Abstract
Background
[18F]PSMA-1007 is a promising tracer for integrated positron emission tomography and computed tomography (PET/CT).
Objective
Our aim was to assess the diagnostic accuracy of [18F]PSMA-1007 PET/CT for primary staging of lymph node metastasis before robotic-assisted laparoscopy (RALP) with extended lymph node dissection (ePLND).
Design, Setting and Participants
The study was a retrospective cohort in a tertiary referral center. Men with prostate cancer that underwent surgical treatment for intermediate- or high-risk prostate cancer between May 2019 and August 2021 were included.
Interventions
[18F]PSMA-1007 PET/CT for initial staging followed by RALP and ePLND.
Outcome measurements and statistical analyses
Sensitivity and specificity were calculated both for the entire cohort and for patients with lymph node metastasis ≥ 3 mm. Positive (PPV) and negative (NPV) predictive values were calculated.
Results and limitations
Among 104 patients included in the analyses, 26 patients had lymph node metastasis based on pathology reporting and metastases were ≥ 3 mm in size in 13 of the cases (50%). In the entire cohort, the sensitivity and specificity of [18F]PSMA-1007 were 26.9% (95% confidence interval (CI); 11.6–47.8) and 96.2% (95% CI; 89.2–99.2), respectively. The sensitivity and specificity of [18F]PSMA-1007 to detect a lymph node metastasis ≥ 3 mm on PET/CT were 53.8% (95% CI; 25.1–80.8) and 96.7% (95% CI; 90.7–99.3), respectively. PPV was 70% and NPV 93.6%.
Conclusions
In primary staging of intermediate- and high-risk prostate cancer, [18F]PSMA-1007 PET/CT is highly specific for prediction of lymph node metastases, but the sensitivity for detection of metastases smaller than 3 mm is limited. Based on our results, [18F]PSMA-1007 PET/CT cannot completely replace ePLND.
Patient summary
This study investigated the use of an imaging method based on a prostate antigen-specific radiopharmaceutical tracer to detect lymph node prostate cancer metastasis. We found that it is unreliable to discover small metastasis.
Similar content being viewed by others
Introduction
Prostate cancer is the most common cancer form in developed countries, and the incidence is increasing [1]. A significant proportion of patients diagnosed with localized prostate cancer is successfully managed with radical prostatectomy or radiotherapy. Accurate assessment of tumor stage, regional lymph node involvement and distant metastasis are essential to recommend proper treatment.
Conventional imaging, such as computed tomography (CT), magnetic resonance imaging (MRI), and bone scintigraphy (BS), have shown limited sensitivity to detect prostate cancer metastasis [2, 3]. Molecular imaging, using different radiopharmaceuticals, has shown promising results in visualizing the presence and extent of prostate cancer lesions [4, 5]. Radiolabeled choline positron emission tomography/computed tomography (choline PET/CT) was previously used to identify lymph node metastasis, but studies have shown a poor accuracy [5,6,7]. Ligands targeting prostate-specific membrane antigen (PSMA) have been introduced in PET/CT for localization of recurrent disease and more recently for primary staging of high-risk tumors. Several tracers have been developed for PSMA PET/CT. Studies have shown promising results with [68 Ga]Ga-PSMA-11 for both staging and disease recurrence [4, 8, 9]. In a recent study by Fendler et al. [10], a [68 Ga]Ga-PSMA-11 PET/CT scan changed management in more than 50% of cases with biochemical recurrence (BCR). Similar results were shown in another study by Sonni et al. in 2020 [11]. [18F]PSMA-1007 is a radiopharmaceutical which has not been evaluated to the same degree as [68 Ga]Ga-PSMA-11. It has the advantage of a longer half-life and offers a low urinary clearance compared to [68 Ga]Ga-PSMA-11 PET/CT. However, a previous study by Vollnberg et al. showed that [18F]PSMA-1007 PET/CT scans often present focal unspecific bone uptake, that should be interpreted carefully to avoid over-staging [12].
A head-to-head comparison by Kuten et al. [13] showed that both [18F]PSMA-1007 and [68 Ga]Ga-PSMA-11 identified all lesions in the prostate in intermediate- or high-risk prostate cancer patients at staging. Similarly, Hoberück et al. [14] found no difference in staging accuracy between the two tracers.
