Abstract
Background
Biological factors are known to influence disease trajectories and treatment effectiveness in alcohol addiction and preclinical and clinical evidence suggests that sex is an important factor influencing disease dynamics in alcohol dependence. Another critical factor is age at first intoxicating drink, which has been identified as a risk factor for later alcohol binging. Preclinical research allows prospective monitoring of rodents throughout the lifespan, providing very detailed information that cannot be acquired in humans. Lifetime monitoring in rodents can be conducted under highly controlled conditions, during which one can systematically introduce multiple biological and environmental factors that impact behaviors of interest.
Methods
Here, we used the alcohol deprivation effect (ADE) rat model of alcohol addiction in a computerized drinkometer system, acquiring high-resolution data to study changes over the course of addictive behavior as well as compulsive-like drinking in cohorts of adolescent vs. adult as well as male vs. female rats.
Results
Female rats drank more alcohol than male rats during the whole experiment, drinking much more weak alcohol (5%) and similar amounts of stronger alcohol solutions (10%, 20%); female rats also consumed more alcohol than male rats during quinine taste adulteration. Increased consumption in females compared to males was driven by larger access sizes of alcohol. Differences in circadian patterns of movement were observed between groups. Early age of onset of drinking (postnatal day 40) in male rats had surprisingly little impact on the development of drinking behavior and compulsivity (quinine taste adulteration) when compared to rats that started drinking during early adulthood (postnatal day 72).
Conclusions
Our results suggest that there are sex-specific drinking patterns, not only in terms of total amount consumed, but specifically in terms of solution preference and access size. These findings provide a better understanding of sex and age factors involved in the development of drinking behavior, and can inform the preclinical development of models of addiction, drug development and exploration of options for new treatments.
Plain language summary
Various factors can influence the development of alcohol addiction, but studying these factors in humans over the long-term is challenging and costly. With modern sensing technologies, rodents can be monitored throughout the lifespan, providing detailed information obtained under controlled conditions. Previous research suggests sex- and age-dependent differences in addiction processes, with female rats consuming more alcohol and age at first drink resulting in heavier later consumption, but a better characterization of these is needed. Using a rodent model of addiction and relapse, collecting high-resolution longitudinal drinking data in a computerized system over ~ 11 months, we studied differences in the development of addiction and compulsive-like drinking in male vs female as well as adult vs adolescent rats. Female rats drank more alcohol than male rats during the whole experiment, drinking much more weaker alcohol (5%) and similar amounts of stronger alcohol solutions (10%, 20%); female rats also consumed more alcohol than male rats in an aversive taste challenge, displaying more compulsive-like drinking. Increased consumption in females compared to males was driven by larger amounts consumed per approach. Little effect of age of onset of drinking was observed. Our results suggest sex-specific differences in the development of drinking patterns and solution preference, not only in terms of total amount consumed. These findings highlight the importance of awareness of sex-specific factors when developing models of addiction, as well as eventual treatment strategies and interventions.
Highlights
-
female rats show more pronounced compulsive-like behavior in response to quinine taste adulteration
-
female rats consumed more alcohol than rats throughout the longitudinal experiment (~ 11 months), driven by differences in access size and frequency of accesses
-
adolescent and adult male rats did not differ in terms of drinking behavior and compulsive-like drinking
-
sex differences in locomotor activity and circadian patterns were observed, with female rats moving more and having later peak activity in the day
Similar content being viewed by others
Background
Excessive alcohol use can have serious effects on health and is a leading cause of preventable death worldwide [1]. Alcohol is the most common substance for which addiction criteria are met. Addiction is characterized by craving, loss of control of amount of or frequency of use, compulsion to use and continued use despite adverse consequences [2]. While some people can consume large amounts of alcohol without coming to harm, others experience ongoing addiction-related problems.
Clinical and preclinical research has documented sexually dimorphic effects of alcohol exposure through life, and both developmental processes and treatment effectiveness may differ accordingly [3]. Epidemiological investigations suggest that men have higher 12 month (17.6%) and lifetime (36%) prevalences for Alcohol Use Disorder (AUD) than women (10.4, 22.7%, respectively) [4], but this may be due to opportunity rather than specific vulnerability [5, 6]. Although men are more likely to drink alcohol and consume excessive amounts, in women who drink excessively, biological differences (e.g., body size, structure, chemistry, etc.) are thought to lead women to absorb more alcohol and take longer to metabolize it [7], leading to more immediate as well as longer lasting effects. Women become addicted to alcohol more rapidly and at lower doses and have a faster progression to dependence [8]; research has identified higher risk of liver diseases, accelerated alcohol-related cognitive decline and shrinkage of the brain in women compared to men [9]. Indeed, in recent years it has been suggested that the ‘gender gap’ in drinking is decreasing [10] and has almost closed [11, 12]. While preclinical research has in large part used adult male animals in models of addiction and its treatment, there have been an increasing number of studies examining the development of alcohol drinking behavior in females, finding that females acquire self-administration of alcohol more rapidly and consume more alcohol, but have reduced severity of withdrawal symptoms than males, possibly tied to differential sex hormones [6, 13, 14].
