Abstract
Background
Premature birth and/or low birthweight have long-lasting effects on cognition. The purpose of the present systematic review is to examine whether the effects of prematurity and/or low birth weight on neurodevelopmental outcomes differ between males and females.
Methods
Web of Science, Scopus, and Ovid MEDLINE were searched for studies of humans born premature and/or of low birthweight, where neurodevelopmental phenotypes were measured at 1 year of age or older. Studies must have reported outcomes in such a way that it was possible to assess whether effects were greater in one sex than the other. Risk of bias was assessed using both the Newcastle–Ottawa scale and the National Institutes of Health Quality assessment tool for observational cohort and cross-sectional studies.
Results
Seventy-five studies were included for descriptive synthesis, although only 24 presented data in a way that could be extracted for meta-analyses. Meta-analyses found that severe and moderate prematurity/low birthweight impaired cognitive function, and severe prematurity/low birthweight also increased internalizing problem scores. Moderate, but not severe, prematurity/low birthweight significantly increased externalizing problem scores. In no case did effects of prematurity/low birthweight differ between males and females. Heterogeneity among studies was generally high and significant, although age at assessment was not a significant moderator of effect. Descriptive synthesis did not identify an obvious excess or deficiency of male-biased or female-biased effects for any trait category. Individual study quality was generally good, and we found no evidence of publication bias.
Conclusions
We found no evidence that the sexes differ in their susceptibility to the effects of severe or moderate prematurity/low birthweight on cognitive function, internalizing traits or externalizing traits. Result heterogeneity tended to be high, but this reflects that one sex is not consistently more affected than the other. Frequently stated generalizations that one sex is more susceptible to prenatal adversity should be re-evaluated.
Plain Language Summary
Early life environmental conditions and adversities affect health into adulthood. For example, it is well-known that premature birth and low birthweight have long-lasting effects on the development and functioning of the brain, affecting various aspects of academic performance, intelligence, and the risk of behavioural problems including depression, anxiety, aggression, impulsivity, and inattention. However, it is not clear if these effects differ between boys and girls. We searched for studies examining the effects of prematurity and/or of low birthweight on cognitive abilities and behavioural problems in children measured at 1 year of age or older, and identified 75 relevant studies. Combining the results of studies found that prematurity/low birthweight decreased measures of intelligence and increased the incidence of behavioural problems, as expected. However, there was no indication that the effects of prematurity/low birthweight consistently differed between males and females, and there were no specific traits where boys appeared to be more or less susceptible to the effects of prematurity/low birthweight than girls. While sex and gender influence health, and in many cases will influence the effects of early life conditions on health, our study shows that prematurity and low birthweight have similar long-term effects on intelligence and behaviour in boys and girls.
Highlights
-
Premature birth and/or low birthweight have long-lasting effects on cognition, but it is not clear if these effects differ between males and females.
-
We searched for studies examining the effects of prematurity and/or of low birthweight on neurodevelopmental phenotypes measured at one year of age or older, and identified 75 studies for descriptive synthesis, and 24 for meta-analyses.
-
Meta-analyses found that prematurity/low birthweight impaired cognitive function and increased internalizing and externalizing problem scores. However, in no case did effects of prematurity/low birthweight differ between males and females.
-
Descriptive synthesis did not identify an obvious excess or deficiency of male-biased or female-biased effects for any type of trait.
-
We found no evidence that the sexes differ in their susceptibility to the effects of severe or moderate prematurity/low birthweight on cognitive function, internalizing traits or externalizing traits.
Similar content being viewed by others
Background
Insults in early life can have far-reaching impacts on health. Numerous systematic reviews have found associations between premature birth and/or low birthweight and cognitive abilities throughout childhood from infancy [1], to preschool age [2,3,4], to later childhood [5,6,7] and even into adulthood [8,9,10,11]. Even being born late preterm (34–36 weeks [12]) or early term (37–38 weeks [13]) has effects on cognition. Moreover, brain sparing (redistribution of blood flow to the brain) in response to intrauterine growth restriction does not fully protect cognitive abilities [14].
While there is clear and consistent evidence that low birthweight and premature birth have lasting effects on cognition, it is not clear whether males or females may be more susceptible to such effects. Many authors have suggested that males may have greater susceptibility to early life conditions [15,16,17,18,19,20,21,22,23]. However, with regard to the effects of low birthweight and prematurity, while studies often adjust for effects of sex (i.e., take into account overall differences between males and females), or acknowledge sex as a potentially confounding factor, relatively few assess sex-dependent effects (e.g., whether males are more or less affected by prematurity than females). Moreover, such sex-dependent effects have not been examined in a systematic review, although two studies using meta-regression found that the effect or prematurity or low birthweight was not related to the sex ratio of study participants [24, 25]. Another examined sex-specific effects of nutritional supplements in these populations [26]. A systematic review of the effects of a variety of prenatal stressors on the hypothalamic–pituitary–adrenal axis of the offspring [27] found females more vulnerable. Other work has also suggested that females may be more susceptible to the effect of prenatal adversity on the risk of developing affective problems [28].
The purpose of the present systematic review is to examine whether the effects of prematurity and/or low birth weight on neurodevelopmental outcomes differ between males and females. Because we are interested in exposures that occur prior to birth, we will use the term “sex” for brevity. However, we acknowledge that outcomes are measured after substantial socialization has occurred, and thus will be affected by both sex and gender.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29]. This study is registered with PROSPERO (CRD42021228814).
Eligibility criteria, information sources and search strategy
Eligible studies were of humans born premature and/or of low birthweight, where unaffected comparators/controls were also included, i.e., individuals born at term, and/or individuals of normal birth weight. Prematurity was defined as birth prior to 37 weeks of gestation. Low birth weight is defined either in terms of a fixed value (e.g., < 2500 g) or in terms of a percentile (e.g., below the 10th percentile for gestational age). There is substantial heterogeneity with regard to the criteria used to define prematurity and low birthweight, but a meta-analysis has previously supported the use of both gestational age and/or birthweight as inclusion criteria for the study of the effects of prematurity on cognition [30]. The original registration in PROSPERO included exposure to prenatal maternal depression or anxiety or stress, but this was later removed to narrow the scope of the study.
Some studies have suggested increased male vulnerability in long-term behavioral and cognitive outcomes [15, 19, 20, 22, 23], and so we focused on such outcomes measured at 1 year of age or older, including assessments of abilities relating to language (including reading and speech), behaviour, memory, learning, thinking and problem solving. Discrete and continuous outcomes were included. Outcomes that were defined primarily in terms of motor skills, vision, hearing and/or brain morphology (e.g., volumes of brain regions) were excluded.
To be included, studies must have reported outcomes in such a way that it was possible to assess whether the effect of prematurity and/or low birthweight was greater in one sex than the other (e.g., presented separately by sex and/or the statistical interaction between sex and exposure was tested and reported). If differences between the sexes were reported separately for different exposure groups (e.g., differences between males and females were reported separately for preterm and term individuals), but differences between exposures are not reported separately for the sexes, results were not included if it was not possible to assess the latter comparison and extract the relevant data.
Web of Science, Scopus, Ovid MEDLINE, were all searched May 25, 2020, and this search was repeated on May 11, 2022, limiting to publication dates of 2020 or later. The search strategy is provided in Additional file 1.
