Abstract
Background
Bone disease in multiple myeloma is characterized by reduced bone formation. The gold standard of bone formation is the mineral apposition rate (MAR), an invasive technique reflecting bone formation at a single site. We compared 18F-fluoride-PET with the MAR in myeloma patients.
Methods
Bone formation was measured before and after bortezomib treatment by determination of the MAR in iliac bone marrow biopsies and the measurement of 18F-uptake.
Results
The inter- and intra-individual variations in 18F-uptake (SUVA50%) were pronounced as 33.50 (range 4.42 to 37.92) and 27.18 (range 4.00 to 31.18), respectively. A significant correlation between the MAR and 18F-uptake was found (r = 0.80, p = 0.017). There was a heterogeneous response after treatment varying from −2.20 to 4.53.
Conclusions
Iliac 18F-uptake was associated with the local MAR in myeloma patients. Furthermore, 18F-fluoride-PET demonstrated the heterogeneity of in vivo bone formation, enabling monitoring during treatment.
Similar content being viewed by others
Background
Bone disease (BD) is a major cause of morbidity and mortality in multiple myeloma (MM) patients. Up to 90 % of patients develop bone pathology throughout the course of their disease, which is not only characterized by increased osteolysis but importantly also by reduced bone formation (BF) [1]. With an increased life expectancy due to novel agents, identifying agents that in addition to their anti-MM effect improve BD is becoming more important. The proteasome inhibitor bortezomib has not only been described to inhibit osteolysis by blocking RANKL-mediated bone resorption by osteoclasts [2] but also to stimulate BF. This has been supported by the observation that the levels of biochemical markers of bone deposition increased after bortezomib treatment [3]. In addition, Giuliani showed an increase in the number of osteoblasts on the bone surface in bone marrow (BM) biopsies after bortezomib treatment [4]. However, to the best of our knowledge, in vivo data on the effect of bortezomib on BF are lacking.
One of the reasons for that is that monitoring the effect of treatment on MM-BD is hampered by the fact that the gold standard of BF, the mineral apposition rate (MAR) determined in tetracycline-labelled bone biopsies, is an invasive, laborious technique. Moreover, since MM-BD is known to have a focal and heterogeneous character with a single bone biopsy, important information will be missed. Obtaining multiple biopsies is impossible for both practical and ethical reasons. Therefore, the exploration of non-invasive, whole body techniques for the measurement of BF is of great interest. Experimental 18F-fluoride-PET imaging offers a potential method for the assessment of bone turnover. 18F-ions exchange with hydroxyl groups in the hydroxyl-apatite crystal of bone, resulting in fluoro-apatite [5]. Hence, the uptake of 18F reflects in vivo BF. Previous studies have shown that the quantification of 18F-fluoride-PET uptake had a direct relationship with local bone morphometrical changes indeed [6, 7]. Furthermore, 18F-fluoride-PET yields simplified quantitative parameters such as a standardized uptake value (SUV), which may be useful in the assessment of treatment response [8].
In the present study, we evaluated the accuracy of 18F-fluoride-PET-CT for monitoring in vivo BF by comparing 18F-uptake with the invasive gold standard, MAR, in MM patients being treated with bortezomib.
Methods
This study was designed as a prospective, non-randomized single-centre pilot study. The review board approved the study, which was conducted according to the provisions of the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice. All patients provided written informed consent. Key inclusion criteria were bortezomib naive, relapsed MM after at least one treatment line and the presence of at least one manifest focal (osteolytic) lesion. Corticosteroid treatment in the previous 4 weeks and the presence of at least grade 2 or painful polyneuropathy were the main exclusion criteria.
Intravenous (IV) bortezomib (1.3 mg/m2) was administered on days 1, 4, 8 and 11 of every 21-day cycle for up to four cycles. There was no concurrent use of corticosteroids and bisphosphonates. Before treatment, 18F-fluoride-PET-CT was performed, with BM biopsy obtained on the first day of cycle one. These examinations were repeated at the end of the fourth treatment cycle.
Response after treatment was defined according to the International Myeloma Working Group (IMWG) response criteria.