Alcohol is widely used in youth and underage drinking is a serious problem, and early onset of drinking increases alcohol use in adulthood [15, 16]. Youth drink less often than adults but binge when they do; 90% of alcohol drinks consumed by youth are during binge drinking [17]. This can have serious health consequences as adolescent alcohol use can cause long-term changes in brain function and also brain structure [18, 19], and it has been reported that alcohol misuse is the leading cause of death for youth (15–24 years old, [20]). Human and rodent studies have both found that adolescent ethanol exposure results in deficits during adulthood, including cognitive impairments and altered development of gray and white matter [18, 19]. A facilitatory effect of adolescent exposure to alcohol on adult intake has been suggested in several studies [21, 22], but there are also conflicting reports showing no increased consumption [23] or sensitivity to aversion resistance [24]. There are also reports suggesting that age of drinking onset is not a strong predictor of prospective alcohol intake and relapse-like drinking [25].
Preclinical models of voluntary drug intake offer the ability to study the longitudinal development of addiction processes [26] and have the advantage of controlling for contributing individual genetic, behavioral and environmental influences seen in people [12]. Rats develop quickly and are considered sexually mature around 6 weeks of age; one day for a rat is the approximate equivalent of one month of human life [27]. The alcohol deprivation effect (ADE) paradigm is a longitudinal model of alcohol addiction development in which rats are given voluntary access to different concentrations of alcohol [28]. The experimental procedure consists of repeated deprivation and reintroduction phases; reintroduction phases approximate relapse after abstinence and over time, rats show addiction-like drinking patterns, developing increased preference for stronger solutions of alcohol. In addition, once addiction-like behavior has stabilized, rats also develop compulsive-like drinking (aversion resistance, e.g., taste adulteration of alcohol solutions using bitter quinine), which is one important measure of addiction [29, 30].
Using modern high-resolution sensing and recording systems, it is possible to follow rats as they develop addiction-like behavior [28], building profiles to understand the development of drinking behavior, response to treatment [31], and compulsive drinking at a greater level of detail [32]. There is a continued need for animal models to characterize compulsive alcohol intake towards the identification of mechanisms that promote pathological drinking [33], and it is particularly important to extend this research to female and adolescent groups.
In the present study, we examined the longitudinally assessed drinking behavior of female and male (adult and adolescent) rats acquired via digital “drinkometer” as they underwent a four bottle (H2O, 5%, 10%, 20% ethanol) free-choice ADE paradigm (5 cycles over ~ 11 months) and a subsequent quinine challenge. The drinkometer system continuously measured alcohol consumption at high resolution; locomotor activity was also concurrently acquired. We characterized and compared how drinking and movement behavior in the different groups evolved over the experiment, taking a closer look at consumption patterns (e.g., breaking down consumption by solution strength) during a regular ADE and during the quinine challenge.
Methods
Animals
Drinking and locomotor data from n = 30 adult male Wistar rats (postnatal day (PND) = 72) are reported in [32]. Data from adult females (n = 14; PND 66) and adolescent males (n = 16, PND 44) from the breeding colony at the CIMH were collected and included. The CIMH Wistar rat line was developed at the Max-Planck-Institute for Psychiatry in Munich ("Crl:WI(Han)" (RS:0001833)) and has been selectively bred at the CIMH Mannheim for a robust alcohol drinking and ADE phenotype for over 15 years. Adult females and adolescent males were housed in the same experimental room.
At the outset of the experiment, a group of female adolescent rats was also included. However, it was soon discovered that these adolescent females were hyperactive in the drinkometer setup and kept knocking the alcohol solution and water bottles out of the system, compromising the data measurement and posing an ethical issue as constant access of water could not be provided. The decision was made to continue the experiment without adolescent females.
Rats were housed individually in standard rat cages (Eurostandard Type III; Ehret) dimensions: top (outside) 425 mm × 276 mm, bottom (inside) 390 mm × 230 mm, height: 153 mm, floor area: ~ 820 cm2 with feeders at the cover and followed by a 5-cm raised area, on a 12 h:12 h light–dark cycle (lights on at 07:30). During the experiment, all rats had ad libitum access to standard laboratory rat food (LASQCdiet® Rod16-Auto, Lasvendi and soy-free) and tap water. Bottles hold 250 ml of liquid with ball nipples. The light intensity was max 130 lx in the drinking room and 50 lx within every cage. The relative humidity in all rooms was 45–65% and room temperature was constant between 22 and 24 °C. Bedding material in the cages had steam-sterilized aspen wood (2–3 mm, Abedd) with no environmental enrichment. Cages were changed once per week.
