Abstract
Background
Sex differences exist not only in the efficacy but also in adverse event rates of many vaccines. Here we compared the safety of BNT162b2 vaccine administered off-label in female and male children younger than 5 years in Germany.
Methods
This is a retrospective cohort study, in which we performed a post-hoc analysis of a dataset collected through an authentication-based survey of individuals having registered children aged 0-<5 years for vaccination against SARS-CoV-2 in six private practices and/or two lay person-initiated vaccination campaigns. We analyzed the safety profiles of the first 3 doses of 3–10 µg BNT162b2. Primary outcome was comparison in frequencies of 4 common post-vaccination symptom categories such as local, general, musculoskeletal symptoms and fever. Data were analyzed according to sex in bivariate analyses and regression models adjusting for age, weight, and dosage. Interaction between sex and BNT162b2 dosage was assessed. An active-comparator analysis was applied to compare post-vaccination symptoms after BNT162b2 versus non-SARS-CoV-2 vaccines.
Results
The dataset for the present analysis consisted of 7801 participants including 3842 females (49%) and 3977 males (51%) with an age of 3 years (median, interquartile: 2 years). Among individuals receiving 3 µg BNT162b2, no sex differences were noted, but after a first dose of 5–10 µg BNT162b2, local injection-site symptoms were more prevalent in girls compared to boys. In logistic regression, female sex was associated with higher odds of local symptoms, odds ratio (OR) of 1.33 (95% confidence interval [CI]: 1.15–1.55, p < 0.05) and general symptoms with OR 1.21 (95% CI: 1.01–1.44, p < 0.05). Following non-BNT162b2 childhood vaccinations, female sex was associated with a lower odds of post-vaccination musculoskeletal symptoms (OR: 0.29, 95% CI: 0.11–0.82, p < 0.05). An active comparator analysis between BNT162b2 and non-SARS-CoV-2 vaccinations revealed that female sex positively influenced the association between BNT162b2 vaccine type and musculoskeletal symptoms.
Conclusions
Sex differences exist in post-vaccination symptoms after BNT162b2 administration even in young children. These are of importance for the conception of approval studies, for post-vaccination monitoring and for future vaccination strategies (German Clinical Trials Register ID: DRKS00028759).
Plain English summary
For many childhood vaccines, the immune responses are different between the sexes. For the COVID-19 vaccine BNT162b2, the symptoms occurring after vaccination in children have not sufficiently been investigated. In this study, we performed an analysis of a dataset of retrospectively collected information on symptoms occurring after vaccinations with BNT162b2 and regular childhood vaccines in 7801 children under the age of 5 years in Germany, with a focus on the sex differences. Among the four categories assessed, local injection-site and general symptoms were more frequent in girls compared to boys. In contrast, following the administration of other childhood vaccines, the caregivers more frequently reported muscle-related symptoms in boys than in girls. In conclusion, sex differences exist in symptoms occurring after vaccinations even in young children, which is important to consider for future studies evaluating vaccine safety.
Registration
The present work was registered at the German Clinical Trials Register ID: DRKS00028759.
Highlights
Data on mRNA vaccine safety are abundant, but few studies have investigated children aged under 5 years.
The CoVacU5 study was a retrospective cohort of off-label vaccinations with BNT162b2 SARS-CoV-2 vaccine in Germany, from which we here present a detailed sex-disaggregated reanalysis of post-vaccination symptoms in 7801 children.
Females were more likely than males to experience local injection-site or general symptoms after being administered BNT162b2.
Implications of the present data highlight the importance to assess the sex differences in future mRNA vaccine licensure studies and post-vaccination monitoring.
Similar content being viewed by others
Introduction
Biological sex introduces a variability in immune responses, and this is therefore pertinent to sex-specific reactions of adaptive immune system following vaccination [1, 2]. As a consequence, the humoral immune responses of vaccines in humans and in experimental models are more pronounced in females than males as reported for the vaccines against influenza [3, 4], hepatitis A or dengue virus [2]. The potential underlying mechanisms have been attributed to the immunosuppressive effect of male sex hormones [5, 6]. In contrast, several observations favor that some vaccines have increased efficacy against infection in men [2, 7,8,9].