Selection process
Three reviewers (AB, JKC, SA) screened non-overlapping sets of titles and abstracts from the first search to exclude studies where the exposure and/or outcome clearly did not meet inclusion criteria (i.e., each title and abstract was screened by one reviewer at this stage). Two reviewers (AB, RH) independently examined the full texts of the remaining studies to assess whether they met eligibility criteria. Disagreements were resolved by a third reviewer (JKC). One reviewer (GMP) screened titles and abstracts from the updated search, and two reviewers (GMP, JKC) independently examined full texts of the remaining studies.
Data collection and data items
Data collected included type and severity of exposure and comparator group (e.g., < 33 weeks vs term; < 1500 g vs controls), sample size per group, outcome studied, age at which the outcome was studied, the effect of exposure on outcome in males (e.g., means, odds ratios, including standard deviations and/or confidence intervals, as available), the effect of exposure on outcome in females, and the approach to test for sex dependent effects (e.g., presented separately by sex or the statistical interaction between sex and exposure was tested). Where multiple scores were summarized (e.g., multiple measures of cognitive function combined into IQ, or multiple problem scores combined into internalizing and externalizing problems), we extracted only the summary score. Where both continuous scores and proportions above/below a cut off were reported, we extracted only continuous scores. Two reviewers (MAM, NMN) independently extracted data, and disagreements were resolved by a third reviewer (JKC). Where data were provided in figures, they were extracted them WebPlotDigitizer [31].
Study risk of bias assessment
Studies were assessed for quality and risk of bias by AB and EVL using two scoring systems [32, 33]; criteria are listed in Additional file 2: Table S1. These assessments were only used to assess the quality of the studies, but were not included in meta-analyses.
Effect measures and synthesis methods
We collected means and standard deviations for continuous outcomes, and odds ratios, relative risks or hazard ratios for discrete outcomes. Upon extraction of data, we found that there was no type of neurodevelopmental outcome for which 5 or more studies reported results for discrete outcomes, and so only studies presenting means for continuous outcomes were included in meta-analyses. Similarly, less than 5 studies reported outcomes for a given type of neurodevelopmental outcome assessed at an age of less than 5 years in such a way that data could be extracted for meta-analyses, and so such studies were excluded from meta-analyses.
Meta-analyses were performed separately on cognitive, internalizing and externalizing traits (see Table 1 for examples of each type of trait). For all traits, values were first scaled, so that the average value for the 4 groups in a given study (exposed males, control males, exposed females, control females) was 100; means and standard deviations were scaled by multiplying all values by a fixed value for a given study. Where a given study measured a trait at multiple ages, or measured multiple traits in the same category (cognitive, internalizing, or externalizing), values were averaged across ages/traits, such that each study contributed only one set of 4 values to the meta-analysis for each category of outcome.
To reduce heterogeneity among studies for meta-analyses, we analysed studies using severe (birthweight < 1500 g and/or gestational age < 34 weeks) and moderate (birthweight < 2500 g and/or gestational age < 37 weeks) criteria separately. Where a study examined two categories of exposure that both fit our categorization of severe or moderate, we selected the exposure expected to be more debilitating, e.g., one study [35] categorized preterm (< 30 weeks) infants by whether they were intrauterine growth restricted (IUGR) or appropriate for gestational age (AGA) and so we only included the IUGR group. Another examined the effects of prematurity and small for gestational age (SGA) separately [36], and we included only the prematurity results, since these were more comparable with most other studies.
Meta-analysis were implemented in the R package ‘metafor’, using the ‘escalc’ function to calculate the standardized mean difference (SMD) for each study and sex, where SMD is the difference in mean value between affected and control children, divided by the pooled standard deviation of the two groups. We used the ‘rma.mv’ function to fit a random effects model, with sex and study as random effects [37]. The effect of exposure was estimated in males and females separately in each study, and then averaged over all studies, allowing us to test whether the effect of exposure differs by sex. Age at assessment was included as a moderator. Where subjects were within a range of ages upon assessment (and not measured at multiple discrete timepoints), the average age at assessment was used, if provided, and otherwise the midpoint of the range was used. Residual heterogeneity was assessed using the QE test, and the I2 statistic was calculated using the ‘rma’ function without random effects. Forest plots were used to visualize the results of individual studies.
For studies not included in the meta-analysis, because means and standard deviations could not be extracted, we performed a descriptive synthesis, summarizing traits where males were more affected, where females were more affected, where the sexes were affected equally and where there was no effect of exposure. We also considered whether results depended on how the outcome was assessed (e.g., researcher, parent, teacher or self), since bias in behavioural assessment may vary among these approaches.
Reporting bias assessment and certainty assessment
Reporting bias assessment was performed by inspection of funnel plots. Since we did not seek to use specific estimates of effect to support clinical decisions or recommendations, we did not assess certainty of evidence [38].
Results
Study selection, study characteristics and results of individual studies
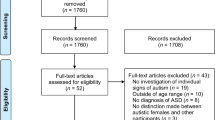
Results of the search are shown in Fig. 1. In total, 75 studies matched our criteria [19, 35, 36, 39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110], although only 24 presented data in a way that could be extracted for meta-analyses [19, 35, 36, 41, 42, 51, 52, 61,62,63,64,65,66, 68, 73, 74, 76, 81, 85, 87, 89, 93, 105, 107]. Study characteristics and results of all studies are summarized in Additional file 2: Table S2, and the results categorized into cognitive function, internalizing behaviour, externalizing behaviour or language skills by age at assessment are provided in Additional file 2: Table S3. Data extracted from studies included in meta-analyses are provided in Additional file 2: Table S4.
Risk of bias in studies
The results of assessment of quality and risk of bias for the 24 studies included in meta-analyses are shown in Additional file 2: Table S1. Given that we examined studies of the effects of preterm birth/low birthweight on neurodevelopmental outcomes, a number of the assessment criteria were necessarily true, whereas another assessment criterion was never met (please see Additional file 2: Table S1 for details). In other cases, our inclusion criteria ensured that the assessment criteria were met. With these caveats, the average score was 9.25 out of a possible 14 marks (66%) on the National Institutes of Health NHLBI Quality assessment tool for observational cohort and cross-sectional studies scale, which was considered intermediate quality [27]. The average score was 7.29 out of a possible score of 9 (81%) on the Newcastle–Ottawa scale, which was considered good [111]. A common weakness was that many studies used self- or parent-reported data, and so the assessment was not blind to exposure status. Even where an investigator or clinician was administering the test, it was generally not clear if they were blind to exposure status. Loss to follow up of over 20% was also common, occurring in over half of the studies.
Descriptive synthesis
Figures 2, 3, 4 and 5 summarize whether effects of prematurity/low birthweight were sex-dependent, significant in both sexes, or not significant in either sex, for traits related to cognitive function, internalizing behaviour, externalizing behaviour or language skills, respectively; trait categories are described in Table 1. Only 4 studies [45, 50, 53, 60] reported autism-related traits, and so these were not included in figures. Overall, sex-dependent effects tended to be less frequent than findings of effects in both sexes or findings of no effect in either sex. In general, there was no obvious excess or deficiency of male-biased or female-biased effects for any trait category. However, four studies [63, 67, 90, 100] found internalizing and emotional problems to be more affected in females in childhood (10 years of age or lower), whereas no study found males to be more affected at this age (Fig. 3). However, two of these studies [63, 90] observed other internalizing traits to be unaffected in either sex. Language traits in childhood also were also more often affected in females [19, 36, 45, 69, 74, 104] than in males [56, 69] (Fig. 5). However, all but one of the studies that found traits more affected in females [19] also found other traits to be affected in both sexes or unaffected in either sex.