Our health monitoring program checks on all FELASA-recommended pathogens together with an external CRO (mfd Diagnostics) at a 3-month interval. We use at least one bedding/food- and water sentinel rat/per holding room. Experimental procedures were conducted according to the ethical guidelines for the care and use of laboratory animals. Experiments were approved by the local animal care committee (Regierungspräsidium Karlsruhe, Germany: AZ:35-9185.81/G-227/20).
ADE paradigm, drinkometer system, quinine challenge
The ADE paradigm was carried out as previously described, with drinking behavior recorded using a digital drinkometer system implemented in the home cage [28]. Rats first underwent a long-term (4 weeks) period of baseline voluntary alcohol consumption. During this time, they are presented with four bottles, containing three different concentrations of EtOH (5%, 10%, 20%, prepared using 96% EtOH; VWR international #83804.360 diluted to the correct concentration) and water. Positions of bottles were changed regularly to avoid place preference.
After the first baseline period, rats are deprived of alcohol for 2 weeks, which is followed by reintroduction of alcohol. Upon reintroduction, the alcohol deprivation effect (ADE), a robust increase in alcohol drinking as well as a shift to stronger solutions, is observed. This process, with successive 2-week deprivations and 4-week reintroduction periods, was repeated 4 more times, for a total of 5 ADE cycles.
In the earlier study in adult male rats [32], during the ADE6 cycle, a preliminary quinine test of different concentrations (0.01, 0.03 and 0.05 g/L) was conducted, finding that 0.05 g/L was appropriate for use in the drinkometer system bottles and had a larger effect than 0.01 and 0.03 g/L on reducing consumption during the ADE. A full quinine challenge was conducted during ADE7 using 0.05 g/L for the first three days of reintroduction. In the adult females and adolescent males, given the previous results, no preliminary test was performed; the quinine challenge was then conducted using 0.05 g/L during the first 3 days of reintroduction during ADE6.
Data collection
A computerized Drinkometer system (TSE Systems) which records liquid consumption by amount continuously was used to monitor drinking behavior. The system is implemented in standard rat home cages (Eurostandard Type III) and has four drinking stations to enable choice of solution. Each of these consists of a glass vessel containing a liquid, and a high-precision sensor that detects the amount of liquid removed from the vessel. Special bottle caps are used to prevent evaporation and spills. Bottle weights are measured in 200-ms steps and can be registered every second, and the system can detect volume changes as small as 0.01 g. For these experiments, the sampling interval was set at 1 min.
Locomotor activity was monitored using an activity detection sensor (Mouse-E-Motion, Infra-e-motion) mounted above each cage. These devices use an infrared sensor to detect rat body movements of min. 1.5 cm at any position inside the cage at the resolution of 1 s. For these experiments, locomotor activity was registered in 5-min bins.
Statistical analysis
Data were examined over the whole experiment and at the individual ADE levels. Analyses were conducted in R v3.63, and IBM SPSS 27.
The weekly level was examined to investigate overall developmental patterns of drinking and movement behavior. Average daily drinking during each BL and ADE week was calculated. In the adult females and adolescent males, technical issues led to lost data during the 4th BL period. In the young males, drinking data from 4 rats were lost from ADE4 until the end of the experiment.
To examine group differences during the whole experiment (from BL1 to BL6), a random-intercepts mixed model was used. In this model, consumption was specified as the dependent variable. Group (female, male, adolescent male) and period were specified as fixed factors. Restricted maximum likelihood estimation was used, with a diagonal covariance structure, which assumes heterogeneous variance and no correlation between time points. Pairwise comparisons were examined using estimated marginal means with Bonferroni correction.
ADE1, ADE5 and ADE with quinine (ADEQ) were further considered as the periods of interest. The first ADE has previously been shown to be an initial transition towards addiction-like behavior [34], while ADEs are thought to stabilize by the 5th ADE. ADEQ was examined to study compulsive-like drinking behavior. During these periods, magnitude of ADEs were quantified by comparing total consumption (i.e., sum of all solutions) on the first day of reintroduction (the ADE) to the average consumption on the last 3 days of baseline (BL) using paired T-tests (Cohen’s d was calculated for significant comparisons). Welch’s tests were used to compare groups to account for uneven sample sizes (variances), with effect size calculated using omega squared (ω2). Pairwise multiple comparisons tests with Games–Howell post hoc tests were performed to compare groups (females, males, adolescent males).
