Abstract
Background
Tail vein injection under short anesthesia is the most commonly used route for administering radiopharmaceuticals. However, the small caliber of the vein in rodents may lead to tracer extravasation and thereby compromise quantitative accuracy of PET. We aimed to evaluate a method for correction of interstitial radiotracer leakage in the context of pre-clinical therapeutic response assessment.
Methods
In two separate studies involving 16 nude rats, a model of human ovarian cancer was xenografted and each was treated with a Phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor or used as a control. Tracer injections were performed via the tail vein by a single operator. Two observers qualitatively evaluated the resulting images and if appropriate drew a volume of interest (VOI) over the injection site to record extravasated activities. Uncorrected and corrected tumors’ mean standardized uptake value (SUV)mean was computed (corrected injected activity = calibrated activity − decay corrected residual syringe activity − decay corrected tail extravasated activity). Molecular analyses were taken as a gold standard. The frequency and magnitude of extravasation were analyzed, as well as the inter-observer agreement and the impact of the correction method on tumor uptake quantification.
Results
Extravasation never exceeded 20 % of the injected dose but occurred in more than 50 % of injections. It was independent of groups of animals and protocol time points with p values of 1.00 and 0.61, respectively, in the first experiment and 0.47 and 0.13, respectively, in the second experiment. There was a good inter-observer agreement for qualitative analysis (kappa = 0.72) and a moderate agreement when using quantitative analysis (ρ c = 0.94). In both experiments, there was significant difference between uncorrected and corrected SUVmean. Despite this significant difference, mean percent differences between uncorrected and corrected SUVmean in the first and the second experiments were -3.61 and -1.78, respectively. Concerning therapy assessment, in both experiments, significant differences in median %SUVmean between control and treated groups were observed over all time points with either uncorrected and corrected data (p < 0.05).
Conclusions
Although extravasation is common and can be reproducibly corrected, this is probably not required for validation of response to drugs that induce large SUV changes. However, further studies are required to evaluate the impact of extravasation in situations where less marked metabolic responses are observed or important extravasations occur.
Similar content being viewed by others
Background
In cancer research, 18fluorodeoxyglucose small animal positron emission tomography/computed tomography (18FDG SA-PET/CT) is a recognized non-invasive tool for in vivo assessment of therapeutic response to novel therapies [1–4]. To this end, accurate quantitative values, such as standardized uptake values (SUVs), are mandatory. However, there are numerous confounding factors, including the mode of tracer administration.
Tail vein injection (with or without an intravenous catheter) under short anesthesia is the most commonly used route for administering radiopharmaceuticals. The small caliber of the vein in rodents may, however, predispose to tracer extravasation into the interstitium. Other validated administration routes are available, including retro-orbital and intraperitoneal injection [5, 6]. The main limitation of the first technique relates to restrictions imposed by many animal care committees. The second type of injection has several advantages. It is reproducible in longitudinal studies and can be performed on conscious animals, thereby reducing stress levels. However, a risk of failure remains (intra-digestive injection, [7, 8]), and it has not been validated in animals bearing intraperitoneal tumors in which peritoneal tracer absorption could be impaired. Moreover, even though delayed images (60 min) have been shown to be equivalent to intravenous injection [9–11], retro-orbital and intraperitoneal injections do not allow dynamic imaging [5].
Methods for measuring tail vein injection failure quantitatively have been developed [12, 13]. As far as we know, however, the impact of extravasation on quantitative measures of tumor uptake and whether these can be reliably corrected by estimation of extravasated activity has not been evaluated in rats. Therefore, we aimed to evaluate the impact of such a correction method in the context of therapeutic response assessment of a dual Phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor (BEZ-235) on a chemoresistant model of human ovarian cancer xenografted in nude rats.
Methods
Animal model
The regional Ethics Committee granted approval to conduct this study (no. N/02-10-09/18/10-12). All procedures performed in this study involving animals were in accordance with the ethical standards of the regional research committee and with the 1964 Helsinki Declaration and its later amendments.