Qualitative synthesis of effects of prematurity/low birthweight on measures of cognitive function. Numbers indicate studies that found measures of cognitive function to be affected in both sexes, to be affected more in females, to be affected more in males, or to be affected in neither sex in childhood (1–10 years), adolescence (11–18 years), and adulthood (over 19 years). In each age group, the y-axis indicates the logarithm (base 10) of the sample size. A single study could show different results for different traits and so may appear in more than one cell. Study numbering is the same as in text. Circles indicate studies that examined sex-dependence using statistical interaction terms, whereas squares indicate studies that analyzed the sexes separately
Studies that did not find an effect of prematurity/low birthweight did not have obviously lower sample sizes than those reporting significant effects (Figs. 2, 3, 4 and 5). For most types of traits, more studies assessed traits in childhood, although for internalizing traits, there were similar number of assessments at adolescence.
Testing whether effects are sex-dependent by testing the sexes separately, rather than a more formal test (e.g., of the interaction between sex and exposure) increases the frequency of false positives [112, 113]. We therefore expected that studies testing the sexes separately would show more sex-dependent effects than studies that tested for interactions between sex and exposure. However, this was not observed (Figs. 2, 3, 4 and 5).
Effects of observer
A few studies had traits assessed by both youth and parents, or by parents and teachers, allowing the effects of different observers to be compared directly. The results of self-reports often differed from those of parent/care-giver reports, although not in consistent ways. For internalizing problems, two studies found sex-dependent effects of prematurity/low birthweight in self reports but not parent reports [52, 66], whereas this pattern was reversed in a third [64]. For externalizing problems, effects of prematurity/low birthweight in both sexes were observed in self reports but in neither sex in parent reports [52], whereas another study found the reverse [66], and a third study found no effects in either self reports or parent reports [64]. Taylor et al. found sex by observer interactions for a variety of internalizing and externalizing traits that indicated that differences between parent and self reports were larger for females than for males [102].
Parent and teacher reports both found effects on autism and ADHD symptoms in both sexes [60]. However, in another study, teacher-reported disattention showed a greater effect in females, and teacher-reported hyperactivity/impulsivity was not affected in either sex, whereas both of these traits were affected in both sexes when reported by parents [87]. Thus, the observer may be a source of heterogeneity in such studies, although its effects do not appear to be consistent.
Quantitative synthesis—severe prematurity/low birthweight on cognitive function
Ten studies examined the effects of severe prematurity/low birthweight on cognitive function, generally measured as IQ [19, 35, 36, 61, 62, 65, 73, 89, 93, 107]. Age was not a significant moderator (P = 0.80; Additional file 3: Fig. S1) and so was removed from the model. Severe prematurity/low birthweight significantly reduced cognitive function (Fig. 6), but this effect did not differ between males and females (P = 0.31). There was significant, high heterogeneity among studies (I2 = 76%, QE = 68, P < 0.0001), although the overall result is generally consistent with the results of individual studies, most of which found both sexes to be affected [19, 35, 36, 62, 65, 93, 107]. However, two studies found a significant effect in males but not females [61, 73], while another found no effect in either sex [89].
Meta-analysis of the effects of severe prematurity/low birthweight on cognitive function. Squares represent estimates (with confidence intervals) and marker size indicates weight. Diamonds represent estimates for each sex and for the difference between sexes, with the width of the diamond indicating the confidence interval. SMD standardized mean difference
Quantitative synthesis—moderate prematurity/low birthweight on cognitive function
Five studies examined the effects of moderate prematurity/low birthweight on cognitive function [36, 51, 68, 74, 81], and age was not a significant moderator (P = 0.24; Additional file 3: Fig. S2). Moderate prematurity/low birthweight reduced cognitive function (Fig. 7), but males and females did not differ in estimated effect size (P = 0.50). There was significant heterogeneity among studies (I2 = 80%, QE = 35, P < 0.0001). The two individual studies with sample sizes greater than 200 in all groups found no effect [36, 68], while another found an effect in both sexes [81], and two others found a variety of patterns depending on which aspect of cognitive function was examined [51, 74].
Quantitative synthesis—severe prematurity/low birthweight on internalizing problems
Seven studies examined the effects of severe prematurity/low birthweight on internalizing problem scores [19, 41, 52, 61, 62, 64, 66]. Age was not a significant moderator (P = 0.11; Additional file 3: Fig. S3) and so was removed from the model. Severe prematurity/low birthweight significantly increased internalizing problem scores (Fig. 8), and this effect tended to be larger in females, but the difference between the sexes was not significant (P = 0.12). The heterogeneity among studies was marginally non-significant (I2 = 19%, QE = 20, P = 0.06). Within individual studies, results varied among different internalizing traits or were sometimes sex-dependent [41, 52, 62, 64, 66], although two individual studies found no significant effects [19, 61]. Only four studies examined the effects of moderate prematurity/low birthweight on internalizing problems [63, 68, 81, 105], and so these results were not synthesized.
Quantitative synthesis—severe and moderate prematurity/low birthweight on externalizing problems
Nine and 7 studies examined the effects of severe [19, 41, 42, 52, 61, 62, 64, 66, 85] and moderate [42, 51, 63, 68, 76, 81, 87] prematurity/low birthweight on externalizing problem scores, respectively. Age was not a significant moderator in either analysis (P = 0.17 and 0.42, respectively; Additional file 3: Figs. S4 and S5) and so was removed from the models. Surprisingly, moderate prematurity/low birthweight significantly increased externalizing problem scores (Fig. 9), whereas severe prematurity/low birthweight did not (Fig. 10). However, in neither case did effect sizes differ between the sexes (P = 0.42 and 0.78 for severe and moderate, respectively). There was significant heterogeneity among studies (I2 = 67%, QE = 46, P < 0.0001, and I2 = 59%, QE = 34, P = 0.0007 for severe and moderate, respectively). Among studies examining effects of severe prematurity/low birthweight on externalizing traits, three individual studies found no effects [19, 41, 64], while others found greater effects in females [61] or effects in both sexes [62], or variable effects in different traits [42, 52, 66, 85]. Among studies examining effects of moderate prematurity/low birthweight, most reported variable patterns among different externalizing traits [42, 51, 63, 87], although two, including one with sample sizes greater than 200 in all groups, found no significant effects [68, 76].
Quantitative synthesis by observer
Because we found that observer (e.g., self vs parent) may be a source of heterogeneity, we repeated meta-analyses of internalizing and externalizing traits separately for self-reports and parent-reports. In no case did the effect of prematurity/low birth weight differ between males and females (Additional file 4). However, for the effects of severe prematurity/low birthweight on both internalizing problem scores and externalizing problem scores, heterogeneity was higher for self-reports (I2 = 76% and 85% for internalizing and externalizing, respectively) than for parent reports (0% and 46%; Additional file 4). Severe prematurity/low birthweight significantly increased parent-reported internalizing problem scores, as in the combined analysis (described above), but did not have a significant effect on self-reported internalizing problem scores (Additional file 4). Severe prematurity/low birthweight did not have significant effects on self-reported or parent-reported externalizing problem scores (Additional file 4), as in the combined analysis. The effect of moderate prematurity/low birthweight on parent-reported externalizing problem scores was marginally non-significant (P = 0.06; Additional file 4), whereas it was significant in the combined analysis. Heterogeneity was similar in the parent-reported studies (I2 = 61%; Additional file 4) as in the combined analysis (I2 = 59%). There were no studies of the effects of moderate prematurity/low birthweight on self-reported externalizing problem scores.
Reporting biases
Funnel plots did not show asymmetry (Additional file 3: Figs. S6–S10), suggesting no evidence of reporting bias.