Drinking profiles were calculated for each rat. Consumption (g/kg) of each solution (5%, 10% and 20%) was calculated and total consumption (g/kg) was calculated by summing the solutions. Furthermore, we calculated the average access size and frequency of consumption of the different solutions (during the first day of ADE).
During ADEQ, compulsive-like drinking was quantified using percent change versus baseline of the same ADE cycle (BLQ). That is:
In addition, for an additional measure of compulsivity, comparing the size of the ADEQ to a regular one, we calculated the difference of magnitude in change:
Locomotor activity
Locomotor activity was examined to characterize the different phases in a similar way to drinking data. Mean activity levels were calculated hourly and daily during the whole experiment and different periods. Association of hourly drinking by solution with locomotor activity was also examined. Mixed models were used to test for group differences as above.
In addition, we explored the circadian pattern of rats using a cosinor analysis. Cosinor analysis is often used to model circadian patterns in time series [35]. The package “ActCR” implemented in R was used to calculate the mesor (rhythm adjusted mean activity), amplitude (half the extent of predictable variation during a cycle) and acrophase (time to reach peak activity) of hourly locomotor activity during ADE1, ADE5, and ADEQ. For these analyses, 5 days of data (e.g., 120 h) were used. (The first day of ADE until midnight was not used given the effect of the experimenter entering the room to change alcohol bottles.) For mesor, amplitude and acrophase, Welch’s tests were used to compare differences between groups with multiple comparisons using Games–Howell post hoc tests.
Locomotor activity and drinking
The association between hourly locomotor activity and alcohol consumption during ADE5 and ADEQ were examined using random-intercepts mixed models. Locomotor activity was specified as the dependent variable, with 5%, 10%, and 20% consumption (g/kg) specified as independent variables. Separate models were calculated for the different groups.
Results
General drinking behavior between groups
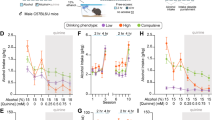
The weekly total alcohol consumption is illustrated in Fig. 1a, showing the average daily total consumption in different groups by week phase during the experiment. Significant effects of group (F(2,55.76501) = 15.382, p < 0.001) and period (increased consumption over time, F(1,312.017) = 53.194, p < 0.001) were observed. Adult female rats drank significantly higher amounts of alcohol than both adult male and adolescent male rats (both p < 0.001) throughout the duration of the experiment, descriptively starting even at the first BL. Adolescent males (starting drinking on PND44) and adult males (starting drinking on PND72) had similar drinking patterns throughout the entire experiment and consumption did not significantly differ (n.s.).
Drinking behavior between groups throughout the experiment. The total alcohol consumption a as well as individual bottles (b 5% c 10% and d 20%) are summarized for the different baseline (BL) and relapse (ADE) phases of the experiment. Data are presented as mean values ± SEM. Gray bars represent experimental deprivation phases, where only water was accessible
Underlying this, as shown in Fig. 1b–d, the higher levels of drinking observed in females compared to the other two groups was due to a significantly higher consumption of 5%.
An effect of group on 5% drinking (F(2,56.798) = 8.044, p < 0.001) was observed. Females drank significantly more 5% than males (p < 0.001), and adolescent males (p = 0.038). Descriptively, an increase in 5% drinking in females was seen after the first ADE. 5% drinking between males did not significantly differ throughout the experiment (n.s.). An effect of period was not observed (n.s.). For both 10% and 20%, effects of period were observed (increased drinking over time, 10% (F(1,207.211) = 32.615, p < 0.001; 20% (F(1,229.385) = 35.604, p < 0.001), but no effect of group (both n.s.)
Comparison of ADEs over time
In ADE1, significant increases in intake (ADEs) were observed in all groups: females (F) (t(13) = − 6.660, p < 0.001, Cohen’s d = 1.780), males (M) (t(29) = − 10.116, p < 0.001, d = 1.847) and adolescent males(AM) (t(15) = − 12.095, p < 0.001, d = 3.024) (Fig. 2a). Group differences were observed in both BL1 (Welch (2,27.685) = 3.554, p = 0.042, ω2 = 0.065) and ADE1 (Welch(2,33.247) = 4.117, p = 0.025, ω2 = 0.075), driven by differences between adult males and females (BL1: p = 0.070 (n.s.); ADE1, p = 0.030); drinking in adult and adolescent males did not significantly differ (both n.s.).