The aim of this study was to evaluate the accuracy of [18F]PSMA-1007 in lymph node staging in intermediate- and high-risk prostate cancer, using histopathology after extended pelvic lymph node dissection (ePLND) as reference method. We investigated a consecutive, retrospective cohort in a tertiary referral center.
Material and methods
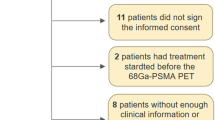
All patients who underwent primary staging of intermediate- or high-risk prostate cancer with [18F]PSMA-1007 PET/CT followed by robotic-assisted laparoscopic prostatectomy (RALP), with extended pelvic lymph node dissection (ePLND) from May 2019 until August 2021, were eligible to be included in the study. The study was approved by the Regional Ethics Committee in Lund (nr 2016/417, 2018/753), and all patients signed an informed consent. Data extracted from medical records included age, date of diagnosis, treatment modality, PSA, Gleason score, results of PSMA PET/CT and details from pathology reports. Patients were classified as intermediate- or high risk based on the EAU guidelines risk groups. Surgery was performed by experienced surgeons at Skåne University Hospital in Malmö using a detailed template. Bilateral ePLND was defined as removal of the tissue from the bifurcation of the common iliac artery and distally along the external iliac vessels, above the internal iliac artery and deep in the obturator fossa. Tissue from the left and right side was sent separately for histopathological examination. ePLND was performed in conjunction with RALP in every case.
PET/CT imaging
Patients were administered 4 MBq/kg [18F]PSMA-1007 (median 4.0, range 3.7–6.7) through intravenous bolus injection. A head-to-knee PET/CT scan was performed using GE Discovery MI PET-CT systems (Discovery MI; GE Healthcare, Milwaukee, WI, USA) 120 min (median 120, range 115–130) after administration of the radiopharmaceutical [15]. No forced diuresis was applied as [18F]PSMA-1007 is mainly eliminated through bile. Acquisition time was 2 min/bed position (3 min when BMI > 40). The PET/CT-system has a 128-slice CT. The Q.Clear reconstruction algorithm was used, including time-of-flight, point spread function and CT-based attenuation correction with a 256 × 256 matrix (pixel size, 2.7 × 2.7 mm2, slice thickness, 2.8 mm [16]. The noise-regularization parameter (β) was set to 800 [17]. An adaptive statistical iterative reconstruction technique was used for the CT images.
PET/CT images were evaluated for lymph node metastases by a nuclear medicine physician with > 5 years' experience of whole-body PET/CT. Evaluation was done blinded and compared with a previous evaluation done for clinical purposes. If there was disagreement between the evaluations or if the clinical evaluation was inconclusive a third evaluation was performed by a nuclear medicine specialist with > 10 years' experience of PET/CT. Lymph nodes were graded 1–5 according to the E-PSMA reader confidence scale [18]. Grade 1–2 lymph nodes were considered non-pathological, while grade 4–5 were considered pathological. Grade 3 includes “faint uptake in a site typical for prostate cancer.” We considered lymph node uptake clearly visible (with standardized uptake value threshold 0–10) but clearly below spleen as faint. Grade 3 was considered pathological when deviating from known patterns of unspecific uptake (mainly faint symmetric uptake along the external iliac vessels or intense uptake in thorax or axilla). Maximum standardized uptake value (SUVmax) calculated by body weight was measured in the prostate.
Histopathology
Lymph node specimens from the left and right side were examined in a blinded fashion by experienced pathologists at Skåne University Hospital using routine methods. According to standard of practice in Sweden, the presence and size of metastases and the total number of lymph nodes extracted from each side were reported in detail.