Discussion
This is the first systematic review and meta-analysis to examine whether the effects of prematurity/low birthweight on neurodevelopmental outcomes are sex-dependent, and one of the first to examine sex-dependent long-term effects of prenatal adversity in humans (e.g., [27]). In our quantitative synthesis, we found no significant sex-dependence of effects of severe or moderate prematurity/low birthweight on cognitive function, internalizing traits or externalizing traits. Severe prematurity/low birthweight tended to have greater effects on internalizing problem scores in females, but this was not significant and effect sizes were small, i.e., a SMD of 0.15 in males and 0.31 in females. In most meta-analyses, heterogeneity was significant and moderate (I2 > 50%) to high (I2 > 75%). We used a random effects model, which accounts for variability between studies [114], but nevertheless it appears that the effects of prematurity/low birthweight on cognitive function, internalizing problems and externalizing problems are not consistent. While this precludes a definitive conclusion on the overall effects of prematurity/low birthweight, we can conclude with confidence that one sex is not consistently more affected than the other.
To assess potential sources of heterogeneity, we analysed effects of severe and moderate prematurity/low birthweight separately, and included age at assessment as a moderator, which was not found to be significant. Recently, it was found that the type of test used to assess cognitive abilities (e.g., full-scale vs short-form assessments of general intelligence) contributed to 14% of between-study variance in the effects of prematurity [115]. In the present study, this may have contributed to the heterogeneity between studies, although a number of studies were ambiguous about whether the assessment was full-scale or short-form, and so we could not assess this formally. We also examined studies which reported traits assessed by different observers (i.e., youth and parents or parents and teachers) and found results often differed depending on observer, but not in a consistent way, although one study found that differences between parent and self reports were larger for females than for males [102]. Gendered expectations of behaviour may also contribute to variability in assessments [76], and this effect may depend on specific social context.
In our descriptive synthesis of 75 studies, we found that sex-dependent effects tended to be less frequent than findings of effects in both sexes or neither sex, and generally there did not appear to be an excess or deficiency of male-biased or female-biased effects. There were slight excesses of results finding internalizing and language problems to be more affected in females in childhood. However, even in these categories, there were many more results of effects in both sexes and/or neither sex, even among studies with substantial sample sizes. This is consistent with the results of the meta-analyses, which found no overall sex dependence.
Numerous studies in this field examine sex differences in responses to early life adversity without explicitly testing whether effects differ between males and females (e.g., using an interaction) [20, 60]. Testing males and females separately would be expected to generate spurious sex-dependent effects [36, 112, 113]. In the present study, approximately two-thirds of included studies tested interactions between prematurity/low birthweight and sex, while the remainder of the studies tested effects in males and females separately. Surprisingly, studies that tested the sexes separately did not show an excess of sex-dependent effects.
Limitations
Definitions of prematurity/low birthweight varied among studies, which may explain some of the heterogeneity among studies, although a previous meta-analysis has supported the use of both gestational age and/or birthweight as inclusion criteria [30]. The wide variety of tools used to assess outcomes likely contributed to heterogeneity as well. The lack of consistency in how data were analysed and how results were reported made synthesis challenging. While our results show that one sex is not consistently more affected than the other, it is possible that there are combinations of exposure severity, outcome, age and mode of assessment, postnatal environment, etc., where one sex is consistently more vulnerable. However, the lack of identifiable causes of heterogeneity, resulting from variability in both study design and reporting [115], means that we are currently unable to identify consistent sex-dependent effects, if they occur. Variable effects of gender in different populations may have also contributed to heterogeneity. While we used the term “sex” for brevity and because the exposure occurred prior to birth, we acknowledge that outcomes may have been heavily influenced by gender; we did not identify any studies that sought to disentangle effects of sex and gender. Gendered treatment of children may have diminished or enhanced biologically based sex differences in the effects of prematurity/low birthweight, e.g., where one gender receives more support in the development of certain traits and behaviours, or is subject to more rigid social expectations, observed effects of adverse early life conditions may be reduced in that gender.
Our conclusions are also limited by the underlying studies. Many studies used self- or parent-reported data and/or it was not clear if the assessor was blind to exposure status. Combined with gendered expectations, this lack of blinding may have obscured or exaggerated sex-dependent effects. Loss to follow up of over 20% was also common, occurring in over half of the studies. However, it is not clear whether bias introduced by loss to follow up would affect the sexes and genders differently. Moreover, a recent meta-regression found that attrition rate did not contribute to variation in effect sizes in studies of preterm birth and cognitive ability [115].
A difficulty in interpreting sex-dependent effects of early life adversity is that the prevalence and severity of adversity may differ between the sexes. Indeed, males are at increased risk of preterm birth [116, 117]. This may create a selection bias, e.g., if male newborns are more likely to die and thus be lost to follow-up. This might dampen sex-dependent effects if, for example, males were more impacted by prematurity, but the most-severely affected males did not survive, whereas females did. However, such effects of selection would be expected to be reduced in cases of moderate prematurity/low birthweight, because a greater proportion of infants would be expected to survive. We did not observe greater sex-dependence in our analysis of moderate complications. The issue of selection bias is also difficult to address, because mortality is female-biased early in gestation [118], i.e., while there may be an observable bias, where males are more likely to be lost to follow-up, there may also be an unobserved bias, where females are more likely to be lost at earlier stages of gestation and so not included in studies at all.
Perspectives and significance
It has often been suggested that males have greater susceptibility to early life conditions [15,16,17,18,19,20,21,22,23]. Our results show that this is not the case with regard to the lasting effects of premature birth and/or low birthweight on cognitive function, internalizing problems and externalizing problems. Thus, the view that males are more vulnerable in general should be re-evaluated. Specific insults may have sex-dependent effects on specific phenotypes [27], but care should be taken in generalizing such observations. While sex and gender clearly influence health and disease, as well as the effects of early life adversity, it is also important to acknowledge that many traits may not show such differences.
Availability of data and materials
This review was based on published data. All data extracted for this study are included in the article and its Additional files.
References
Burstein O, Zevin Z, Geva R. Preterm birth and the development of visual attention during the first 2 years of life: a systematic review and meta-analysis. JAMA Netw Open. 2021;4: e213687. https://doi.org/10.1001/jamanetworkopen.2021.3687.
Arpi E, D’Amico R, Lucaccioni L, Bedetti L, Berardi A, Ferrari F. Worse global intellectual and worse neuropsychological functioning in preterm-born children at preschool age: a meta-analysis. Acta Paediatr. 2019;108:1567–79. https://doi.org/10.1111/apa.14836.
Dean B, Ginnell L, Boardman JP, Fletcher-Watson S. Social cognition following preterm birth: a systematic review. Neurosci Biobehav Rev. 2021;124:151–67. https://doi.org/10.1016/j.neubiorev.2021.01.006.
Sandoval CC, Gaspardo CM, Linhares MBM. The impact of preterm birth on the executive functioning of preschool children: a systematic review. Appl Neuropsychol Child. 2022;11:873–90. https://doi.org/10.1080/21622965.2021.1915145.
Sacchi C, Marino C, Nosarti C, Vieno A, Visentin S, Simonelli A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: a systematic review and meta-analysis. JAMA Pediatr. 2020;174:772–81. https://doi.org/10.1001/jamapediatrics.2020.1097.
McBryde M, Fitzallen GC, Liley HG, Taylor HG, Bora S. Academic outcomes of school-aged children born preterm: a systematic review and meta-analysis. JAMA Netw Open. 2020;3: e202027. https://doi.org/10.1001/jamanetworkopen.2020.2027.