In two experiments, 16 4-week-old female nude rats (Harlan Laboratories, Indianapolis, IN, USA) bearing subcutaneous SKOV3 human ovarian tumors were used. Ten rats were treated with BEZ-235, a dual Phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor (Selleck Chemicals, Houston, TX, USA) from day 0 to day 3, with treatment being discontinued on day 4. Six rats were used as controls. Each animal had four tumors: two in the shoulders and two in the upper thighs, providing 64 lesions for evaluation.
In the first experiment, five rats underwent PET imaging on days 0, 3, and 7. This cohort consisted of two groups: untreated controls (n = 2) and treated rats (n = 3). A second cohort was treated at the same time points and used for molecular analysis. In the second experiment, a cohort of 11 animals, consisting of untreated controls (n = 7) and rats receiving BEZ-235 (n = 4), was used for both PET imaging and molecular analysis. For the latter aim, one rat each from the treated and untreated groups was sacrificed on days 0, 3, and 4, and all remaining animals were sacrificed after the last PET examination had been performed on day 5. For general anesthesia, heated inhaled isoflurane was administered with an anesthesia device dedicated to small animals (Minerve, France).
Molecular analyses were performed in both experiments, including cell proliferation assessment (Ki-67 immunostaining), and Phosphoinositide 3-kinase/mammalian target of rapamycin target expression studies (p4E-BP1 immunostaining), which were taken as gold standards for therapeutic assessment in the present work.
Cross-calibration
A cross-calibration among the SA-PET/CT system and the dose calibrator was performed. A 10-MBq 18F-FDG solution (as assessed by the dose calibrator) was used to fill a vial of exact known volume, which resulted in a solution of known concentration. This solution was used to fill a cylinder phantom that was scanned for 20 min on the SA-PET/CT scanner. A large volume of interest (VOI) was used to determine the mean activity concentration as assessed by the SA-PET/CT scanner. Cross-calibration factors were then derived and used to synchronize counts/measurements for the two pieces of equipment.
SA-PET/CT acquisitions, reconstructions, and analysis
Animals were kept fasting for 6 h. As detailed above, SA-PET/CT (Inveon system, Siemens Medical Solution, Knoxville, TN, USA) examinations were performed on days 0, 3, and 7 in the first experiment and days 0, 3, 4, and 5 in the second experiment. The same individual (NA, with a 15-year experience in tail vein injection) performed tracer injections. Injections were performed intravenously through the tail vein, under general anesthesia, using a 29-gauge needle. Injected volume was always kept below 0.4 mL. The average calibrated activities were 38 ± 7 and 39 ± 5 MBq with uptake times of 91 ± 8 and 105 ± 14 min following injection, respectively, for the first and second experiments. Animals were imaged in prone position with the tail positioned on their right side. Reconstructions were performed using a NEMA NU 4-Optimized Maximum A Posteriori (MAP) Reconstruction [14] with scatter and attenuation corrections.
SA-PET/CT analysis
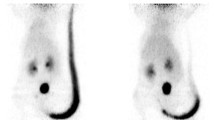
Intravenous injections were qualitatively evaluated from reconstructed images. When the observer concluded to the presence of extravasation, a three-dimensional volume of interest (VOI) with a visually adapted isocontour was drawn over the tail injection site (Fig. 1). Activity in the tail (Bq and Bq/cc) was recorded. Two independent observers made these analyses to determine the inter-observer variability. The activity of tail vein injection extravasation was corrected for decay assuming that there was no interstitial absorption of the tracer in the tail between the injection and the imaging session. Corrected injected activity was calculated by the following formula:
Representative images in animals with and without misinjection. Maximum intensity projection (MIP) visualization of the small animal positron emission tomography/computed tomography (SA-PET/CT) reconstruction for a treated rat in the second experiment without tracer extravasation on day 0 (a) and with tracer extravasation on day 3 (b). On day 3, a volume of interest (VOI) region was manually drawn and a visually adapted. Isocontour was applied (displayed in green) to quantify and correct for tail vein extravasation. Yellow arrows show the subcutaneous tumors
For therapeutic response assessment in each experiment, a cylindrical VOI was drawn over each tumor. The uncorrected and corrected mean pixel values were extracted for each VOI and SUVmean were computed as follows, assuming a 1 g/mL density:
Data were processed with a dedicated Siemens station (Inveon Research Workplace 2.2, Siemens Molecular Imaging, Knoxville, TN, USA).