Along with the reported sex differences in vaccination efficacy, a plethora of data has revealed sex differences in the rates of adverse events post vaccination [10]. For instance, anaphylaxis is reported to be 4 times more frequent in females than in males [8, 11]. In the case of SARS-CoV-2 vaccines it is opposite, individual undesired sequelae such as myocarditis are more frequently observed in males [12]. However, symptomatic presentation post SARS-CoV-2 vaccination is dominated by reports of females [13,14,15]. In support, vaccine monitoring data and pooled analyses of cross-sectional studies revealed a higher probability of adverse effects in women after receiving the Pfizer-BioNTech vaccine (Comirnaty®) [16, 17]. However, further studies are warranted to understand the outcome post SARS-CoV2 vaccination and more studies that disaggregate data by sex are needed [13].
A population that was the last to become eligible for SARS-CoV-2 vaccines were young children. We have previously reported on the overall safety and effectiveness of BNT162b2 in the retrospective CoVacU5 cohort of children vaccinated off-label in Germany [18, 19]. Such inclusion and assessment represented a unique opportunity to detect otherwise inaccessible dose-response relationships in post-vaccination symptoms.
Even if female sex in adulthood is associated with more frequent adverse events after SARS-CoV-2 vaccination [20], nothing is known in young children. We hypothesized that female sex would be associated with a greater odds of experiencing adverse symptoms after BNT162b2 vaccination in young children, and that sex-specific effects may be dependent on dose.
To test this hypothesis, we aimed to compare 4 common post-vaccination symptom categories such as local, general, musculoskeletal symptoms and fever after BNT162b2 vaccination in children of both sexes younger than 5 years of age, and we aimed to determine if the safety profile was dependent on the dose of BNT162b administered.
Methods
Study design
The present study was a post-hoc analysis of the CoVacU5 study. The CoVacU5 study was a retrospective cohort study that assessed symptoms occurring after vaccination with BNT162b2 in children aged less than 5 years old.
Participants
Participants of the CoVacU5 study were recruited through e-mail addresses of the vaccination registration databases of 21 healthcare institutions and two German vaccination promotion programs “Bildung Aber Sicher” and “U12Schutz”. Inclusion criteria for respondents of the CoVacU5 study survey were having registered a child younger than 5 years old for off-label vaccination with BNT162b2 mRNA vaccine and informed consent of parent or guardian. Exclusion criteria were duplicate entries that had overlaps in all of the four variables age, sex, weight and height, unless the duplicate entry was explained as twins or triplets. Respondents without a correct invitation code were excluded. SARS-CoV-2 vaccinations that were administered before May 2021 or administered using vaccines other than BNT162b2 were also excluded.
Study procedure
The original survey was available on a web-based REDCap platform [21] from April 15th to May 9th 2022. The survey procedure was pseudonymized and restricted to invited participants providing an authentication code, as previously described [18]. In brief, all eligible potential participants were recruited via the e-mail addresses by which they had previously registered a child aged less than 5 years old for a vaccination with BNT162b2. The invitation e-mails were sent out twice to the eligible individuals, containing an individual access code to allow pseudonymized but authentication-based survey participation only.
The vaccinations with BNT162b2 mRNA vaccine had previously been administered off-label following German law and outside of any study protocol. In addition, the physicians responsible for each vaccinating site were mandated by German law to report each unexpected or severe side effect to the authorities as part of routine medical care.
The study was conducted according to the principles of the Declaration of Helsinki and is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline for observational studies. The study protocol was assessed and approved by the Ethics Committee of University of Rostock, Germany (ID: A 2022-0065). The study was registered in the German Clinical Trials Register (ID: DRKS00028759).
Variables
The survey included close-ended questions gathered demographic information, basic medical data, and timing and dosage of vaccinations with BNT162b2, and all non-SARS-CoV-2 vaccinations in the three months preceding the first BNT162b2 administration. Next, questions covered post-vaccination symptoms occurring after BNT162b2 or non-SARS SARS-CoV-2 vaccinations in 9 symptom categories with close-ended and open-ended follow-up questions to narrow down the characteristics of each individual symptom as described in the initial CoVacU5 study [18].
Outcomes
The primary outcome of this post-hoc analysis was the sex-specific frequency of symptoms within the four categories of local reaction (injection site), general symptoms (e.g. fatigue, feeling of weakness, general feeling of illness), musculoskeletal symptoms and fever that occurred after first, second and/or third vaccinations with BNT162b2, further stratified by age group and dosage. As secondary analyses, we tested whether an interaction between sex and BNT162b2 dosage was present in association with the primary outcome. Next, we investigated symptoms occurring after non-SARS-CoV-2 vaccinations, and we analyzed symptoms post-BNT162b2 administration in comparison with those occurring after non-SARS-CoV-2 vaccine administration in the period between January 15th, 2022 and May 9th, 2022.