Upadhyay RP, Naik G, Choudhary TS, Chowdhury R, Taneja S, Bhandari N, et al. Cognitive and motor outcomes in children born low birth weight: a systematic review and meta-analysis of studies from South Asia. BMC Pediatr. 2019;19:35. https://doi.org/10.1186/s12887-019-1408-8.
Bolbocean C, van der Pal S, van Buuren S, Anderson PJ, Bartmann P, Baumann N, et al. Health-related quality-of-life outcomes of very preterm or very low birth weight adults: evidence from an individual participant data meta-analysis. Pharmacoeconomics. 2023;41:93–105. https://doi.org/10.1007/s40273-022-01201-2.
Fernández de Gamarra-Oca L, Ojeda N, Gómez-Gastiasoro A, Peña J, Ibarretxe-Bilbao N, García-Guerrero MA, et al. Long-term neurodevelopmental outcomes after moderate and late preterm birth: a systematic review. J Pediatr. 2021;237:168-176.e11. https://doi.org/10.1016/j.jpeds.2021.06.004.
Eves R, Mendonça M, Baumann N, Ni Y, Darlow BA, Horwood J, et al. Association of very preterm birth or very low birth weight with intelligence in adulthood: an individual participant data meta-analysis. JAMA Pediatr. 2021;175: e211058. https://doi.org/10.1001/jamapediatrics.2021.1058.
Krishna M, Jones S, Maden M, Du B, Mc R, Kumaran K, et al. Size at birth and cognitive ability in late life: a systematic review. Int J Geriatr Psychiatry. 2019;34:1139–69. https://doi.org/10.1002/gps.5138.
Martínez-Nadal S, Bosch L. Cognitive and learning outcomes in late preterm infants at school age: a systematic review. Int J Environ Res Public Health. 2020;18:74. https://doi.org/10.3390/ijerph18010074.
Nielsen TM, Pedersen MV, Milidou I, Glavind J, Henriksen TB. Long-term cognition and behavior in children born at early term gestation: a systematic review. Acta Obstet Gynecol Scand. 2019;98:1227–34. https://doi.org/10.1111/aogs.13644.
Benítez-Marín MJ, Marín-Clavijo J, Blanco-Elena JA, Jiménez-López J, González-Mesa E. Brain sparing effect on neurodevelopment in children with intrauterine growth restriction: a systematic review. Child (Basel, Switzerland). 2021;8:745. https://doi.org/10.3390/children8090745.
Bale TL. The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialogues Clin Neurosci. 2016;18:459–64.
Nugent BM, O’Donnell CM, Epperson CN, Bale TL. Placental H3K27me3 establishes female resilience to prenatal insults. Nat Commun. 2018;9:2555. https://doi.org/10.1038/s41467-018-04992-1.
Devaskar SU, Chu A. Intrauterine growth restriction: hungry for an answer. Physiology. 2016;31:131–46. https://doi.org/10.1152/physiol.00033.2015.
Pérez-Cerezales S, Ramos-Ibeas P, Rizos D, Lonergan P, Bermejo-Alvarez P, Gutiérrez-Adán A. Early sex-dependent differences in response to environmental stress. Reproduction. 2018;155:R39–51. https://doi.org/10.1530/REP-17-0466.
Kozhemiako N, Nunes AS, Vakorin VA, Chau CMY, Moiseev A, Ribary U, et al. Sex differences in brain connectivity and male vulnerability in very preterm children. Hum Brain Mapp. 2020;41:388–400. https://doi.org/10.1002/hbm.24809.
DiPietro JA, Voegtline KM. The gestational foundation of sex differences in development and vulnerability. Neuroscience. 2017;342:4–20. https://doi.org/10.1016/j.neuroscience.2015.07.068.
Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJP. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–5. https://doi.org/10.1002/ajhb.20995.
Sandman CA, Glynn LM, Davis EP. Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75:327–35. https://doi.org/10.1016/j.jpsychores.2013.07.009.
Sutherland S, Brunwasser SM. Sex differences in vulnerability to prenatal stress: a review of the recent literature. Curr Psychiatry Rep. 2018;20:102. https://doi.org/10.1007/s11920-018-0961-4.
van Houdt CA, Oosterlaan J, van Wassenaer-Leemhuis AG, van Kaam AH, Aarnoudse-Moens CSH. Executive function deficits in children born preterm or at low birthweight: a meta-analysis. Dev Med Child Neurol. 2019;61:1015–24. https://doi.org/10.1111/dmcn.14213.
Twilhaar ES, Wade RM, De Kieviet JF, Van Goudoever JB, Van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 2018;172:361–7. https://doi.org/10.1001/JAMAPEDIATRICS.2017.5323.
Lin L, Crowther C, Gamble G, Bloomfield F, Harding JE. Sex-specific effects of nutritional supplements in infants born early or small: protocol for an individual participant data meta-analysis (ESSENCE IPD-MA). BMJ Open. 2020;10: e033438. https://doi.org/10.1136/bmjopen-2019-033438.
Carpenter T, Grecian SM, Reynolds RM. Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans suggest increased vulnerability in females: a systematic review. J Dev Orig Health Dis. 2017;8:244–55. https://doi.org/10.1017/S204017441600074X.
Davis EP, Pfaff D. Sexually dimorphic responses to early adversity: implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology. 2014;49:11–25. https://doi.org/10.1016/j.psyneuen.2014.06.014.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. https://doi.org/10.1136/BMJ.N71.
Sentenac M, Chaimani A, Twilhaar S, Benhammou V, Johnson S, Morgan A, et al. The challenges of heterogeneity in gestational age and birthweight inclusion criteria for research synthesis on very preterm birth and childhood cognition: an umbrella review and meta-regression analysis. Paediatr Perinat Epidemiol. 2022;36:717–25. https://doi.org/10.1111/ppe.12846.
Rohatgi A. Webplotdigitizer: version 4.4. 2020. https://automeris.io/WebPlotDigitizer.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa (NOS) for assessing the quality of nonrandomized studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 10 Apr 2021.
National Institutes of Health NHLBI. Quality assessment tool for observational cohort and cross-sectional studies. http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort. Accessed 10 Apr 2021.
Goodman A, Lamping DL, Ploubidis GB. When to use broader internalising and externalising subscales instead of the hypothesised five subscales on the strengths and difficulties questionnaire (SDQ): data from british parents, teachers and children. J Abnorm Child Psychol. 2010;38:1179–91. https://doi.org/10.1007/S10802-010-9434-X/TABLES/6.
Morsing E, Asard M, Ley D, Stjernqvist K, Marsal K. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics. 2011;127:E874–82. https://doi.org/10.1542/peds.2010-1821.
Christians JK, Chow NA. Are there sex differences in fetal growth strategies and in the long-term effects of pregnancy complications on cognitive functioning? J Dev Orig Health Dis. 2022;13:766–78. https://doi.org/10.1017/S2040174422000204.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. https://doi.org/10.18637/jss.v036.i03.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13. https://doi.org/10.1016/J.JCLINEPI.2017.05.006.
Adrian JA, Bakeman R, Akshoomoff N, Haist F. Cognitive functions mediate the effect of preterm birth on mathematics skills in young children. Child Neuropsychol. 2020;26:834. https://doi.org/10.1080/09297049.2020.1761313.
Alati R, Lawlor DA, Mamun AA, Williams GM, Najman JM, O’Callaghan M, et al. Is there a fetal origin of depression? Evidence from the Mater University Study of Pregnancy and its outcomes. Am J Epidemiol. 2007;165:575–82. https://doi.org/10.1093/aje/kwk036.