Statistical analysis
Statistical analysis was performed using STATA (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) including 95% confidence intervals (CI) were calculated for [18F]PSMA-1007 PET/CT scan using the histology results after ePLND as gold standard. Two patient-based scenarios were analyzed: firstly, the ability of [18F]PSMA-1007 PET/CT scanning to detect the presence of lymph node metastasis of any size in a patient, and secondly the ability to detect lymph node metastasis ≥ 3 mm in diameter. The size of the metastasis and not the lymph node itself was reported. Considering all lymph nodes obtained at ePLND as separate observations, the ability of [18F]PSMA-1007 PET/CT scan to detect the presence of metastasis of any size in lymph nodes was analyzed (lesion-based analyses). The Mann–Whitney U-test was used for comparison of SUVmax in the prostate. A p value less than 0.05 was considered statistically significant.
Results
We evaluated a consecutive series of 106 intermediate- or high-risk prostate cancer patients who were evaluated with [18F]PSMA-1007 PET/CT prior to RALP with ePLND between May 2019 and August 2021. Two patients were excluded since a follow-up [18F]PSMA-1007 PET/CT was positive for lymph nodes on the exact location of the initial [18F]PSMA-1007 PET/CT, suggesting these lymph nodes were not removed during ePLND. After exclusions, 104 patients remained for final analysis. Patient characteristics are summarized in Table 1. In total, 2519 lymph nodes were removed by ePLND and prostate cancer metastases were found by histopathological examination in 41 lymph nodes from 26 patients (Table 2).
Lymph node metastasis was detected pre-operatively by [18F]PSMA-1007 in 7 of these 26 patients resulting in a sensitivity of 26.9% (95% CI; 11.6–47.8) and a specificity of 96.2% (95% CI; 89.2–99.2) (Table 3). PPV and NPV were 70% and 79.8%, respectively (Table 3).
For lymph nodes metastases ≥ 3 mm in diameter, [18F]PSMA-1007 PET/CT detected 7 out of 13 metastases, resulting in a sensitivity and specificity of 53.8% (95% CI; 25.1–80.8) and 96.7% (95% CI; 90.7–99.3), respectively. PPV and NPV for detecting lymph nodes ≥ 3 mm were 70% and 93.6%, respectively (Table 3). The area under curve (AUC) in receiver operating characteristics (ROC) analysis for [18F]PSMA-1007 PET/CT to detect lymph node metastases larger than 3 mm in size was 0.75 (95% CI; 0.61–0.89) compared to 0.62 (95% CI; 0.53–0.71) for metastases of any size.
In high-risk prostate cancers, the sensitivity to detect lymph node metastasis of any size with [18F]PSMA-1007 PET/CT pre-operatively was 35% (95% CI: 15.4–59.2) (Tables 4 and 5). Only one patient had a positive [18F]PSMA-1007 PET/CT in the intermediate risk group. The specificity was high in both groups; 96.7% (95% CI; 88.5–99.6) for high-risk and 94.4% (95% CI; 72.7–99.9) for intermediate prostate cancers. In the high-risk group, PPV and NPV for detecting lymph nodes were 77.8% and 81.7%, respectively, and AUC ROC was 0.66 (95% CI; 0.55–0.77).
Quantification of tracer uptake in lymph nodes and in the prostate
Three lymph nodes were classified as false positives on [18F]PSMA-1007 PET/CT using histopathology as gold standard. They all displayed faint uptake in non-enlarged lymph nodes asymmetrically distributed along the external iliac vessels. All true positives showed moderate (close to or above spleen) or higher uptake on [18F]PSMA-1007 PET/CT along the external iliac vessels or faint or higher uptake along the internal iliac vessels (Fig. 1).
Examples of true and false positives and negatives with [18F]PSMA-1007 PET/CT. a True positive [18F]PSMA-1007 PET/CT with low grade uptake in two lymph nodes along the right external and internal iliac vessels. PAD showed one 5 mm metastasis from the right side (presumably from the internal iliac since the lymph node along the external vessels measured 12 mm and was PSMA + in its entirety) and one 1,5 mm from the left (not detected by PET/CT). b False positive [18F]PSMA-1007 PET/CT with low grade uptake in one lymph node along the right external iliac vessels. c True negative [18F]PSMA-1007 PET/CT with low grade uptake in several lymph nodes along the external vessels bilaterally. d False negative [.18F]PSMA-1007 PET/CT with faint uptake in three lymph nodes along the external vessels bilaterally (only on shown in image)
There was no significant difference in SUVmax in the prostate between true positives (median 20.2, range 8.0–53.9, n = 7) and false negatives (median 17.0, range 5.2–47.5, n = 19), p = 0.59.