Statistical analysis
These analyses aimed to determine (1) if tail vein extravasation corrected data performed better than uncorrected data for predicting target inhibition and (2) if there was inter-observer variability in the tail vein injection correction process. Statistical analyses were performed on a per-lesion basis without taking into account intra-rat correlation. Fisher’s exact probability tests, with the Freeman–Halton extension when necessary [15], were performed to test if the occurrence of tail vein extravasation was different according to groups (treated or not) and protocol time points. Inter-observer agreement was assessed by means of Cohen’s kappa coefficients (qualitative analysis) and Lin’s concordance coefficient (quantitative analysis). Tail vein activity measurements (Bq) of observers A and B were compared using Bland–Altman method comparisons. Wilcoxon tests and Bland–Altman plots were used for paired comparisons of corrected and uncorrected data amongst treated and control groups. To assess the ability to discriminate between control and treated groups, relative SUVmean values of day 0 for each group were compared over the different time points (day 3, 4, 5, and 7), for corrected and uncorrected data, using Mann–Whitney tests. For each test, the alpha risk was set at 0.05. Statistical analysis, graphs, and plots were performed with GraphPad Prism version 5.0 for Mac (GraphPad Software, La Jolla, CA, USA; www.graphpad.com).
Results
Tail vein extravasation frequency and magnitude
Both observers recorded that extravasation was visible in at least 50 % of injections. According to observer A, tail vein extravasation occurred in 92.3 and 59.4 % of SA-PET/CT examinations in the first and second experiments, respectively. In the first experiment, the percentage of injected activity remaining in the tail vein never exceeded 20 %, with a maximum of 16.9 % in a control rat on day 0.
According to observer B, tail vein extravasation occurred in 69.2 and 50.0 % of SA-PET/CT examinations in the first and second experiments, respectively. The maximum tail vein remaining activity was 14.8 % and was observed in the same animal described above.
Ranges of tail vein extravasation activity values and their frequency are described in Table 1 for both observers. As shown in Fig. 2, the extravasation rate was independent of groups and protocol time points in the first and second experiments.
Frequency of tail vein extravasations depending on group and protocol time points. Panels a and b display the first experiment results and panels c and d display the second experiment results. For groups and protocol time point studies, only data of observer A is used. Of note, only three animals were scanned on day 7 because two treated rats had died from treatment toxicity
Inter-observer variability for tail vein activity measurement
Bland–Altman analysis is shown in Fig. 3 and was conducted on all measurements of both experiments. This analysis demonstrated that the mean difference between tail vein activity measurements obtained by observer A and B was 18 kBq with narrow 95 % confidence intervals. Overall, there was a good inter-observer agreement when using qualitative analysis with a kappa value of 0.72 (95 % confidence interval (CI) 0.52–0.92) and a moderate agreement when using quantitative analysis with ρ c value of 0.94 (95 % CI 0.88–0.97). Discordant results between observers occurred in 23.1 and 21.9 % of the first and second experiment SA-PET/CT examinations, respectively. In all but one case, observer A, indicated the presence of extravasation whereas observer B did not. Discordant results related to cases of low remaining activities in the tail, ranging from 0.04 to 0.73 % of theoretical injected activity.
As there was low inter-observer variability for tail vein activity measurement, the following results were assessed only for observer A, who identified more episodes of extravasation.