Allin M, Rooney M, Cuddy M, Wyatt J, Walshe M, Rifkin L, et al. Personality in young adults who are born preterm. Pediatrics. 2006;117:309–16. https://doi.org/10.1542/peds.2005-0539.
Ask H, Gustavson K, Ystrom E, Havdahl KA, Tesli M, Askeland RB, et al. Association of gestational age at birth with symptoms of attention-deficit/hyperactivity disorder in children. JAMA Pediatr. 2018;172:749–56. https://doi.org/10.1001/jamapediatrics.2018.1315.
Baron IS, Erickson K, Ahronovich MD, Baker R, Litman FR. Cognitive deficit in preschoolers born late-preterm. Early Hum Dev. 2011;87:115–9. https://doi.org/10.1016/j.earlhumdev.2010.11.010.
Bjuland KJ, Rimol LM, Løhaugen GCC, Skranes J. Brain volumes and cognitive function in very-low-birth-weight (VLBW) young adults. Eur J Paediatr Neurol. 2014;18:578–90. https://doi.org/10.1016/j.ejpn.2014.04.004.
Bowers K, Wink LK, Pottenger A, McDougle CJ, Erickson C. Phenotypic differences in individuals with autism spectrum disorder born preterm and at term gestation. Autism. 2015;19:758–63. https://doi.org/10.1177/1362361314547366.
Boyle MH, Miskovic V, Van Lieshout R, Duncan L, Schmidt LA, Hoult L, et al. Psychopathology in young adults born at extremely low birth weight. Psychol Med. 2011;41:1763–74. https://doi.org/10.1017/S0033291710002357.
Chatterji P, Lahiri K, Kim D. Fetal growth and neurobehavioral outcomes in childhood. Econ Hum Biol. 2014;15:187–200. https://doi.org/10.1016/j.ehb.2014.09.002.
Colman I, Ataullahjan A, Naicker K, Van Lieshout RJ. Birth weight, stress, and symptoms of depression in adolescence: evidence of fetal programming in a national Canadian cohort. Can J Psychiatry. 2012;57:422–8.
Cosentino-Rocha L, Klein VC, Linhares MBM. Effects of preterm birth and gender on temperament and behavior in children. Infant Behav Dev. 2014;37:446–56. https://doi.org/10.1016/j.infbeh.2014.04.003.
Crump C, Sundquist J, Sundquist K. Preterm or early term birth and risk of autism. Pediatrics. 2021. https://doi.org/10.1542/PEDS.2020-032300.
Cserjési R, Van Braeckel KNJA, Butcher PR, Kerstjens JM, Reijneveld SA, Bouma A, et al. Functioning of 7-year-old children born at 32 to 35 weeks’ gestational age. Pediatrics. 2012;130:E838–46. https://doi.org/10.1542/peds.2011-2079.
Dahl LB, Kaaresen PI, Tunby J, Handegård BH, Kvernmo S, Rønning JA. Emotional, behavioral, social, and academic outcomes in adolescents born with very low birth weight. Pediatrics. 2006;118:e449–59. https://doi.org/10.1542/peds.2005-3024.
Dooley N, Clarke M, Cotter D, Cannon M. Birth weight and childhood psychopathology in the ABCD cohort: association is strongest for attention problems and is moderated by sex. Res child Adolesc Psychopathol. 2022;50:563–75. https://doi.org/10.1007/S10802-021-00859-0.
Duerden EG, Card D, Lax ID, Donner EJ, Taylor MJ. Alterations in frontostriatal pathways in children born very preterm. Dev Med Child Neurol. 2013;55:952–8. https://doi.org/10.1111/dmcn.12198.
Ernst M, Reiner I, Fieß A, Tibubos AN, Schulz A, Burghardt J, et al. Sex-dependent associations of low birth weight and suicidal ideation in adulthood: a community-based cohort study. Sci Rep. 2020;10:12969. https://doi.org/10.1038/S41598-020-69961-5.
Esteban-Cornejo I, Tejero-González CM, Castro-Piñero J, Conde-Caveda J, Cabanas-Sanchez V, Sallis JF, et al. Independent and combined influence of neonatal and current body composition on academic performance in youth: the UP & DOWN study. Pediatr Obes. 2015;10:157–64. https://doi.org/10.1111/ijpo.239.
Factor-Litvak P, Straka N, Cherkerzian S, Richards M, Liu X, Sher A, et al. Associations between birth weight, preeclampsia and cognitive functions in middle-aged adults. J Dev Orig Health Dis. 2011;2:365–74. https://doi.org/10.1017/S2040174411000596.
Farooqi A, Adamsson M, Serenius F, Hãgglöf B. Executive functioning and learning skills of adolescent children born at fewer than 26 weeks of gestation. PLoS ONE. 2016;11: e0151819. https://doi.org/10.1371/journal.pone.0151819.
Farooqi A, Hägglöf B, Sedin G, Gothefors L, Serenius F. Mental health and social competencies of 10- to 12-year-old children born at 23 to 25 weeks of gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics. 2007;120:118–33. https://doi.org/10.1542/peds.2006-2988.
Fevang SKE, Hysing M, Markestad T, Sommerfelt K. Mental health in children born extremely preterm without severe neurodevelopmental disabilities. Pediatrics. 2016. https://doi.org/10.1542/peds.2015-3002.
Gäddlin P-O, Finnström O, Samuelsson S, Wadsby M, Wang C, Leijon I. Academic achievement, behavioural outcomes and MRI findings at 15 years of age in very low birthweight children. Acta Paediatr Int J Paediatr. 2008;97:1426–32. https://doi.org/10.1111/j.1651-2227.2008.00925.x.
Grunau RE, Whitfield MF, Fay TB. Psychosocial and academic characteristics of extremely low birth weight (≤800 g) adolescents who are free of major impairment compared with term-born control subjects. Pediatrics. 2004;114:e725–32. https://doi.org/10.1542/peds.2004-0932.
Datta Gupta N, Deding M, Lausten M. The effect of low birth weight on height, weight and behavioral outcomes in the medium-run. Econ Hum Biol. 2013;11:42–55. https://doi.org/10.1016/j.ehb.2011.06.002.
Hack M, Youngstrom EA, Cartar L, Schluchter M, Gerry Taylor H, Flannery D, et al. Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics. 2004;114:932–40. https://doi.org/10.1542/peds.2003-1017-L.
Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–57. https://doi.org/10.1056/NEJMoa010856.
Hille ETM, Dorrepaal C, Perenboom R, Gravenhorst JB, Brand R, Verloove-Vanhorick SP. Social lifestyle, risk-taking behavior, and psychopathology in young adults born very preterm or with a very low birthweight. J Pediatr. 2008;152:793-800.e4. https://doi.org/10.1016/j.jpeds.2007.11.041.
Hosoki M, Bruckert L, Borchers LR, Marchman VA, Travis KE, Feldman HM. Associations of behavioral problems and white matter properties of the cerebellar peduncles in boys and girls born full term and preterm. Cerebellum. 2023;22:163–72. https://doi.org/10.1007/S12311-022-01375-7.
Huang C, Martorell R, Ren A, Li Z. Cognition and behavioural development in early childhood: the role of birth weight and postnatal growth. Int J Epidemiol. 2013;42:160–71. https://doi.org/10.1093/ije/dys207.
Jennische M, Sedin G. Gender differences in outcome after neonatal intensive care: speech and language skills are less influenced in boys than in girls at 6.5 years. Acta Paediatr Int J Paediatr. 2003;92:364–78. https://doi.org/10.1080/08035250310009310.