A sensitivity analysis was performed to investigate if sensitivity and specificity were affected if all grade 3 lymph nodes were either regarded as pathological or non-pathological. Regarding all grade 3 lymph nodes as pathological gave a sensitivity of 31%, specificity of 78% and AUC of 0.55, compared to a sensitivity of 17%, specificity of 100% and AUC of 0.56 when all grade 3 lymph nodes were classified as non-pathological.
Discussion
In this study of intermediate- and high-risk prostate cancer patients evaluated with [18F]PSMA-1007 PET/CT pre-operatively it was found that the sensitivity for detection of lymph node metastasis of any size was 26.9% and 53.8% for lymph node metastasis > 3 mm in size. However, [18F]PSMA-1007 PET/CT was found to be highly specific, ruling out metastasis in 96.2% of patient with no metastases on histopathological examination.
Correct staging of prostate cancer is essential to select the most appropriate treatment for every patient. Conventional imaging such as CT, MRI, 18F-choline PET/CT and BS has shown poor accuracy in both detecting and ruling out metastases in the lymph nodes [2, 7]. In the EAU guidelines for prostate cancer, lymph node dissection is recommended for staging of prostate cancer in some patients based on risk prediction by nomograms; however, the procedure is associated with significant side effects, and no survival benefit has been demonstrated [19,20,21].
There is a need for improvement in prostate cancer staging, and different tracers used in PET/CT have been shown to provide additive value. [68 Ga]Ga-PSMA-11 has been investigated in several studies with promising results for both staging and recurrence detection [4, 8,9,10]. In a recent study by Moreira et al. [22], the sensitivity and specificity for lymph node metastases were 75% and 90%, respectively. Due to the longer half-life and lower urinary clearance, presumably causing less artifacts in PET/CT, [18F]PSMA-1007 PET/CT might further improve staging, but the method has not yet been sufficiently investigated. Prior studies evaluating the role of [18F]PSMA-1007 PET/CT in primary staging of prostate cancer have reported on limited patient cohorts [23, 24]. Giesel et al. [23] found a high sensitivity of 94.7%, and however, their results were based on only eight patients. Similarly, Kesch et al. [24] analyzed ten patients and found a sensitivity and specificity of 71% and 81%, respectively. To the best of our knowledge, only three larger studies on prostate cancer staging using [18F]PSMA-1007 PET/CT have been published. In line with the results of our study, Meijer et al. [25] analyzed 757 patients and found a sensitivity of 38% and a specificity of 94%. However, they used three different tracers, [68 Ga]Ga-PSMA-11, 18F-DCFPyL and 1[18F]PSMA-1007, which makes it difficult to compare with our present results. In a study by Hermsen et al. [26], 99 men were evaluated with a sensitivity of 53.3% and a specificity of 89.9% which is in line with our study. In the third study by Sprute et al. [27], the accuracy of [18F]PSMA-1007 PET/CT in lymph node staging was investigated in 96 prostate cancer patients. They found a significantly higher sensitivity of 73.5% and somewhat higher specificity of 99.4% than in our study. The differences between our results and those reported by Sprute et al. can to some extent be explained by patient selection. While our study included only patients that had been staged with [18F]PSMA-1007 PET/CT prior to ePLND performed simultaneously with RALP, Sprute et al. included [18F]PSMA-1007 PET/CT scannings performed both for staging and salvage ePLND. For patients with BCR, another tracer, [18F]-rhPSMA-7, has also been investigated [28,29,30] showing a high detection efficacy. In a study on 58 patients by Kroenke et al. [31] using this tracer, the sensitivity and specificity for detecting lymph node metastasis prior to salvage surgery were 72.2% and 92.5%, respectively. These results are difficult to compare to the results of our study due to the diverse patient base. On another note, a prior study analyzing pitfalls of PSMA-PET/CT found that PSMA-ligand uptake in benign lesions was considerably higher with [18F]PSMA-1007 compared to [68 Ga]Ga-PSMA-11 [32]. Overall, due to the lack of head-to-head studies, it is still unclear if one PSMA-tracer offers significant advantages over the others in prostate cancer staging.
