Abstract
Purpose
An 18F-labeled PEGylated arginine-glycine-aspartic acid (RGD) dimer {[18F]FPP(RGD)2} has been used to image tumor αvβ3 integrin levels in preclinical and clinical studies. Serial positron emission tomography (PET) studies may be useful for monitoring antiangiogenic therapy response or for drug screening; however, the reproducibility of serial scans has not been determined for this PET probe. The purpose of this study was to determine the reproducibility of the integrin αvβ3-targeted PET probe, [18F]FPP(RGD)2, using small animal PET.
Methods
Human HCT116 colon cancer xenografts were implanted into nude mice (n = 12) in the breast and scapular region and grown to mean diameters of 5–15 mm for approximately 2.5 weeks. A 3-min acquisition was performed on a small animal PET scanner approximately 1 h after administration of [18F]FPP(RGD)2 (1.9–3.8 MBq, 50–100 μCi) via the tail vein. A second small animal PET scan was performed approximately 6 h later after reinjection of the probe to assess for reproducibility. Images were analyzed by drawing an ellipsoidal region of interest (ROI) around the tumor xenograft activity. Percentage injected dose per gram (%ID/g) values were calculated from the mean or maximum activity in the ROIs. Coefficients of variation and differences in %ID/g values between studies from the same day were calculated to determine the reproducibility.
Results
The coefficient of variation (mean±SD) for %IDmean/g and %IDmax/g values between [18F]FPP(RGD)2 small animal PET scans performed 6 h apart on the same day were 11.1 ± 7.6% and 10.4 ± 9.3%, respectively. The corresponding differences in %IDmean/g and %IDmax/g values between scans were −0.025 ± 0.067 and −0.039 ± 0.426. Immunofluorescence studies revealed a direct relationship between extent of ανβ3 integrin expression in tumors and tumor vasculature with level of tracer uptake. Mouse body weight, injected dose, and fasting state did not contribute to the variability of the scans; however, consistent scanning parameters were necessary to ensure accurate studies, in particular, noting tumor volume, as well as making uniform: the time of imaging after injection and the ROI size. Reanalysis of ROI placement displayed variability for %IDmean/g of 6.6 ± 3.9% and 0.28 ± 0.12% for %IDmax/g.
Conclusion
[18F]FPP(RGD)2 small animal PET mouse tumor xenograft studies are reproducible with relatively low variability.



Similar content being viewed by others
References
Humphries MJ. Integrin structure. Biochem Soc Trans 2000;28(4):311–39.
Akiyama SK. Integrins in cell adhesion and signaling. Hum Cell 1996;9(3):181–6.
Pasqualini R, Koivunen E, Ruoslahti E. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol 1997;15(6):542–6.
Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prévost N, et al. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med 2009;15(10):1163–9.
Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol 2004;64:207–38.
Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA. Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J Cell Biol 2003;162(5):933–43.
Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell 1994;77(4):477–8.
Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 1996;12:697–715.
Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol 2007;25(13):1651–7.
Maubant S, Saint-Dizier D, Boutillon M, Perron-Sierra F, Casara PJ, Hickman JA, et al. Blockade of alpha v beta3 and alpha v beta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood 2006;108(9):3035–44.
Perron-Sierra F, Saint Dizier D, Bertrand M, Genton A, Tucker GC, Casara P. Substituted benzocyloheptenes as potent and selective alpha(v) integrin antagonists. Bioorg Med Chem Lett 2002;12(22):3291–6.
Nisato RE, Tille JC, Jonczyk A, Goodman SL, Pepper MS. alphav beta 3 and alphav beta 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis 2003;6(2):105–19.
Wu Y, Cai W, Chen X. Near-infrared fluorescence imaging of tumor integrin alpha v beta 3 expression with Cy7-labeled RGD multimers. Mol Imaging Biol 2006;8(4):226–36.
Beer AJ, Schwaiger M. Imaging of integrin alphavbeta3 expression. Cancer Metastasis Rev 2008;27(4):631–44.
Eskens FA, Dumez H, Hoekstra R, Perschl A, Brindley C, Böttcher S, et al. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer 2003;39(7):917–26.
Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med 2009;15(4):392–400.