In ADE5, group differences continued to be observed (BL5 Welch (2,20.952) = 8.647, p = 0.002, ω2 = 0.285; ADE5: Welch (2,16.565) = 12.181, p = 0.001, ω2 = 0.376) drinking in females was much higher than in adult (BL5: p = 0.001; ADE5: p < 0.001) and adolescent males (BL5: p = 0.005, ADE5: p = 0.024) (Fig. 2b). BL and ADE drinking in adolescent and adult males did not significantly differ (n.s.). Significant ADEs were observed in all groups (all p < 0.001; F: t(13) = − 4.572, d = 1.222, M: t(28) = − 9.242, d = 1.716, AM: t(10) = − 8.978, d = 2.707). We note that the average magnitude of ADEs was larger in males than females (M: + 62%, AM: + 76%, F: + 38.5%), potentially due to a ceiling effect.
With the introduction of quinine as compulsivity measure during ADEQ (Fig. 2c), sizes of ADEs in all rats were decreased; only in adult males was a significant ADE (i.e., compulsive-like drinking) observed (t(29) = − 2.289, p = 0.030, d = 0.418; AM (t(10) = − 1.899, p = 0.087; F (t(13) = − 1.033, p = 0.321), however the larger sample size likely plays a role in this statistical significance. As in other periods, group differences were observed (BLQ: Welch(2,22.852) = 4.655, p = 0.020, ω2 = 0.195; ADEQ: Welch(2,20.665) = 3.995, p = 0.034, ω2 = 0.208) and the amount of drinking was higher in females than both adult males (BLQ: p = 0.042; ADEQ: p = 0.033), and adolescent males (BLQ: p = 0.016; ADEQ: p = 0.035), with no significant differences between adult and adolescent males observed (n.s.).
Comparing regular and quinine ADEs by individual alcohol solutions
Differences between last regular relapse (ADE5) and the following quinine-adulterated relapse (ADEQ) are depicted in Fig. 3. For the 5% bottle, significant differences were observed during ADE5 (Welch(2,19.369) = 3.858, p = 0.039, ω2 = 0.180), i.e., higher consumption in females than males (p = 0.030) and adolescent males (p = 0.068, n.s.) were observed. These differences between groups disappeared in ADEQ (Welch (2,18.814) = 2.665, p = 0.173).
Significant decreases between ADE5 and ADEQ were observed in all groups (M: t(28) = 6.427, p < 0.001, d = 1.193; AM: (t(9) = 2.371, p = 0.042, d = 0.750, F: t(14) = 4.410, p < 0.001, d = 1.139).
For the 10% bottle, consumption decreased in all groups but this decrease did not reach significance (M: t(28) = 2.028, p = 0.052, AM: t(9) = 2.126, p = 0.062, F: t(14) = 0.325, p = 0.750). No significant group differences were observed.
For 20%, descriptively, consumption increases in females (the most pronounced, but with high variance) and adult males were observed; while a decrease was observed in adolescent males, none of these changes reached significance. No significant group differences were observed.
Access sizes and frequency of accesses in regular ADE and ADEQ
Looking at ADE5 (Fig. 4a), access sizes differed between groups for 5% (Welch (2,18.044) = 3.732, p = 0.044) but not 10% and 20% and were largest in females for all solutions. This was similar during ADEQ, effects of group approached but did not reach significance for 5% (Welch (2,18.354) = 2.920, p = 0.079) and 20% (Welch (2,19.519) = 3.124, p = 0.067). Comparing only adult and male and female rats found that female rats had significantly larger access sizes of 5% during ADE5 (Welch (1,15.666) = 6.407, p = 0.022) and both 5% and 20% during ADEQ (5% Welch (1,15.964) = 5.251, p = 0.036; 20% Welch (1,18.194) = 6.458, p = 0.020)).
When comparing across ADEs, in ADEQ (Fig. 4b), access sizes of 5% decreased in all animals compared to ADE5 (adult males: p < 0.001, d = 1.914; adolescent males: p = 0.002, d = 1.510; females: p = 0.028, d = 0.692). Only adolescent males experienced a significant decrease in 10% access size (p = 0.002, d = 1.282), and no group had significant decreases in 20% access size (adolescent males and females had non-significant increases).
In ADE5 (Fig. 4c), no effect of group was observed on frequency of accesses, but descriptively, the frequency of 5% accesses was highest in females, while frequency of 10% accesses was higher in the male groups. In ADEQ, no effect of group on frequency of accesses was observed.
Comparing across ADEs, in ADEQ (Fig. 4d), compared to ADE5, frequency of accesses was more affected than access size. Notably, the frequency of 5% accesses in females decreased almost threefold (p = 0.003, d = 0.965), while 10% and 20% increased but not significantly. In adolescent males, frequencies of accesses to all solutions did not change significantly. In adult males, the frequency of 5% decreased (p < 0.001, d = 1.063), while 10% decreased non-significantly; frequency of 20% accesses increased (p = 0.023, d = 0.438).