Our subanalysis of the metastasis size showed a significantly higher sensitivity and specificity with a larger metastasis size. This was also seen in Sprute et al. where the detection of nodes more than 3 mm in size the sensitivity and specificity was 85.9% and 99.5%, respectively. It is unclear if Sprute et al. measured the size of the metastasis or the size of the lymph node, which makes the results difficult to compare. Nevertheless, in line with the study by Sprute et al., as well as studies of [68 Ga]Ga-PSMA-11, we found that [18F]PSMA-1007 PET/CT has a lower detection rate for small lymph node metastasis [27, 33]. In fact, no metastasis < 3 mm in size was identified on [18F]PSMA-1007 PET/CT in our study.
We attempted to analyze the benefit of [18F]PSMA-1007 PET/CT in different risk categories, according to the EAU risk groups for prostate cancer patients. Due to low numbers in the intermediate group, only one patient had a positive [18F]PSMA-1007 PET/CT, which makes it impossible to draw any conclusion. Regarding the interpretation of [18F]PSMA-1007 PET/CT scans we found that faint tracer uptake along the external iliac vessels is unspecific and should be interpreted cautiously. False negative findings were not associated with lower SUVmax in the prostate compared to true positives. Again, the low number of true positive cases makes our results uncertain.
Our study utilizes PET scanners with silicon photomultiplier technology and the Q.Clear reconstruction algorithm. Both these technologies can improve image quality and potentially lesion detection [34,35,36]. In prostate cancer, this has, to our knowledge, only been studied in BCR with promising results [37, 38]. It is possible that a lower sensitivity would have been found with conventional PET scanners.
The strengths of this study are the population-based design, the meticulous information collection, and the blinded assessment of [18F]PSMA-1007 PET/CT scans by one nuclear medicine physician. The main limitations are the retrospective design of the study and the limited number of patients.
Conclusion
We found [18F]PSMA-1007 PET/CT to be highly specific for prediction of lymph node metastases in patients with intermediate- or high-risk prostate cancer, but with low sensitivity for detection of metastatic lesions smaller than 3 mm in size. Currently, [18F]PSMA-1007 PET/CT cannot completely replace staging with ePLND in patients with no signs of distant metastases.
Availability of data and materials
The datasets from the study can be made available from the corresponding author on reasonable request.
References
Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–16.
Briganti A, Abdollah F, Nini A, Suardi N, Gallina A, Capitanio U, et al. Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. Eur Urol. 2012;61(6):1132–8.
Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77(4):403–17.
Umbehr MH, Muntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64(1):106–17.
Kjolhede H, Ahlgren G, Almquist H, Liedberg F, Lyttkens K, Ohlsson T, et al. Combined 18F-fluorocholine and 18F-fluoride positron emission tomography/computed tomography imaging for staging of high-risk prostate cancer. BJU Int. 2012;110(10):1501–6.
Puterman C, Bjoersdorff M, Amidi J, Anand A, Soller W, Jiborn T, et al. A retrospective study assessing the accuracy of [18F]-fluorocholine PET/CT for primary staging of lymph node metastases in intermediate and high-risk prostate cancer patients undergoing robotic-assisted laparoscopic prostatectomy with extended lymph node dissection. Scand J Urol. 2021;55(4):293–7.
Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C, et al. Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: a multicenter prospective phase 3 imaging trial. JAMA Oncol. 2021;7(11):1635–42.
Esen T, Falay O, Tarim K, Armutlu A, Koseoglu E, Kilic M, et al. (68)Ga-PSMA-11 positron emission tomography/computed tomography for primary lymph node staging before radical prostatectomy: central review of imaging and comparison with histopathology of extended lymphadenectomy. Eur Urol Focus. 2021;7(2):288–93.
Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5(6):856–63.
Sonni I, Eiber M, Fendler WP, Alano RM, Vangala SS, Kishan AU, et al. Impact of (68)Ga-PSMA-11 PET/CT on staging and management of prostate cancer patients in various clinical settings: a prospective single-center study. J Nucl Med. 2020;61(8):1153–60.