Schottelius M, Laufer B, Kessler H, Wester HJ. Ligands for mapping alphavbeta3-integrin expression in vivo. Acc Chem Res 2009;42(7):969–80.
Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin alphavbeta3 targeted radiotracers for tumor imaging. Mol Pharm 2006;3(5):472–87.
Haubner R, Decristoforo C. Radiolabelled RGD peptides and peptidomimetics for tumour targeting. Front Biosci 2009;14:872–86.
Beer AJ, Niemeyer M, Carlsen J, Sarbia M, Nährig J, Watzlowik P, et al. Patterns of alphavbeta3 expression in primary and metastatic human breast cancer as shown by 18F-Galacto-RGD PET. J Nucl Med 2008;49(2):255–9.
Beer AJ, Grosu AL, Carlsen J, Kolk A, Sarbia M, Stangier I, et al. [18F]galacto-RGD positron emission tomography for imaging of alphavbeta3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 2007;13(22 Pt 1):6610–6.
Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, Grosu AL, et al. Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin alpha(v)beta3 expression in man. Clin Cancer Res 2006;12(13):3942–9.
Liu S, Liu Z, Chen K, Yan Y, Watzlowik P, Wester HJ, et al. 18F-labeled galacto and PEGylated RGD dimers for PET imaging of αvβ3 integrin expression. Mol Imaging Biol 2010;12(5):530–8.
Wu Z, Li ZB, Cai W, He L, Chin FT, Li F, et al. 18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): synthesis and microPET imaging of alphavbeta3 integrin expression. Eur J Nucl Med Mol Imaging 2007;34(11):1823–31.
Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR, Gambhir SS, et al. Quantitative PET imaging of tumor integrin alphavbeta3 expression with 18F-FRGD2. J Nucl Med 2006;47(1):113–21.
Wu Z, Li ZB, Chen K, Cai W, He L, Chin FT, et al. microPET of tumor integrin alphavbeta3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4). J Nucl Med 2007;48(9):1536–44.
Chin FT, Shen B, Liu SL, Berganos RA, Chang E, Mittra E, Chen XY, Gambhir SS. Initial experience with clinical-grade [18F]FPPRGD2: an automated multi-step radiosynthesis for human PET studies. Submitted to Mol Imaging Biol 2010.
Dijkgraaf I, Beer AJ, Wester HJ. Application of RGD-containing peptides as imaging probes for alphavbeta3 expression. Front Biosci 2009;14:887–99.
Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther 2006;5(11):2624–33.
Tseng JR, Dandekar M, Subbarayan M, Cheng Z, Park JM, Louie S, et al. Reproducibility of 3'-deoxy-3'-(18)F-fluorothymidine microPET studies in tumor xenografts in mice. J Nucl Med 2005;46(11):1851–7.
Knoess C, Siegel S, Smith A, Newport D, Richerzhagen N, Winkeler A, et al. Performance evaluation of the microPET R4 PET scanner for rodents. Eur J Nucl Med Mol Imaging 2003;30(5):737–47.
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 1994;13(4):601–9.
Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2003;2(3):131–7.
Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med 1998;26(4):217–38.
Dandekar M, Tseng JR, Gambhir SS. Reproducibility of 18F-FDG microPET studies in mouse tumor xenografts. J Nucl Med 2007;48(4):602–7.
Weber WA, Ziegler SI, Thödtmann R, Hanauske AR, Schwaiger M. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med 1999;40(11):1771–7.
Minn H, Zasadny KR, Quint LE, Wahl RL. Lung cancer: reproducibility of quantitative measurements for evaluating 2-[F-18]-fluoro-2-deoxy-D-glucose uptake at PET. Radiology 1995;196(1):167–73.
Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 2006;11(17–18):812–8.