Compulsive-like drinking at the individual level
In order to assess the individual level of compulsive-like drinking in the three groups, we compared relapse rates of quinine-adulterated ADEs to baseline consumption at the same cycle, as well as the previous regular ADE. This painted a different picture of distributions of the different groups. Looking at %change during ADEQ, all groups had rats who exhibited compulsive-like drinking (i.e., positive change > 0%; Fig. 5a), and means did not significantly differ between groups (Welch (2,16.990) = 0.969, p = 0.400), although we note high variability in the female group. Looking at the magnitude of change between ADEQ and ADE5, an effect of group was observed (Fig. 5b, Welch (2,22.526) = 5.747, p = 0.010, ω2 = 0.159), driven by females showing greater magnitude of change between ADEs than males (p = 0.006). Descriptively, using this metric, we also observe that male rats could be naturally broken into two “more” and “less” compulsive-like groups (i.e., around − 75% change, Fig. 5b).
Overall locomotor activity
Average daily locomotor counts during the whole experimental period were higher in females, lower in adolescents, and lower still in adult males (main effect of group F(2,54.769) = 31.759, p < 0.001; pairwise comparisons all p < 0.01). Over the whole experimental period, activity decreased in all groups (F(1,322.729) = 81.530, p < 0.001), descriptively, the most in adolescent males.
Daily movement patterns
Examining hourly locomotor activity on the daily (Fig. 6) and hourly (Fig. 7) levels during ADE1, it was observed that females and adolescent males initially had similar movement patterns, while adult males moved less. By ADE5 movement in adolescent males had decreased to a level more similar to that in adult males; female activity levels remained comparatively constant.
During ADEQ, activity in both male groups was similar and comparable to activity during ADE5. While still higher than males, female activity during ADEQ was reduced when compared to ADE5.
Locomotor activity and drinking
In ADE5 in adult males, significant associations were seen between locomotor activity and consumption of 5% (F(1,3001.401) = 69.578, p < 0.001) and 10% (F(1,3003.060) = 11.371, p = 0.001), but not 20% solutions (n.s.). In ADE5 in females and adolescent males, significant associations were seen between locomotor activity and consumption of all solutions (F: 5% F(1,1139.227) = 358,193; 10% F(1,1147,820) = 128.821;20% F(1,1146.265) = 34.911 all p = 0.001; AM: 5% F(1,1105.858) = 157.117; 10% F(1,1172.229) = 114.826, 20% F(1,1180.917) = 59.629, all p = 0.001). In all groups at ADE5, association effects were stronger with weaker solutions (5% > 10% > 20%).
During ADEQ, significant associations were seen between locomotor activity and consumption of all solutions in all groups (M: 5% F(1,2195.349) = 38.227, p < 0.001; 10% F(1,2688.383) = 7.564, p = 0.006; 20% F(1,2943.603) = 5.853, p = 0.016; AM: 5% F(1,1523.208) = 248.750; 10% F(1,1530.938) = 223.852; 20% F(1,1529.931) = 189.271, all p < 0.001; F: 5% F(1,1334.724) = 113.974; 10% F(1,1339.266) = 123.806; 20% F(1,1338.372) = 94.576, all p < 0.001). Association strength in both groups of males followed the pattern (5% > 10% > 20%), but in females, was 10% > 5% > 20%.
Cosinor analysis
Figure 8 shows the mesor, amplitude and acrophase as determined by cosinor analysis for the different periods. Significant group differences were observed in all measures during all periods (all p < 0.005). Females had significantly higher mesor and amplitude than males during all periods (all p < 0.01). Descriptively, adolescent males began the experiment with more movement rhythms to females and finished with more similar patterns to adult males. We note that the acrophase of female rats was later in the day compared to both groups of males (in ADE1: adult males p = 0.002; in ADE5: adolescent males p = 0.001; in ADEQ: both males p < 0.05) and this can also be seen in the hourly activity traces in Fig. 7.
Discussion
In the present study, we investigated sex and age differences in the development of alcohol consumption and compulsive-like drinking. Our results point to considerably different male and female consumption patterns over time, differences which were also reflected in response to alcohol adulteration with quinine. Adolescent and adult males developed largely similar patterns of consumption.
Females consumed more alcohol than males during the whole experiment, which is in line with the wider literature; our longitudinal results are in line with the idea of a “telescoping effect”, which suggests an accelerated course in females vs. males in the development of substance use disorders [36]. Interestingly, this difference was already apparent prior to the first ADE, which is consistent with the idea of the presence of different innate biological mechanisms promoting intake [37], and possibly the mediation of rewarding effects of alcohol by sex hormones [38]. While females consumed similar amounts of 10% and 20% as males, females consumed the 5% alcohol solution in much higher amounts. It is possible that this reflected taste preference; the 5% solution is slightly sweet and it has been shown that female rats prefer sweet solutions compared to male rats [39]. Interestingly, the difference in 5% between males and females widened considerably after the first ADE cycle.