Comparison between uncorrected and corrected data
In each experiment, there was a significant difference between uncorrected and corrected SUVmean values with p values <0.0001 (Fig. 4a, c). Bland–Altman analyses showed that mean percent differences between uncorrected and corrected SUVmean values in the first and second experiments were −3.61 and −1.78, respectively (Fig. 4b, d).
Comparison of corrected and uncorrected SUVmean values. a, b Upper panels display the SUVmean values of the first experiment, and c, d lower panels display the SUVmean values of the second experiment, as recorded by observer A. Data is shown as Tukey boxplots (left panels, lines displaying median, 25th and 75th percentiles; cross represents the mean value) and Bland–Altman plots (right panels)
Impact of tail vein extravasation on assessment of therapeutic response
Molecular analysis
On day 3, immunohistochemistry studies showed decreases in p4E-BP1 phosphorylation, a downstream marker of mTOR activation in treated animals. On days 4 and 5, Phosphoinositide 3-kinase/mammalian target of rapamycin pathways exhibited partial recovery with almost complete recovery by day 7. When focusing on cell proliferation, Ki-67 staining was statistically and significantly lower in the treated group as compared with controls on days 3, 4, and 5. By day 7, there was no difference between control and treated groups. These results were detailed in another publication [16] and were used as the reference standard in the present work.
SA-PET analysis
Both corrected and uncorrected data showed significant decreases in 18F-FDG uptake on day 3 in treated groups in each experiment with a partial uptake recovery by day 5 (second experiment), which became more pronounced on day 7 (first experiment). In both experiments, significant differences in median %SUVmean values between control and treated groups were observed across all times points, either with corrected or uncorrected data. It is also noteworthy that quartiles were almost similar with corrected and uncorrected data (Fig. 5).
Uncorrected and corrected quantitative values in two therapy assessment experiments. Standard uptake values (SUVmean) relative to day 0 (medians, quartiles and means (star) for control and treated groups across all times points for the first and second experiments with uncorrected (a) and corrected (b) data. Quantitative values were extracted from observer A data. Legend for p values: ***p < 0.001, **p < 0.01, and *p < 0.05
Discussion
Gaining venous access in animals can be challenging, even for experienced researchers, leading to tracer extravasation and potentially biased quantitative values. The present study evaluated whether data corrected for tail vein extravasation using a subtraction method performed better than uncorrected data in predicting therapy response in a large panel of animals in two therapeutic response assessment protocols. The main findings were that (1) tail vein extravasations occurred frequently (in at least in 50 % of cases as assessed by each observer across both experiments) and (2) that its frequency was not linked to treatment, time point within a given experiment, nor the cumulative number of injections. One would have expected the treatment-associated side effects, such as dehydration and cachexia, as well as repetitive injections, to impact on the quality of tail vein injections. Observed extravasation frequencies ranged from 50 to 92.3 % within the experiments described, which is much higher than those observed in other publications, reaching 40 % at worst [12, 13, 17]. However, it is likely that these authors did not look for extravasation with the same scrutiny. For example, in the study of Groman et al. [12], the presence of a misinjection was considered only when it exceeded 10 % of the injected dose (Table 2). This led to a more limited assessment of the problem by not accounting for the smallest extravasations. However, our results demonstrated that, despite one observer identifying extravasation more frequently, there was little inter-observer variability in quantifying the amount of activity extravasated using the tail vein correction process. These findings suggest that this technique is reproducible.