Acknowledgments
This work was supported, in part, by National Cancer Institute (NCI) In Vivo Cellular Molecular Imaging Center (ICMIC) grant P50 CA114747 (SSG), NCI 5R01 CA119053 (ZC), and a grant from Bayer HealthCare (Berlin, Germany). The Stanford University Radiochemistry Facility is acknowledged for radionuclide production and radiochemistry support.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
a Representative immunofluorescent pictographs (×100) of 6 μm ectopic tumor (frozen) sections that were stained with antibodies against human ανβ3 integrins (FITC or green signal) and against murine VEGF-R2 (Flt-1: PE or red signal). Cell nuclei were visualized by DAPI (blue signal). Sections were taken from tumors with high [18F]FPP(RGD)2 uptake (top row, 2.6 ± 0.3) and with low probe uptake (bottom row, 1.0 ± 0.3). Each panel represents a unique tumor. b The extent of integrin signaling was computed with the use of Image J Software, and the samples with high (n = 3) vs low (n = 3) probe uptake were compared. c An immunofluorescent pictograph showing patterns of murine CD31 and human integrin expression around a tumor vasculature (×200) magnification. Correlation of a v b 3 integrin expression to [18F]FPP(RGD)2uptake. In order to validate that changes in %ID/g values reflects changes in integrin expression, tumors with both high %IDmax/g (mean = 2.6 ± 0.3, n = 6) and low %IDmax/g (mean = 1.0 ± 0.3, n = 6) were isolated, 6-μm sections were cut, and then monitored via immunofluorescence staining for the extent of ανβ3 expression. Representative examples of these immunofluorescent photomicrographs are shown in a. Although initial qualitative assessment of integrin distribution and expression (green signal) showed considerable heterogeneity, there did seem to be a trend towards more extensive distribution of green signal in tumor sections with high %IDmax/g samples compared to those with low %IDmax/g. The extent of positive antibody staining was determined by assessing the total area of the field of view at ×40. A region of interest (ROI) was then drawn with best judgment around those regions that were positive for integrin fluorescence staining. A ratio of ROI area for integrin over area of total field of view was then compared to [18F]FPP(RGD)2 uptake. ROI measurements of integrin positive regions (b) revealed that there was indeed a significant difference (p < 0.05) between the extent of integrin expression and high vs low [18F]FPP(RGD)2 uptake values (as represented by %IDmax/g). A plot of this ROI ratio vs %IDmax/g yielded a moderately linear function with R2 = 0.45, p < 0.05, and slope = 0.29. Although most of the signal appeared on tumor tissue directly, some of the signal surrounded murine anti-VEGF-R2-PE positive tubules (red signals, arrow, c). This observation suggests that the tumor tissue around murine neovasculature tends to express ανβ3 and this facilitates the interaction of tumor cells to the neovasculature. (PDF 193 kb)
Supplemental Figure 2
a Full image coronal section scan of mouse shown in Fig. 1 for the first scan (a) and the second scan 6 h later (b). a First full-body coronal scans of nude mouse with a scapular and breast xenograft of HCT116 tumors. Injected tracer is [18F]FPP(RGD)2. Scan was performed 1 h after injection. Note small necrotic region in scapular tumor. (PDF 96 kb)
Supplemental Figure 2
b Full image coronal section scan of mouse shown in Fig. 1 for the first scan (a) and the second scan 6 h later (b). b Second full-body coronal scans of nude mouse with a scapular and breast xenograft of HCT116 tumors. Injected tracer is [18F]FPP(RGD)2 and was injected 5 h after first scan. Second scan was performed 1 h after injection. (PDF 92 kb)
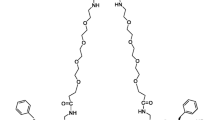
Supplemental Figure 3
Chemical structure of [18F]FPP(RGD)2 (Arg-Gly-Asp) radiotracer used in the reproducibility study. Note multiple repeats of the cyclic RGD motif at the active binding site of the tracer as well as the extended PEGylated “tail.” (PDF 37 kb)
Supplemental Table 1
Table showing lack of change in %ID/g uptake as a function of input of high versus low amount of [18F]FPP(RGD)2. (PDF 20 kb)
Supplemental Table 2
Similar rate of change in %IDmean/g and %IDmax/g parameters of the tracer [18F]FPP(RGD)2 for small (126 ± 50 mm3) vs large (739 ± 150 mm3) HCT116 tumor xenografts at 1 to 4 h post-tail vein injection. A total of four mice werecompared with four large tumors and four small ones. (PDF 20 kb)
Rights and permissions
About this article
Cite this article
Chang, E., Liu, S., Gowrishankar, G. et al. Reproducibility study of [18F]FPP(RGD)2 uptake in murine models of human tumor xenografts. Eur J Nucl Med Mol Imaging 38, 722–730 (2011). https://doi.org/10.1007/s00259-010-1672-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1672-1