Vollnberg B, Alberts I, Genitsch V, Rominger A, Afshar-Oromieh A. Assessment of malignancy and PSMA expression of uncertain bone foci in [(18)F]PSMA-1007 PET/CT for prostate cancer-a single-centre experience of PET-guided biopsies. Eur J Nucl Med Mol Imaging. 2022. https://doi.org/10.1007/s00259-022-05745-5.
Kuten J, Fahoum I, Savin Z, Shamni O, Gitstein G, Hershkovitz D, et al. Head-to-head comparison of (68)Ga-PSMA-11 with (18)F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J Nucl Med. 2020;61(4):527–32.
Hoberuck S, Lock S, Borkowetz A, Sommer U, Winzer R, Zophel K, et al. Intraindividual comparison of [(68) Ga]-Ga-PSMA-11 and [(18)F]-F-PSMA-1007 in prostate cancer patients: a retrospective single-center analysis. EJNMMI Res. 2021;11(1):109.
Rahbar K, Afshar-Oromieh A, Bogemann M, Wagner S, Schafers M, Stegger L, et al. (18)F-PSMA-1007 PET/CT at 60 and 120 minutes in patients with prostate cancer: biodistribution, tumour detection and activity kinetics. Eur J Nucl Med Mol Imaging. 2018;45(8):1329–34.
Ross S. Q.Clear White paper. GE Healthcare; 2014.
Tragardh E, Minarik D, Brolin G, Bitzen U, Olsson B, Oddstig J. Optimization of [(18)F]PSMA-1007 PET-CT using regularized reconstruction in patients with prostate cancer. EJNMMI Phys. 2020;7(1):31.
Ceci F, Oprea-Lager DE, Emmett L, Adam JA, Bomanji J, Czernin J, et al. E-PSMA: the EANM standardized reporting guidelines v.10 for PSMA-PET. Eur J Nucl Med Mol Imaging. 2021;48(5):1626–38.
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–62.
Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, Suardi N, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;61(3):480–7.
Preisser F, van den Bergh RCN, Gandaglia G, Ost P, Surcel CI, Sooriakumaran P, et al. Effect of extended pelvic lymph node dissection on oncologic outcomes in patients with d’amico intermediate and high risk prostate cancer treated with radical prostatectomy: a multi-institutional study. J Urol. 2020;203(2):338–43.
Moreira LF, Mussi TC, Cunha MLD, Filippi RZ, Baroni RH. Accuracy of (68)Ga-PSMA PET/CT for lymph node and bone primary staging in prostate cancer. Urol Oncol. 2022;40(3):104.e17-104.e21.
Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44(4):678–88.
Kesch C, Vinsensia M, Radtke JP, Schlemmer HP, Heller M, Ellert E, et al. Intraindividual comparison of (18)F-PSMA-1007 PET/CT, multiparametric MRI, and radical prostatectomy specimens in patients with primary prostate cancer: a retrospective. Proof Concept Study J Nucl Med. 2017;58(11):1805–10.
Meijer D, van Leeuwen PJ, Roberts MJ, Siriwardana AR, Morton A, Yaxley JW, et al. External validation and addition of prostate-specific membrane antigen positron emission tomography to the most frequently used nomograms for the prediction of pelvic lymph-node metastases: an international multicenter study. Eur Urol. 2021;80(2):234–42.
Hermsen R, Wedick EBC, Vinken MJM, van Kalmthout LWM, Kusters-Vandevelde HVN, Wijers CHW, et al. Lymph node staging with fluorine-18 prostate specific membrane antigen 1007-positron emission tomography/computed tomography in newly diagnosed intermediate- to high-risk prostate cancer using histopathological evaluation of extended pelvic node dissection as reference. Eur J Nucl Med Mol Imaging. 2022. https://doi.org/10.1007/s00259-022-05827-4.
Sprute K, Kramer V, Koerber SA, Meneses M, Fernandez R, Soza-Ried C, et al. Diagnostic accuracy of (18)F-PSMA-1007 PET/CT imaging for lymph node staging of prostate carcinoma in primary and biochemical recurrence. J Nucl Med. 2021;62(2):208–13.