Johnson EO, Breslau N. Increased risk of learning disabilities in low birth weight boys at age 11 years. Biol Psychiatry. 1999;47:490–500. https://doi.org/10.1016/S0006-3223(99)00223-1.
Johnson S, Evans TA, Draper ES, Field DJ, Manktelow BN, Marlow N, et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed. 2015;100:F301–8. https://doi.org/10.1136/archdischild-2014-307684.
Kelly YJ, Nazroo JY, McMunn A, Boreham R, Marmot M. Birthweight and behavioural problems in children: a modifiable effect? Int J Epidemiol. 2001;30:88–94. https://doi.org/10.1093/ije/30.1.88.
Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513-520.e1. https://doi.org/10.1016/j.jpeds.2007.08.009.
Lagerström M, Bremme K, Eneroth P, Magnusson D. Sex-related differences in school and IQ performance for children with low birth weight at ages 10 and 13. J Spec Educ. 1991;25:261–70. https://doi.org/10.1177/002246699102500209.
Lahti J, Räikkönen K, Kajantie E, Heinonen K, Pesonen A-K, Järvenpää A-L, et al. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. J Child Psychol Psychiatry Allied Discip. 2006;47:1167–74. https://doi.org/10.1111/j.1469-7610.2006.01661.x.
Lee M-S, Huang L-Y, Chang Y-H, Huang STY, Yu H-L, Wahlqvist ML. Lower birth weight and diet in Taiwanese girls more than boys predicts learning impediments. Res Dev Disabil. 2012;33:2203–12. https://doi.org/10.1016/j.ridd.2012.06.008.
Leijon I, Bladh M, Finnström O, Gäddlin P-O, Nelson N, Hammar M, et al. Self-reported mental health and cortisol activity at 27–28 years of age in individuals born with very low birthweight. Acta Paediatr Int J Paediatr. 2020;109:948–58. https://doi.org/10.1111/apa.15093.
Liu Y, Mendonça M, Bartmann P, Wolke D. Very preterm birth and trajectories of domain-specific self-concept from childhood into adulthood. Dev Psychopathol. 2021;34:1926–37. https://doi.org/10.1017/S0954579421000432.
Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123:1037–44. https://doi.org/10.1542/peds.2008-1162.
Malatesta-Magai C, Leak S, Tesman J, Shepard B, Culver C, Smaggia B. Profiles of emotional development: individual differences in facial and vocal expression of emotion during the second and third years of life. Int J Behav Dev. 1994;17:239–69. https://doi.org/10.1177/016502549401700202.
Martel MM, Lucia VC, Nigg JT, Breslau N. Sex differences in the pathway from low birth weight to inattention/hyperactivity. J Abnorm Child Psychol. 2007;35:87–96. https://doi.org/10.1007/s10802-006-9089-9.
Møllegaard S. The effect of birth weight on behavioral problems in early adolescence: new evidence from monozygotic twins. Econ Hum Biol. 2020;36: 100828. https://doi.org/10.1016/j.ehb.2019.100828.
Momany AM, Kamradt JM, Ullsperger JM, Elmore AL, Nigg JT, Nikolas MA. Sex moderates the impact of birth weight on child externalizing psychopathology. J Abnorm Psychol. 2017;126:244–56. https://doi.org/10.1037/abn0000238.
Murray E, Matijasevich A, Santos IS, Barros AJD, Anselmi L, Barros FC, et al. Sex differences in the association between foetal growth and child attention at age four: specific vulnerability of girls. J Child Psychol Psychiatry Allied Discip. 2015;56:1380–8. https://doi.org/10.1111/jcpp.12422.
Nosarti C, Allin MP, Frangou S, Rifkin L, Murray RM. Hyperactivity in adolescents born very preterm is associated with decreased caudate volume. Biol Psychiatry. 2005;57:661–6. https://doi.org/10.1016/j.biopsych.2004.12.003.
Olafsen KS, Rønning JA, Kaaresen PI, Ulvund SE, Handegård BH, Dahl LB. Joint attention in term and preterm infants at 12 months corrected age: the significance of gender and intervention based on a randomized controlled trial. Infant Behav Dev. 2006;29:554–63. https://doi.org/10.1016/j.infbeh.2006.07.004.
Perricone G, Regina Morales M, Anzalone G. Neurodevelopmental outcomes of moderately preterm birth: precursors of attention deficit hyperactivity disorder at preschool age. Springerplus. 2013;2:1–7. https://doi.org/10.1186/2193-1801-2-221.
Pharoah POD, Stevenson CJ, Cooke RWI, Stevenson RC. Prevalence of behaviour disorders in low birthweight infants. Arch Dis Child. 1994;70:271–4. https://doi.org/10.1136/adc.70.4.271.
Portnoy S, Callias M, Wolke D, Gamsu H. Five-year follow-up study of extremely low-birthweight infants. Dev Med Child Neurol. 1988;30:590–8. https://doi.org/10.1111/j.1469-8749.1988.tb04796.x.
Potijk MR, De Winter AF, Bos AF, Kerstjens JM, Reijneveld SA. Behavioural and emotional problems in moderately preterm children with low socioeconomic status: a population-based study. Eur Child Adolesc Psychiatry. 2015;24:787–95. https://doi.org/10.1007/s00787-014-0623-y.
Pyhälä R, Lahti J, Heinonen K, Pesonen A-K, Strang-Karlsson S, Hovi P, et al. Neurocognitive abilities in young adults with very low birth weight. Neurology. 2011;77:2052–60. https://doi.org/10.1212/WNL.0b013e31823b473e.
Quesada AA, Tristão RM, Pratesi R, Wolf OT. Hyper-responsiveness to acute stress, emotional problems and poorer memory in former preterm children. Stress. 2014;17:389–99. https://doi.org/10.3109/10253890.2014.949667.
Ritter BC, Perrig W, Steinlin M, Everts R. Cognitive and behavioral aspects of executive functions in children born very preterm. Child Neuropsychol. 2014;20:129–44. https://doi.org/10.1080/09297049.2013.773968.
Rooney R, Hay D, Levy F. Small for gestational age as a predictor of behavioral and learning problems in twins. Twin Res. 2003;6:46–54. https://doi.org/10.1375/136905203762687898.
Sabet F, Richter LM, Ramchandani PG, Stein A, Quigley MA, Norris SA. Low birthweight and subsequent emotional and behavioural outcomes in 12-year-old children in Soweto, South Africa: findings from birth to twenty. Int J Epidemiol. 2009;38:944–54. https://doi.org/10.1093/ije/dyp204.
Salomäki S, Rautava P, Junttila N, Huhtala M, Leppänen MH, Nyman A, et al. Social functioning questionnaires of adolescents born preterm show average profiles and attenuated sex differences. Acta Paediatr. 2021;110:1490–7. https://doi.org/10.1111/APA.15728.
Salvan P, Froudist Walsh S, Allin MPG, Walshe M, Murray RM, Bhattacharyya S, et al. Road work on memory lane-functional and structural alterations to the learning and memory circuit in adults born very preterm. Neuroimage. 2014;102:152–61. https://doi.org/10.1016/j.neuroimage.2013.12.031.
Scott FE, Mechelli A, Allin MP, Walshe M, Rifkin L, Murray RM, et al. Very preterm adolescents show gender-dependent alteration of the structural brain correlates of spelling abilities. Neuropsychologia. 2011;49:2685–93. https://doi.org/10.1016/j.neuropsychologia.2011.05.016.