Statistical analysis
No power analysis was performed for this study. Analyses were conducted using STATA version 15 and MATLAB R2020a. Categorical variables were compared using Fisher’s exact or Chi-Square test. Continuous variables were assessed using Wilcoxon rank sum test. Logistic regression models were used with the four symptoms categories as dependent variables, sex and BNT162b2 vaccine dosage as predictors of interest, and age, height and weight as a priori confounders. Next, sex and dosage were introduced as an interaction term in the models above, and the models with versus without the interaction term were compared using Likelihood Ratio tests. For analyses of non-BNT162b2 vaccines, dosage variable was not included in the models. For the active-comparator analysis comparing post-BNT162b2 symptoms with those occurring after non-BNT162b2 vaccines, we used logistic regression models as above but included vaccine type “BNT162b2” vs. “non-BNT162b2” as a predictor variable. For both bivariate and regression analyses, all p values and 95% confidence intervals underwent adjustment for multiple testing of 4 symptom categories by the Bonferroni method. Statistical significance was assumed at an adjusted p < 0.05.
Results
Study population
The present analysis included 7801 participants comprising 3842 girls (49%) and 3977 boys (51%) aged 3 years (median, interquartile: 2 years) at the time when receiving their first vaccination with BNT162b2. Demographics, health-related information of participants and the dosage of administered BNT162b2 mRNA vaccines are displayed as sex-disaggregated data in Table 1. The age, height, weight, the chosen BNT162b2 doses, and the fraction of children with comorbidities or long-term medication were similar between the sexes.
Sex-stratified occurrence of post-vaccination symptoms reported after BNT162b2 administration
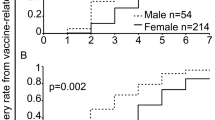
We assessed the most frequently reported symptom categories of the original CoVacU5 study [18] with sex stratification. The sex-stratified occurrence of local injection site, general, musculoskeletal symptoms and fever after the first administration of BNT162b2 mRNA vaccine are shown in Fig. 1. Similarly, symptom categories reported to occur after second or third administration of BNT162b2 mRNA vaccine are shown in Fig. 1 and Supplemental Table 1, respectively. The frequency of local injection-site reactions was higher in girls than in boys, and higher at the dose of 10 µg BNT162b2. However, there was no significant difference in frequencies between sexes or doses for general, musculoskeletal symptoms and fever after BNT162b2 administration. The underlying numerical data are shown in Supplemental Table 1. Overall, descriptive analysis suggested specific sex differences in post-BNT162b2 symptoms.
In logistic regression models adjusted for age, weight and height, female sex was associated with occurrence of local symptoms after BNT162b2 administration (OR: 1.33 [95% CI: 1.15;1.55]). (Table 2) Similarly, female sex was associated with occurrence of general symptoms after BNT162b2 administration (OR: 1.21 [95% CI: 1.01;1.44]). There were no associations between sex and fever or musculoskeletal symptoms (Table 2). The sex-stratified analyses captured associations between BNT162b2 mRNA vaccine dosage and symptoms observed in all symptoms categories (Fig. 1; Table 2). However, we found no significant interactions between sex and dosage for symptoms occurring post BNT162b2 vaccination (Table 2).
Sex-specific symptoms occurring after non-BNT162b2 vaccine administration
As a next step, we tested whether a sex effect was present in symptoms occurring after any on-label administration of non-BNT162b2 vaccinations against viruses and bacteria that were performed in the three months prior to a BNT162b2 vaccination, as shown in Supplementary Table 2. In logistic regression models, we found that fever, general or local symptoms were sex-independent (Table 3). However, female sex was negatively associated with musculoskeletal symptoms after non-BNT162b2 vaccine administration (OR: 0.29 [95% CI: 0.11;0.82]). These data indicate that after on-label childhood vaccines, most symptoms categories except musculoskeletal symptoms were not associated with biological sex.