18F-fluoride-PET-CT was acquired with a Gemini TF PET/CT scanner (Philips, The Netherlands). 18F-fluoride-PET-CT was performed 60 min post injection of 2.1 MBq/kg of 18F-fluoride, PET emission images were collected for 5 min per bed position from groin to skull. Low-dose CT imaging (30 mAs) was used for attenuation correction. PET-CT scans were obtained prior to the BM biopsy. Activity in the region of interest (ROI) and the volume of interest (VOI) were calculated using the standard software ROI tool (Leuven ROI tool) to define the SUV. 18F-uptake was measured with SUVA50% [9]. The effect of bortezomib on bone remodelling was measured both visually and quantitatively per patient in predetermined bone lesions.
A control group consisting of seven healthy controls was used to validate the variability of fixation at the bone level. A comparison was made between the non-involved femoral bone of MM patients and the femoral bone of healthy individuals. In addition, the results of these seven patients were compared to the results of normal individuals as described by Puri et al. [10].
Bone biopsies of the posterior superior iliac crest were taken after tetracycline labelling. The samples were embedded in polymethylmethacrylate without prior decalcification. The histomorphometric assessment of unstained (tetracycline fluorescence) 5-μm sections was performed automatically using Nis Elements (Nikon www.nikoninstruments.com). Nomenclature was used according to the update of the American Society for Bone and Mineral Research nomenclature committee. The tetracycline double labelling of bone was used to calculate kinetic data on bone turnover. Tetracycline binds to newly formed bone at the bone/osteoid (unmineralized bone) interface where it shows as a linear fluorescence. If a second dose is given after 11–14 days, the amount of bone formed during that interval can be calculated by measuring the distance between the two fluorescent labels.
The correlation between MAR and 18F-uptake (SUVA50%) of the posterior superior iliac crest of which the BM biopsy was taken was calculated using Spearman’s correlation coefficient. A statistically significant (p < 0.05) correlation coefficient was used as a primary cut-off value for the acceptable accuracy for 18F-fluoride-PET-CT. The measurements before and after treatment were regarded as two separate outcome measures because of intermediate bortezomib treatment.
Results
We enrolled seven consecutive patients with relapsed MM scheduled for bortezomib treatment. Two patients prematurely discontinued therapy after two cycles of therapy. As a consequence, in these patients, the MAR and PET measurements were not repeated.
The MAR could be quantified in 8 of 12 biopsies. Three of the remaining four biopsies did not reveal any tetracycline labelling, and the fourth biopsy only revealed single-layer labels. In the evaluable eight biopsies, we found a significant correlation between MAR and 18F-uptake (SUVA50%) (r = 0.80, p = 0.017; Fig. 1). When considering the absence of tetracycline labelling as a lack of BF and thereby a MAR of 0, the correlation coefficient was r = 0.65 (n = 11, p = 0.029). An example of MAR and 18F-fluoride-PET-CT in an individual patient is depicted in Fig. 2.
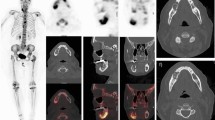
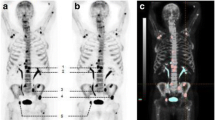
18F-fluoride-PET-CT and MAR (patient BBR01). a 18F-fluoride-PET before and after treatment is shown. It is illustrated that there is a large variety in 18F-uptake within the patient (range 7.34 to 11.34). After treatment, an increase in 18F-uptake in almost all lesions is seen, again with pronounced intra-individual heterogeneity (range 12.50 to 17.49). b CT and 18F-fluoride-PET of the iliac crest are shown. It is illustrated that the increase in 18F-uptake (11.34 to 13.09) after treatment at the site of the biopsy (red arrow) correlates with a decrease in extramedullary plasmacytoma and increase in osteosclerosis. c The biopsy was taken of the right iliac crest (red arrow in panel a and b). The increase in 18F-uptake (11.34 to 13.09) at this site is reflected by an increase in MAR (1.04 to 1.26). The double layers are pointed by white arrows
In addition, the 18F-uptake in the bone of a control group consisting of seven healthy individuals, from our own database, and normal individuals as being described by Puri et al. [10] was compared. There was no difference (Mann-Whitney U test, p = 0.083), indicating that 18F-uptake is a reproducible method for quantification of bone remodelling indeed (Additional file 1: Table S1).