Eiber M, Kroenke M, Wurzer A, Ulbrich L, Jooss L, Maurer T, et al. (18)F-rhPSMA-7 PET for the detection of biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med. 2020;61(5):696–701.
Wurzer A, Di Carlo D, Schmidt A, Beck R, Eiber M, Schwaiger M, et al. Radiohybrid ligands: a novel tracer concept exemplified by (18)F-or (68)Ga-labeled rhPSMA inhibitors. J Nucl Med. 2020;61(5):735–42.
Rauscher I, Karimzadeh A, Schiller K, Horn T, D'Alessandria C, Franz C et al. Detection efficacy of (18)F-rhPSMA-7.3 PET/CT and impact on patient management in patients with biochemical recurrence of prostate cancer after radical prostatectomy and prior to potential salvage treatment. J Nucl Med; 2021.
Kroenke M, Schweiger L, Horn T, Haller B, Schwamborn K, Wurzer A, et al. Validation of (18)F-rhPSMA-7 and (18)F-rhPSMA-7.3 PET imaging results with histopathology from salvage surgery in patients with biochemical recurrence of prostate cancer. J Nucl Med. 2022. https://doi.org/10.2967/jnumed.121.263707.
Rauscher I, Kronke M, Konig M, Gafita A, Maurer T, Horn T, et al. Matched-pair comparison of (68)Ga-PSMA-11 PET/CT and (18)F-PSMA-1007 PET/CT: frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy. J Nucl Med. 2020;61(1):51–7.
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195(5):1436–43.
Oddstig J, Leide Svegborn S, Almquist H, Bitzen U, Garpered S, Hedeer F, et al. Comparison of conventional and Si-photomultiplier-based PET systems for image quality and diagnostic performance. BMC Med Imaging. 2019;19(1):81.
Parvizi N, Franklin JM, McGowan DR, Teoh EJ, Bradley KM, Gleeson FV. Does a novel penalized likelihood reconstruction of 18F-FDG PET-CT improve signal-to-background in colorectal liver metastases? Eur J Radiol. 2015;84(10):1873–8.
Wagatsuma K, Miwa K, Sakata M, Oda K, Ono H, Kameyama M, et al. Comparison between new-generation SiPM-based and conventional PMT-based TOF-PET/CT. Phys Med. 2017;42:203–10.
Alberts I, Hunermund JN, Sachpekidis C, Mingels C, Fech V, Bohn KP, et al. The influence of digital PET/CT on diagnostic certainty and interrater reliability in [(68)Ga]Ga-PSMA-11 PET/CT for recurrent prostate cancer. Eur Radiol. 2021;31(10):8030–9.
Duan H, Baratto L, Hatami N, Liang T, Mari Aparici C, Davidzon GA, et al. (68)Ga-PSMA11 PET/CT for biochemically recurrent prostate cancer: Influence of dual-time and PMT- vs SiPM-based detectors. Transl Oncol. 2022;15(1): 101293.
Acknowledgments
Not applicable.
Funding
Open access funding provided by Lund University. This work was supported by grants from The Swedish Cancer Society (#CAN 2018/522), The Research Funds at Skåne University Hospital, The Governmental Funding (ALF) through The Faculty of Medicine, Lund University (contract numbeF2018:810) and The Swedish Prostate Cancer Federation.
Author information
Authors and Affiliations
Contributions
JI and EH contributed to the data analysis along with interpretation and the manuscript drafting. AS measured the size of the metastasis in the lymph nodes when this was not known. ET and AB contributed with critical review and revision. AB did the design of the study and funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Regional Ethics. Committee in Lund (nr 2016/417, 2018/753) and all patients signed a written informed consent. All methods were carried out in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ingvar, J., Hvittfeldt, E., Trägårdh, E. et al. Assessing the accuracy of [18F]PSMA-1007 PET/CT for primary staging of lymph node metastases in intermediate- and high-risk prostate cancer patients. EJNMMI Res 12, 48 (2022). https://doi.org/10.1186/s13550-022-00918-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13550-022-00918-7