Active comparator analysis of symptoms occurring after BNT162b2 mRNA vaccine versus other vaccine administration in the same population
Further, we aimed to compare the symptoms occurring in a cohort of 4570 children who had received at least one BNT162b2 mRNA vaccine within the last 3 months with those occurring in 1473 individuals of the same population after receiving the non-BNT162b2 vaccinations listed in Supplementary Table 2. Using logistic regression analysis of all vaccinations combined and adjusting for age, weight and height, we found that BNT162b2 mRNA vaccine was associated with higher odds of symptoms of the local injection-site and the musculoskeletal category, but at lower odds of general symptoms or fever (Table 4). In this analysis, female sex was associated with increased odds of post-vaccination local symptoms (OR: 1.35 [95% CI: 1.17–1.56], p < 0.05) but not with any of the other three symptoms categories (Table 4). Secondary analyses revealed an interaction between vaccine type and sex for the musculoskeletal category only (interaction BNT162b2 mRNA vaccine and female sex: OR 4.01 [95% CI: 1.01;16.02], Likelihood ratio test p = 0.021), (current Table 5; final Table 4). Overall, these results indicate that few post-vaccination symptoms showed an association with sex in the combined dataset of BNT162b2 and non-BNT162b2 vaccination, with the exception that after non-BNT162b2 vaccinations the probability of musculoskeletal symptoms was negatively affected by female sex.
Discussion
Principal findings
In the present study, we investigated the sex differences in post-vaccination symptoms in young children using a large retrospective cohort including COVID-19 and non-COVID-19 vaccinations in Germany. Our study supports the notion that biological sex plays an important role in the presentation of post-vaccination symptoms. The study findings demonstrate that post-vaccination symptoms predominantly occur in female children even at very young age far before puberty. Our main findings were that (i) female sex was significantly associated with occurrence of local symptoms and general symptoms after BNT162b2 administration. (ii) Female sex was associated with increased odds of post-vaccination local symptoms among all vaccinations, regardless of type. (iii) Female sex was associated with lower odds of musculoskeletal symptoms occurring after non- BNT162b2 childhood vaccine administration.
The increased probability of local injection-site symptoms in female compared to male sex after any type of vaccination in this pediatric population is in accordance with previous reports on various vaccines [22, 23]. The current BNT162b2 assessment in young children is important as previous reports on safety of BNT162b2 administration in this age group either did not stratify for sex [18, 24, 25] or found no sex differences [26].
Study interpretation
The mechanism underlying the increased odds of local and general symptoms in young girls receiving BNT162b2 is not clear. For vaccination in general, such a sex difference is suspected to be multifactorial and related to pain sensitivity, hypersensitivity reactions or most likely hormonal factors [22]. The observed sex difference for BNT162b2 may also be in part attributable to the innate immune system, as females are known to have stronger innate immune responses than males, sometimes with non-specific adverse vaccine effects [27,28,29]. For instance, in experimental data BNT162b2 stimulates interferon-α/MDA5 signaling, although sex differences in this process are not known and should be further investigated [30]. By contrast, SARS-CoV-2 spike protein stimulates Toll-like receptor (TLR) 2 and TLR 4 signaling [31, 32], and TLR signaling has several sex dimorphisms that might have contributed to the observed findings [2, 27, 33]. In young children, a mini-puberty – a temporary activation of the hypothalamo-pituitary-gonadal axis in the first months to years of life, coincides with the sexual dismorphisms of immune cell counts [34]. However, the sex differences observed in the present findings are less likely caused by inherent differences in the adaptive immune responses, as SARS-CoV-2 neutralizing antibody titers induced by 3 µg of BNT162b2 were comparable between the sexes in this age group according to BNT162b2 manufacturer data [26]. The reason why no sex difference was found in that study may relate to the fact that the manufacturer study used a lower dose.
Strengths and limitations
The strengths of this study include the big sample size of the CoVacU5 dataset and the focus on a very young age group. Sex-stratified analyses in this group are often neglected despite the potential insight to differentiate hormone-dependent from actual sex-specific effects.
The present study also has limitations that need to be acknowledged. These include that no causal effects are investigated between vaccination and symptoms due to the observational design of the study, and that the symptom reports by legal guardians are of subjective nature and underlie both recall bias and potentially depend on the gender of the reporting person which was not reported. Finally, inflammatory post-vaccination symptoms including but not limited to myocarditis that are more frequent in male than female adolescents [35] were not assessed in this study due to the low number of events, and they are of importance and warrant additional investigation in young children.
Perspectives and significance
Increased odds of local and general symptoms in young girls compared to boys are apparent in young children after administration of COVID-19 vaccine. This observation has implications for present and future conceptions of approval studies as well as post-vaccination monitoring and the design of future mRNA applications. The choice of the pro-inflammatory mRNA vaccine delivery vehicle (i.e., types of lipids used for encapsulation) may thus be reevaluated in the future due to sex differences in post-vaccination symptoms affecting even very young children far before puberty. In addition, sex-specific vaccine dosing strategies may be evaluated to find the optimal potential trade-off between vaccine effectiveness and safety in each sex.