Moreover, there was no statistical difference in 18F-uptake (SUVA50%) between the non-involved bone (femur) of MM patients and that of healthy individuals, supporting the fact that in non-affected bone in MM patients BF is normal. (Mann-Whitney U test, p = 0.083) (Additional file 1: Table S1).
Based on conventional imaging, we identified 28 MM bone lesions in the five patients who completed therapy. There was a wide range in 18F-uptake (SUVA50%) before treatment 4.42 to 37.92. In addition, there was a pronounced intra-individual variation as well (minimum range 4.00, BBR01; maximum range 31.18, BBR05) (Table 1).
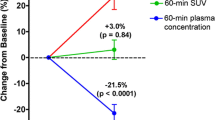
Similar to before treatment, there was a wide range in 18F-uptake (SUVA50%) after treatment (2.71–37.67; median 11.84). Treatment did not affect 18F-uptake, when considering the median 18F-uptake of all involved lesions before and after treatment (11.74 vs 11.84, p = 0.61). However, a detailed analysis of individual patients revealed that the difference in 18F-uptake before and after treatment not only varied between patients (from −2.20 to 4.53 (median 0.48)) (Fig. 3, Table 1) but also between different lesions within the same patient. For example, in BBR04, 18F-uptake in the posterior iliac crest increased from 5.86 to 10.6 (ratio 1.81), with a concomitant increase in MAR from 0 to 1.37, while the BF in the MM-related bone lesions did not improve (ratios 0.77–1.02) (Fig. 3). In only one patient (BBR01), a homogeneous, statistically significant increase in 18F-uptake was shown due to treatment (7.34–11.34 before and 12.50–17.49 after treatment; p = 0.043) (Fig. 3).
Discussion
In order to enable non-invasive in vivo monitoring of BF in MM patients, we compared the gold standard MAR with 18F-fluoride-PET-CT. Although we investigated a limited number of patients only, we found that 18F-fluoride-PET-CT, using a simplified quantitative analysis with SUV, is significantly correlated with MAR. This observation is in line with the results of Messa, who showed a correlation between bone histomorphometric indices and 18F-fluoride-PET in patients with renal osteodystrophy, supporting the implementation of PET for quantifying BF [3].
Moreover, we illustrated the added value of whole body imaging, by showing a large intra-individual heterogeneity in BF, thereby providing more detailed information on the regional BF as compared to MAR, reflecting BF at one site only.
Thirdly, it appears that PET can be used to monitor the effect of bortezomib therapy on BF. Earlier, an increase in osteoblastic activity after bortezomib treatment was shown by technetium (Tc) bone scintigraphy [11]. Since 18F-fluoride-PET-CT has a higher sensitivity and higher resolution than Tc bone scintigraphy [12], it is expected to identify more lesions. This is further supported by the fact that both patients who were described by Lee showed an increase in AP after bortezomib treatment, indicating a pronounced effect on bone remodelling, which is expected to be visualized by Tc bone scintigraphy indeed. In our patient population, AP levels were comparable before and after bortezomib treatment, whereas still changes could be observed by 18F-fluoride-PET-CT. Moreover, there were intra-individual changes. Therefore, 18F-fluoride-PET-CT is a promising technique to further unravel bortezomib-induced bone remodelling.
Thus far, there is only limited circumstantial evidence that bortezomib improves BF. Several investigators suggested an increase in BF during bortezomib treatment based on increased serum BF markers, but only Giulinani has shown an effect on patient osteoblasts. Moreover, there is debate whether this is independent from an anti-MM effect of bortezomib: although serum sclerostin (inhibiting BF) is downregulated independent of an anti-MM effect [13], the increase in the number of osteoblasts was found only in responding patients [4]. Our data, showing a pronounced inter- and intra-individual heterogeneity in BF after treatment, might explain previous controversial results in literature, as serum markers and biopsies at single sites do not reflect such anatomical diversity.
One might argue that in three biopsies no tetracycline labels were observed. This probably reflects very low or absent BF. Therefore, we defined MAR as 0. However, there might also have been a poor compliance of tetracycline intake. In order to exclude any bias, we calculated the correlation coefficient excluding the three biopsies without any label, which did not materially affect the outcome.