Other preclinical research has found that female rats consume equal or larger amounts of alcohol than male rats, dependent on strain and drinking paradigm [40]. Research into potential causes for these differences have found that ovariectomized female rats show no clear-cut change in ethanol intake [41, 42] but do have decreased rewarding effects of ethanol [38]. It is also reported that the estrous cycle itself does not affect alcohol drinking [40], nor the aversive effects of ethanol [38], nor does it account for sex differences in related behavior [21]. On the other hand, it is suggested that there are sex-dependent effects of ethanol on neuronal activity [43, 44], adaptations in diverse brain regions [45], underlying neuroimmune transcriptional signatures and related signaling pathways [46], as well as genetic effects [47] which contribute to differences in alcohol-related behaviors.
Female rats continued to consume more alcohol than males with the introduction of quinine; a few female rats even consumed more alcohol with quinine than in the regular ADE. This is consistent with prior reports of females having enhanced sensitivity to rewarding but blunted sensitivity to aversive effects of ethanol [46, 48, 49]. Previous research suggests that while higher concentrations of quinine are tolerated in female rats, there is no difference in quinine sensitivity [50]. Also, another recent study found that higher concentrations of quinine were needed to suppress ethanol consumption in female as compared to male mice [51]. It has been suggested that this aversion resistance may be related to underlying sex-specific differences in neurocircuitry [52] and genetic factors [47].
Looking at the different solutions, we observed that quinine decreased the consumption of 5% in all rats, with a large effect in female rats (as they had consumed the most 5%). Consumption of 10% was also decreased in all groups, whereas 20% consumption increased in adult animals but not adolescent males (an n.s. decrease). This suggests a possible age-related difference in the development of compulsive-like drinking; it could be that the compulsive-like drinking is not yet developed in younger rats, even as they show the similar consumption patterns to adult males during regular ADEs. In this study, we did not observe that adolescent male rats drank more alcohol or stronger alcohol than their adult male counterparts, which at first glance appears to come in contrast with literature finding that adolescent rats drink more per approach than adults and have more binge-like events [53,54,55]. Many reports have suggested that adolescent ethanol exposure leads to decreased sensitivity to aversive effects and increased sensitive to rewarding properties of alcohol later in life [56], but there are also reports which like the present, do not find these effects of early alcohol exposure [24]. It must be kept in mind that studies have made observations using different paradigms (e.g., 2 bottle choice with 15% alcohol and water; providing different schedules of alcohol access, different routes/types of administration (voluntary/forced)), or during more acute periods of time (e.g., 30 days) than in the present study, or with adolescent rats of different age. In this context, our results support the idea that age and sex are important factors to consider but also that direction and degree of effects observed are also likely to be dependent on specific experimental parameters [12].
One strength of the high-resolution multi-bottle approach is that it gives deeper insight into the mechanisms underlying behavioral effects of drugs and interventions. When comparing access sizes and frequency of accesses of specific solutions, we found differential patterns across groups and regular/quinine ADEs. Access sizes of all solutions were the largest in females. As previously shown [32], the effect of quinine was most apparent on the access size and frequency of accesses to the 5% solution. This effect was observed in all groups and especially prominent in the frequency of accesses in female rats; their original higher frequency of approaches 5% meant that they were the most affected. In contrast, in all animals 10% and 20% were less affected; only in adult males was there a significant increase in frequency of access to 20%. Looking at compulsive-like drinking at the individual level, we find that there is a wider variation of drinking patterns in females than the other groups. As a group, only adult males (with a larger sample) had statistically significant ADEs suggesting compulsive-like drinking; investigation of effects in a larger sample of female rats is warranted.
Our examination of movement patterns found similar results to drinking behavior, with larger differences observed between females and males than adolescent and adult males. The higher levels of activity in females were associated with higher frequency of accesses to 5%. Interestingly, the hourly data show that females and adolescent males started out with more similar movement patterns; while females maintained their activity levels over time, only decreasing mesor and amplitude with the introduction of quinine, both groups of males experienced reductions already during the regular ADE5. By the latter part of the experiment, differences between adolescent and adult males became less prominent. Another interesting observation was that female rats had later peaks of activity. Sex differences in circadian regulation (of sleep and waking cognition [57] and circadian timing systems have been observed and are thought to stem from differences, for example, in (sex) hormonal and stress-responses [58], neurophysiological [59] and neuroendocrine [60] systems. Alterations in circadian timing are thought to be important in determining vulnerability to various types of disease, including addiction [61,62,63] and circadian misalignment may disrupt reward mechanisms, promoting the transition from alcohol use to AUD [64]. One additional consideration when it comes to movement patterns is animal size. Females are smaller in size than males and cages are the same size regardless of animal size—this may have played a determining factor in movement patterns.