In conclusion, sex differences in post-vaccination symptoms after BNT162b2 administration are detectable even in young children. The mechanisms of increased local injection-site and general symptoms reported on the present dataset deserve further investigation in order to better guide future evaluation strategies of emerging vaccines.
Data availability
A restricted dataset is available from the corresponding author on request. Access to the full dataset can be requested upon approval by the Ethics Committee of University of Rostock, as it contains potentially identifying patient information.
References
Fehervari Z. Vaccine sex differences. Nat Immunol. 2019;20(2):111–111.
Klein SL, Jedlicka A, Pekosz A. The xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–49.
Fink AL, Engle K, Ursin RL, Tang WY, Klein SL. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc Natl Acad Sci U S A. 2018;115(49):12477–82.
Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111(2):869–74.
Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78(3):432–7.
D’Agostino P, Milano S, Barbera C, Di Bella G, La Rosa M, Ferlazzo V, et al. Sex hormones modulate inflammatory mediators produced by macrophages. Ann N Y Acad Sci. 1999;876:426–9.
Bignucolo A, Scarabel L, Mezzalira S, Polesel J, Cecchin E, Toffoli G. Sex disparities in efficacy in COVID-19 vaccines: a systematic review and Meta-analysis. Vaccines. 2021;9(8):825.
Jensen A, Stromme M, Moyassari S, Chadha AS, Tartaglia MC, Szoeke C, et al. COVID-19 vaccines: considering sex differences in efficacy and safety. Contemp Clin Trials. 2022;115:106700.
Shapiro JR, Sitaras I, Park HS, Aytenfisu TY, Caputo C, Li M, et al. Association of Frailty, Age, and biological sex with severe Acute Respiratory Syndrome Coronavirus 2 Messenger RNA vaccine–Induced immunity in older adults. Clin Infect Dis. 2022;75(Supplement1):S61–71.
Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the Outcomes of Vaccination over the Life Course. Annu Rev Cell Dev Biol. 2017;33(1):577–99.
Su JR, Moro PL, Ng CS, Lewis PW, Said MA, Cano MV. Anaphylaxis after vaccination reported to the vaccine adverse event reporting system, 1990–2016. J Allergy Clin Immunol. 2019;143(4):1465–73.
Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, et al. Risk of Myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by Age and Sex. Circulation. 2022;146(10):743–54.
Vassallo A, Shajahan S, Harris K, Hallam L, Hockham C, Womersley K, et al. Sex and gender in COVID-19 Vaccine Research: substantial evidence gaps remain. Front Glob Womens Health. 2021;2:761511.
Duijster JW, Lieber T, Pacelli S, Van Balveren L, Ruijs LS, Raethke M, et al. Sex-disaggregated outcomes of adverse events after COVID-19 vaccination: a Dutch cohort study and review of the literature. Front Immunol. 2023;14:1078736.
Mori M, Yokoyama A, Shichida A, Sasuga K, Maekawa T, Moriyama T. Impact of sex and age on mRNA COVID-19 vaccine-related side effects in Japan. Microbiol Spectr. 2022;10(6):e0130922.
Green MS, Peer V, Magid A, Hagani N, Anis E, Nitzan D. Gender differences in adverse events following the Pfizer-BioNTech COVID-19 vaccine. Vaccines. 2022;10(2):233.
Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, et al. First Month of COVID-19 Vaccine Safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–8.
Toepfner N, von Meißner WCG, Strumann C, Drinka D, Stuppe D, Jorczyk M, et al. Comparative safety of the BNT162b2 Messenger RNA COVID-19 vaccine vs other approved vaccines in children younger than 5 years. JAMA Netw Open. 2022;5(10):e2237140–2237140.
Strumann C, Ranzani O, Moor J, Berner R, Töpfner N, Chao CM, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in children under 5 years. J Clin Invest. 2023;133(21):e173329.
Xu W, Ren W, Wu T, Wang Q, Luo M, Yi Y, et al. Real-world safety of COVID-19 mRNA vaccines: a systematic review and Meta-analysis. Vaccines. 2023;11(6):1118.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–81.
Cook IF. Sex differences in injection site reactions with human vaccines. Hum Vaccin. 2009;5(7):441–9.