Sheehan JC, Kerns KA, Müller U. The effect of task complexity on planning in preterm-born children. Clin Neuropsychol. 2017;31:438–58. https://doi.org/10.1080/13854046.2016.1244248.
Stene-Larsen K, Lang AM, Landolt MA, Latal B, Vollrath ME. Emotional and behavioral problems in late preterm and early term births: outcomes at child age 36 months. BMC Pediatr. 2016;16:1–7. https://doi.org/10.1186/s12887-016-0746-z.
Kierulf Strømme K, Strømme P, Bjertness E, Lien L. Intrauterine growth restriction—a population-based study of the association with academic performance and psychiatric health. Acta Paediatr Int J Paediatr. 2014;103:886–91. https://doi.org/10.1111/apa.12657.
Taylor HG, Margevicius S, Schluchter M, Andreias L, Hack M. Persisting behavior problems in extremely low birth weight adolescents. J Dev Behav Pediatr. 2015;36:178–87. https://doi.org/10.1097/DBP.0000000000000139.
Thun-Hohenstein L, Largo RH, Molinari L, Kundu S, Duc G. Early fine motor and adaptive development in high-risk appropriate for gestational age preterm and healthy term children. Eur J Pediatr. 1991;150:562–9. https://doi.org/10.1007/BF02072208.
Tung I, Christian-Brandt AS, Langley AK, Waterman JM. Developmental outcomes of infants adopted from foster care: predictive associations from perinatal and preplacement risk factors. Infancy. 2020;25:84–109. https://doi.org/10.1111/infa.12319.
Van Lieshout RJ, Boylan K. Increased depressive symptoms in female but not male adolescents born at low birth weight in the offspring of a national cohort. Can J Psychiatry. 2010;55:422–30. https://doi.org/10.1177/070674371005500705.
Vederhus BJ, Markestad T, Eide GE, Graue M, Halvorsen T. Health related quality of life after extremely preterm birth: a matched controlled cohort study. Health Qual Life Outcomes. 2010;8:1–8. https://doi.org/10.1186/1477-7525-8-53.
Walker SM, Melbourne A, O’Reilly H, Beckmann J, Eaton-Rosen Z, Ourselin S, et al. Somatosensory function and pain in extremely preterm young adults from the UK EPICure cohort: sex-dependent differences and impact of neonatal surgery. Br J Anaesth. 2018;121:623–35. https://doi.org/10.1016/j.bja.2018.03.035.
Wolke D, Samara M, Bracewell M, Marlow N. Specific language difficulties and school achievement in children born at 25 weeks of gestation or less. J Pediatr. 2008;152:256-262.e1. https://doi.org/10.1016/j.jpeds.2007.06.043.
Ye J, Wu C, Chu X, Wen Y, Li P, Cheng B, et al. Evaluating the effect of birth weight on brain volumes and depression: an observational and genetic study using UK Biobank cohort. Eur Psychiatry. 2020;63:e73. https://doi.org/10.1192/J.EURPSY.2020.74.
Hall J, Jaekel J, Wolke D. Gender distinctive impacts of prematurity and small for gestational age (SGA) on age-6 attention problems. Child Adolesc Ment Health. 2012;17:238–45. https://doi.org/10.1111/j.1475-3588.2012.00649.x.
Broere-Brown ZA, Adank MC, Benschop L, Tielemans M, Muka T, Gonçalves R, et al. Fetal sex and maternal pregnancy outcomes: a systematic review and meta-analysis. Biol Sex Differ. 2020;11:26. https://doi.org/10.1186/s13293-020-00299-3.
Chin EH, Christians JK. When are sex-specific effects really sex-specific? J Dev Orig Health Dis. 2015;6:438–42. https://doi.org/10.1017/S2040174415001348.
Christians JK, Shergill HK, Albert AYK. Sex-dependent effects of prenatal food and protein restriction on offspring physiology in rats and mice: systematic review and meta-analyses. Biol Sex Differ. 2021;12:21. https://doi.org/10.1186/s13293-021-00365-4.
Schroll JB, Moustgaard R, Gøtzsche PC. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med Res Methodol. 2011;11:1–8. https://doi.org/10.1186/1471-2288-11-22/FIGURES/5.
Sentenac M, Twilhaar S, Benhammou V, Morgan AS, Johnson S, Chaimani A, et al. Heterogeneity of design features in studies included in systematic reviews with meta-analysis of cognitive outcomes in children born very preterm. Paediatr Perinat Epidemiol. 2023;37:254–62. https://doi.org/10.1111/PPE.12957.
Verburg PE, Tucker G, Scheil W, Erwich JJHM, Dekker GA, Roberts CT. Sexual dimorphism in adverse pregnancy outcomes—a retrospective Australian population study 1981–2011. PLoS ONE. 2016;11: e0158807. https://doi.org/10.1371/journal.pone.0158807.
Al-Qaraghouli M, Fang YMV. Effect of fetal sex on maternal and obstetric outcomes. Front Pediatr. 2017;5:144. https://doi.org/10.3389/fped.2017.00144.
Orzack SH, Stubblefield JW, Akmaev VR, Colls P, Munné S, Scholl T, et al. The human sex ratio from conception to birth. Proc Natl Acad Sci USA. 2015;112:E2102–11. https://doi.org/10.1073/pnas.1416546112.
Acknowledgements
We thank two anonymous reviewers for constructive comments.
Funding
This study was funded by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (JKC; Grant Number RGPIN-2016-04047 and RGPIN-2021-02853). The funding bodies had no role in study design, collection and interpretation of data or in manuscript preparation.
Author information
Authors and Affiliations
Contributions
JKC performed the literature searches, AB, GMP, JKC, and SA screened titles and abstracts, AB, GMP, JKC, and RH examined full texts, JKC, MAM, and NMN extracted data, and AB and EVL assessed studies for quality and risk of bias. JKC conceived of the study, performed the meta-analyses, and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was based on published work and, therefore, did not require approval by an institutional committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search strategy.
Additional file 2.
Additional Tables S1–S4.
Additional file 3: Figure S1.
Effect of age as a moderator on the effect of severe prematurity/low birthweight on cognitive function. Squares and circles are estimates for males and females, respectively, and marker size indicates weight. Figure S2. Effect of age as a moderator on the effect of moderate prematurity/low birthweight on cognitive function. Figure S3. Effect of age as a moderator on the effect of severe prematurity/low birthweight on internalizing problems. Figure S4. Effect of age as a moderator on the effect of severe prematurity/low birthweight on externalizing problems. Figure S5. Effect of age as a moderator on the effect of moderate prematurity/low birthweight on externalizing problems. Figure S6. Funnel plot of residuals (observed–fitted values) and standard errors for the effect of severe prematurity/low birthweight on cognitive function. Squares and circles are estimates for males and females, respectively, and marker size indicates weight. Figure S7. Funnel plot for the effect of moderate prematurity/low birthweight on cognitive function. Figure S8. Funnel plot for the effect of severe prematurity/low birthweight on internalizing problems. Figure S9. Funnel plot for the effect of severe prematurity/low birthweight on externalizing problems. Figure S10. Funnel plot for the effect of moderate prematurity/low birthweight on externalizing problems.
Additional file 4.
Additional results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Christians, J.K., Ahmadzadeh-Seddeighi, S., Bilal, A. et al. Sex differences in the effects of prematurity and/or low birthweight on neurodevelopmental outcomes: systematic review and meta-analyses. Biol Sex Differ 14, 47 (2023). https://doi.org/10.1186/s13293-023-00532-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13293-023-00532-9