Remaining activity in the tail was measured on in vivo imaging data because a cross-calibration between the SA-PET system and the dose calibrator was undertaken and because we had previously shown that ex vivo counting (taken as gold standards) and Inveon SA-PET/CT data was well correlated [18]. Moreover, Vines et al. had also previously shown an excellent correlation between in vivo imaging measurements and ex vivo gamma-well counter data [13]. Based on the SUV formulae, residual activity in the tail (equal to 16.9 % at worst in the present study) resulted in an underestimation of SUV values when uncorrected, as shown in Fig. 4. Chang et al. showed similar results [17]. In the current experiments, the molecular targeted therapy that was tested induced a marked decrease in 18F-FDG uptake. The median relative changes in the first and second experiments for treated animals were −70.02 and −61.69 %, respectively, using uncorrected data. Therefore, one could argue that the bias induced by tail vein extravasation would have a more pronounced impact when testing other therapies by inducing subtle changes in 18F-FDG uptakes. However, differences between control and treated groups were less important on days 4 to 7, when the therapy had been withdrawn, and there was still no difference between uncorrected and corrected data at these time points. Also, the situation of important extravasations, that could occur when junior researchers with little experience perform tail vein injection, or in the case of treatments inducing major cachexia, may require correction for extravasation.
The technique used in the present study was based on the assumption that there was no interstitial absorption of the tracer in the tail between the injection and the imaging session. Groman et al. [12] found that the tracer was retained at the injection site over an observation period of 7 days, but they used a 20-nm colloidal reagent tracer that had a higher steric hindrance than 18F-FDG. To the best of our knowledge, there are no available studies evaluating this phenomenon with 18F-FDG. Regarding the relatively short uptake period (<120 min), lymphatic clearance was considered negligible in the present study.
Finally, this study was performed in rats bearing tumors, whereas the most frequently used animal model in cancer research is the mouse. Given that we found that a percentage of injected activity remained in the tail (range 0.04–16.9 %) in the same or lower order of magnitude than in other studies on mice, we are confident that similar results would be expected with mice (Table 2).
Conclusions
Despite the relatively high frequency of tail vein extravasation, the low activity remaining in the tail vein following extravasation (never higher than 20 % of the injected dose) led to a negligible magnitude of error and did not interfere with the cancer therapy assessment in rats bearing subcutaneous tumors undergoing targeted therapy. Assessment of therapeutic response using data uncorrected for tail vein extravasation provided similar results to those following correction. Nevertheless, there was no inter-observer variability when quantifying tail vein activity suggesting that the correction of data for tail vein extravasation can be reproducibly performed and may be important when assessing therapies inducing more subtle SUV changes between groups or when important extravasations occur.
Ethics
The regional Ethics Committee granted approval to conduct this study (no. N/02-10-09/18/10-12). All procedures performed in this study involving animals were in accordance with the ethical standards of the regional research committee and with the 1964 Helsinki Declaration and its later amendments.
References
Abourbeh G, Itamar B, Salnikov O, Beltsov S, Mishani E. Identifying erlotinib-sensitive non-small cell lung carcinoma tumors in mice using [(11)C]erlotinib PET. EJNMMI Res. 2015;5:4. doi:10.1186/s13550-014-0080-0.
Chapman DW, Jans HS, Ma I, Mercer JR, Wiebe LI, Wuest M, et al. Detecting functional changes with [(18)F]FAZA in a renal cell carcinoma mouse model following sunitinib therapy. EJNMMI Res. 2014;4(1):27. doi:10.1186/s13550-014-0027-5.
Emonds KM, Swinnen JV, Lerut E, Koole M, Mortelmans L, Mottaghy FM. Evaluation of androgen-induced effects on the uptake of [18F]FDG, [11C]choline and [11C]acetate in an androgen-sensitive and androgen-independent prostate cancer xenograft model. EJNMMI Res. 2013;3(1):31. doi:10.1186/2191-219X-3-31.
Hovhannisyan N, Guillouet S, Fillesoye F, Dhilly M, Patin D, Galateau F, et al. Evaluation of the specificity of [(18)F]fludarabine PET/CT in a xenograft model of follicular lymphoma: comparison with [(18)F]FDG and impact of rituximab therapy. EJNMMI Res. 2015;5:23. doi:10.1186/s13550-015-0101-7.
Nanni C, Pettinato C, Ambrosini V, Spinelli A, Trespidi S, Rubello D, et al. Retro-orbital injection is an effective route for radiopharmaceutical administration in mice during small-animal PET studies. Nucl Med Commun. 2007;28(7):547–53. doi:10.1097/MNM.0b013e3281fbd42b.