Conclusions
Notwithstanding the limitations of a pilot study, the current results support the use of 18F-fluoride-PET-CT for the detection and evaluation of BF in MM patients. In addition, 18F-fluoride-PET-CT provides whole body information regarding bone remodelling. Given the pronounced intra-individual heterogeneity of bone remodelling both before and following treatment, whole body information is important and expected to be of added value to serum markers of BF to evaluate the effect of (new) treatment regimes. Our data show that 18F-fluoride-PET-CT instead of the gold standard MAR is an attractive technique for this evaluation.
References
Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood. 2006;108:3992–6.
Von Metzler I, Krebbel H, Hecht M, et al. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;9:2025–34.
Terpos E, Heath DJ, Rahemtulla A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135:688–92.
Giuliani N, Morandi F, Tagliaferri S, et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–8.
Hawkins RA, Choi Y, Huang SC, et al. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med. 1992;33:633–42.
Messa C, Goodman WG, Hoh CK, et al. Bone metabolic activity measured with positron emission tomography and [18F]fluoride ion in renal osteodystrophy: correlation with bone histomorphometry. J Clin Endocrinol and Metab. 1993;77:949–55.
Piert M, Zittel TT, Becker GA, et al. Assessment of porcine bone metabolism by dynamic [18F]fluoride ion PET: correlation with bone histomorphometry. J Nucl Med. 2001;42:1091–100.
Raijmakers P, Temmerman OP, Saridin CP, et al. Quantification of 18F-fluoride kinetics: evaluation of simplified methods. J Nucl Med. 2014;7:1122–7.
Kobe C, Scheffler M, Holstein A, et al. Predictive value of early and late residual 18F-fluorodeoxyglucose and 18F-fluorothymidine uptake using different SUV measurements in patients with non-small-cell lung cancer treated with erlotinib. Eur J Nucl Med Mol Imaging. 2012;39:1117–27.
Puri T, Frost ML, Curran KM, et al. Differences in regional bone metabolism at the spine and hip: a quantitative study using (18)F-fluoride positron emission tomography. Osteoporos Int. 2013;2:633–9.
Lee SE, Min CK, Yahng SA, et al. Bone scan images reveal increased osteoblastic function after bortezomib treatment in patients with multiple myeloma. Eur J of Haem. 2010;86:83–6.
Mick CG, James T, Hill JD, et al. Molecular imaging in oncology: (18)F-sodium fluoride PET imaging of osseous metastatic disease. Am J Roentgenol. 2014;203:263–71.
Terpos E, Christoulas D, Katodritou E, et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodelling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer. 2012;131:1466–71.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
This study was supported by Janssen-Cilag. The authors declare that they have no competing interests.
Authors’ contributions
JCR developed and wrote the manuscript and analysed the data. PGR designed the study, analysed the data an made important conceptual contributions and assisted in final stages of manuscript preparation. NB designed the study, analysed the data and made important conceptual contributions and assisted in final stages of manuscript preparation. RM made important conceptual contributions and assisted in final stages of manuscript preparation. NJH analysed the data and made important conceptual contributions and assisted in final stages of manuscript preparation. AMdK designed the study and made important conceptual contributions and assisted in final stages of manuscript preparation. MvD made important conceptual contributions and assisted in final stages of manuscript preparation. MJW designed the study and made important conceptual contributions and assisted in final stages of manuscript preparation. JMZ made important conceptual contributions and assisted in final stages of manuscript preparation. PL designed the study, analysed the data and made important conceptual contributions and assisted in final stages of manuscript preparation. PS made important conceptual contributions and assisted in final stages of manuscript preparation. SZ developed and wrote the manuscript, designed the study and analysed the data. All authors read and approved the final manuscript.
Additional file
Additional file 1: Table S1.
18F-uptake (SUVA50%) in (non-involved femoral) bone of MM patients and healthy individuals. (DOCX 13 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Regelink, J.C., Raijmakers, P.G., Bravenboer, N. et al. 18F-fluoride-PET for dynamic in vivo monitoring of bone formation in multiple myeloma. EJNMMI Res 6, 46 (2016). https://doi.org/10.1186/s13550-016-0197-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13550-016-0197-4