicate the lengths of molecular weight standards. M DL2000 marker; LF chickens L1-L12; EF chickens E1-E12; positive control +; negative control −; NC negative control. Note: When the supernatant p27 results for DF-1 cells incubated with the sample plasma were positive, the cell genome was amplified with the ALV-J-specific primer to obtain the target fragment (545 bp) (Additional file 1A). Other subgroups of ALV-, MDV- and REV-specific primers were used for amplification, and no relevant target fragments were obtained (data not shown). The target fragments were not obtained in the individuals with a negative result for the supernatant p27 test (Additional file 1B). To further confirm that the selected chickens were infected with the ALV-J subgroup, the positive samples were subjected to IFA verification. The plasma samples were used to infect DF-1 cells and showed obvious green fluorescence, indicating that the positive EF and LF chickens were infected with ALV-J, while the negative control group showed no green fluorescence (Additional file 1C). Furthermore, the plasma samples were analysed with a p27 test for each collection, and the cell supernatant p27 test results are shown in Additional file 4.
Additional file 2.
Detection of the ev21 and dSPEF2/dPRLR genes in sampled chickens. A The amplification results for the ev21 gene. B The amplification results for the dSPEF2/dPRLR gene. M DL2000 marker; bp base pairs; LF late feathering chicken; 1-6 LF chickens infected with ALV-J; 7-12 LF chickens not infected with ALV-J; EF early feathering chicken; 1-6 EF chickens infected with ALV-J; 7-12 EF chickens not infected with ALV-J. Note: Two target fragments (515 and 390 bp) produced with ev21 gene primers and a 1434-bp target fragment produced with dSPEF2/dPRLR gene primers were found for all LF chickens. Only one target fragment (515 bp) produced with ev21 gene primers and no target fragment produced with dSPEF2/dPRLR gene primers were found for all EF chickens (Additional file 2A and B).
Additional file 4.
ALV-J viremia was detected by ALV group-specific antigen (p27) ELISA. Note: LF Po LF chickens infected with ALV-J; LF Ne LF chickens not infected with ALV-J; EF Po EF chickens infected with ALV-J; EF Ne EF chickens not infected with ALV-J; W, week. The results for viremia are expressed as the S/P value. An S/P value > 0.2 indicates the presence of ALV-J viremia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mo, G., Hu, B., Zhang, Q. et al. dPRLR causes differences in immune responses between early and late feathering chickens after ALV-J infection. Vet Res 53, 1 (2022). https://doi.org/10.1186/s13567-021-01016-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-021-01016-7