Kiely M, Tadount F, Lo E, Sadarangani M, Wei SQ, Rafferty E, et al. Sex differences in adverse events following seasonal influenza vaccines: a meta-analysis of randomised controlled trials. J Epidemiol Community Health. 2023;77(12):791–801.
Hause AM, Marquez P, Zhang B, Moro PL, Myers TR, Bradley C, et al. Safety Monitoring of mRNA COVID-19 vaccine third doses among children aged 6 Months-5 years - United States, June 17, 2022-May 7, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(23):621–6.
Muñoz FM, Sher LD, Sabharwal C, Gurtman A, Xu X, Kitchin N, et al. Evaluation of BNT162b2 Covid-19 vaccine in children younger than 5 years of age. N Engl J Med. 2023;388(7):621–34.
Vaccines and Related Biological Products Advisory Committee June 14–15. 2022 Meeting Briefing Document- FDA- Pfizer- COVID19 Vaccine for Pediatrics | FDA [Internet]. [cited 2023 Apr 17]. https://www.fda.gov/media/159195/download
Aaby P, Benn CS, Flanagan KL, Klein SL, Kollmann TR, Lynn DJ, et al. The non-specific and sex-differential effects of vaccines. Nat Rev Immunol. 2020;20(8):464–70.
Aaby P, Ravn H, Fisker AB, Rodrigues A, Benn CS. Is diphtheria-tetanus-pertussis (DTP) associated with increased female mortality? A meta-analysis testing the hypotheses of sex-differential non-specific effects of DTP vaccine. Trans R Soc Trop Med Hyg. 2016;110(10):570–81.
Ciarambino T, Para O, Giordano M. Immune system and COVID-19 by sex differences and age. Womens Health Lond Engl. 2021;17:17455065211022262.
Li C, Lee A, Grigoryan L, Arunachalam PS, Scott MKD, Trisal M, et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol. 2022;23(4):543–55.
Zhao Y, Kuang M, Li J, Zhu L, Jia Z, Guo X, et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021;31(7):818–20.
Zheng M, Karki R, Williams EP, Yang D, Fitzpatrick E, Vogel P, et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunol. 2021;22(7):829–38.
Roberts BJ, Moussawi M, Huber SA. Sex differences in TLR2 and TLR4 expression and their effect on coxsackievirus-induced autoimmune myocarditis. Exp Mol Pathol. 2013;94(1):58–64.
Karaoglan M, Nacarkahya G. Immunological interpretation of minipuberty: Minipuberty as the driving force of sexual dimorphism in the immune response. Clin Endocrinol (Oxf). 2021;94(4):575–82.
Lee CW, Sa S, Hong M, Kim J, Shim SR, Han HW. Adverse events and Safety Profile of the COVID-19 vaccines in adolescents: Safety Monitoring for adverse events using real-World Data. Vaccines. 2022;10(5):744.
Acknowledgements
The authors want to thank all participating vaccination institutions, legal guardians, and children. We are thankful to the initiatives U12Schutz and Bildung Aber Sicher for help with recruitment of respondents.
Funding
JM was funded by the Bangerter-Rhyner Foundation. MBM was supported by the Swiss National Science Foundation (grant number 214187). Work of CMC was supported by University Medical Center Rostock and Helios Universitätsklinikum Wuppertal. Work of NT was supported by University Medical Center Dresden. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. CS and CMC are co–senior authors. Concept and design: JM, CS, MBM, CMC Acquisition, analysis, or interpretation of data: JM, NT, WCGvM, RB, MBM, KK, CS, CMC Drafting of the manuscript: JM, MBM, KK Critical revision of the manuscript for important intellectual content: JM, NT, WCGvM, RB, MBM, KK, CS, CMC Statistical analysis: CS Administrative, technical, or material support: NT, RB, CMC Supervision: KK, CS, CMC.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was assessed and approved by the Ethics Committee of University of Rostock, Germany (ID: A 2022-0065). All participants gave informed consent via their legal guardian.
Consent for publication
Not applicable (no individual data).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christoph Strumann and Cho-Ming Chao co-senior authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moor, J., Toepfner, N., von Meißner, W.C.G. et al. Sex differences in symptoms following the administration of BNT162b2 mRNA COVID-19 vaccine in children below 5 years of age in Germany (CoVacU5): a retrospective cohort study. Biol Sex Differ 15, 74 (2024). https://doi.org/10.1186/s13293-024-00651-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13293-024-00651-x