Steel CD, Stephens AL, Hahto SM, Singletary SJ, Ciavarra RP. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim (NY). 2008;37(1):26–32. doi:10.1038/laban0108-26.
Arioli V, Rossi E. Errors related to different techniques of intraperitoneal injection in mice. Appl Microbiol. 1970;19(4):704–5.
Gaines Das R, North D. Implications of experimental technique for analysis and interpretation of data from animal experiments: outliers and increased variability resulting from failure of intraperitoneal injection procedures. Lab Anim. 2007;41(3):312–20. doi:10.1258/002367707781282802.
Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med. 2006;47(6):999–1006.
Schiffer WK, Mirrione MM, Dewey SL. Optimizing experimental protocols for quantitative behavioral imaging with 18F-FDG in rodents. J Nucl Med. 2007;48(2):277–87.
Wong KP, Sha W, Zhang X, Huang SC. Effects of administration route, dietary condition, and blood glucose level on kinetics and uptake of 18F-FDG in mice. J Nucl Med. 2011;52(5):800–7. doi:10.2967/jnumed.110.085092.
Groman EV, Reinhardt CP. Method to quantify tail vein injection technique in small animals. Contemp Top Lab Anim Sci. 2004;43(1):35–8.
Vines DC, Green DE, Kudo G, Keller H. Evaluation of mouse tail-vein injections both qualitatively and quantitatively on small-animal PET tail scans. J Nucl Med Technol. 2011;39(4):264–70. doi:10.2967/jnmt.111.090951.
Lasnon C, Dugue AE, Briand M, Blanc-Fournier C, Dutoit S, Louis MH, et al. NEMA NU 4-Optimized Reconstructions for Therapy Assessment in Cancer Research with the Inveon Small Animal PET/CT System. Mol Imaging Biol. 2015;17(3):403–12. doi:10.1007/s11307-014-0805-5.
Freeman GH, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38(1–2):141–9.
Lheureux S, Lecerf C, Briand M, Louis MH, Dutoit S, Jebahi A, et al. (18)F-FDG is a surrogate marker of therapy response and tumor recovery after drug withdrawal during treatment with a dual Phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor in a preclinical model of cisplatin-resistant ovarian cancer. Transl Oncol. 2013;6(5):586–95.
Chang E, Liu S, Gowrishankar G, Yaghoubi S, Wedgeworth JP, Chin F, et al. Reproducibility study of [(18)F]FPP(RGD)2 uptake in murine models of human tumor xenografts. Eur J Nucl Med Mol Imaging. 2011;38(4):722–30. doi:10.1007/s00259-010-1672-1.
Lasnon C, Quak E, Briand M, Gu Z, Louis MH, Aide N. Contrast-enhanced small-animal PET/CT in cancer research: strong improvement of diagnostic accuracy without significant alteration of quantitative accuracy and NEMA NU 4-2008 image quality parameters. EJNMMI Res. 2013;3(1):5. doi:10.1186/2191-219X-3-5.
Acknowledgments
Prof. Aide and Dr. Lasnon are indebted to Prof. Rodney Hicks, Peter MacCallum Cancer Institute, East Melbourne, Australia, for his expert review of the manuscript.
Sources of support
This work was supported by a grant from the French Ligue contre le cancer, Comité du Calvados.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CL participated in the design of the study and its coordination, helped to perform statistical analysis, and drafted the manuscript. AED performed the statistical analysis. MB participated in the coordination of the study and in animal handling. SD carried out the molecular biology analysis. NA conceived of the study, participated in its design and coordination, and helped to draft the manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lasnon, C., Dugué, A.E., Briand, M. et al. Quantifying and correcting for tail vein extravasation in small animal PET scans in cancer research: is there an impact on therapy assessment?. EJNMMI Res 5, 61 (2015). https://doi.org/10.1186/s13550-015-0141-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13550-015-0141-z