This experiment had certain limitations. First, females and adolescent males underwent their assessments at the same time, while adult males underwent the same procedure earlier. Although every effort was made to keep experimental factors constant, batch differences may have been introduced. Given the similarities observed between drinking patterns in the two male groups, however, this effect might be minimal. Sample sizes also differed, with the adult males being the largest group; with equivalent sample sizes, statistically significant compulsive-like drinking might be more apparent in other groups. Next, it would be desirable to also include female adolescent rats in this design. As noted in the methods section, this was not possible in the current experiment, as hyperactivity of the adolescent females prevented proper measurement of consumption and resulted in stoppage of their inclusion due to ethical considerations. We have made similar observations in previous experiments; the development of refined assessment methods will be needed to tackle this issue when it occurs. Developmental differences (i.e., puberty) in stress and gonadal sex hormones might be expected to introduce additional physiological and behavioral differences, (although it has been observed that estrous cycle phase does not account for all sex differences [21]). It has been previously suggested that female rats beginning drinking in adolescence may be more susceptible to stress-induced alcohol consumption [65]. Finally, females and adolescent males were housed together, and we did not control for any effects of estrous cycle on the adolescent males. However, drinking data from adolescent males were remarkably similar to those in adult males (not housed with females, i.e., Fig. 1), suggesting a limited effect on drinking behavior, if any. Perhaps relatedly, in the female rats, we note the variability observed in compulsive-like drinking (i.e., Fig. 5A). One possibility is the involvement of estrous cycles or circulating hormones (not assessed in the current sample). Although it has been shown that females do not show greater variability than males due to estrous cycle [66, 67], and is thought that female rats housed in proximity are likely to be synchronized [68], exceptions to this may have contributed to variability; closer investigation of potential mediating factors is needed.
Perspectives and significance
Gaining a more complete understanding of sex and age factors involved in the development of drinking behavior will be important in preclinical development of models of addiction, drug development and exploration of options for treatment. Further refined exploration of the differences observed will be required to arrive at robust translational insights. The continued collection (and combination) of well-characterized longitudinal datasets investigating different aspects of dynamic processes in addiction will help to achieve a more comprehensive picture of the development of addiction processes.
Conclusions
We observed clear sex differences in drinking patterns, solution preference, response to aversive stimuli and movement patterns in male and female rats, suggesting potential areas of focus for future research.
Availability of data and materials
The datasets used and/or analyzed during the current study can be made available from the corresponding author on reasonable request.
References
(NCCDPHP) NCfCDPaHP. Excessive Alcohol Use Fact Sheet 2022. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/alcohol.htm. Accessed 10 Jan 2023.
WHO. Alcohol Fact Sheet 2022 [Available from: https://www.who.int/news-room/fact-sheets/detail/alcohol.
Finn DA. The endocrine system and alcohol drinking in females. Alcohol Res. 2020;40(2):02.
Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiat. 2015;72(8):757–66.
Van Etten ML, Neumark YD, Anthony JC. Male-female differences in the earliest stages of drug involvement. Addiction. 1999;94(9):1413–9.
Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47.
(NCCDPHP) NCfCDPaHP. Excessive Alcohol Use is a Risk to Women’s Health 2022. https://www.cdc.gov/alcohol/fact-sheets/womens-health.htm. Accessed 10 Jan 2023.
Zilberman M, Tavares H, el-Guebaly N. Gender similarities and differences: the prevalence and course of alcohol- and other substance-related disorders. J Addict Dis. 2003;22(4):61–74.
Erol A, Karpyak VM. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015;156:1–13.
Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008;93(1–2):21–9.
Stelander LT, Høye A, Bramness JG, Selbæk G, Lunde LH, Wynn R, et al. The changing alcohol drinking patterns among older adults show that women are closing the gender gap in more frequent drinking: the Tromsø study, 1994–2016. Subst Abuse Treat Prev Policy. 2021;16(1):45.
Robinson DL, Amodeo LR, Chandler LJ, Crews FT, Ehlers CL, Gómez-A A, et al. The role of sex in the persistent effects of adolescent alcohol exposure on behavior and neurobiology in rodents. Int Rev Neurobiol. 2021;160:305–40.
Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol Rev. 2016;68(2):242–63.
Surakka A, Vengeliene V, Skorodumov I, Meinhardt M, Hansson AC, Spanagel R. Adverse social experiences in adolescent rats result in persistent sex-dependent effects on alcohol-seeking behavior. Alcohol Clin Exp Res. 2021;45(7):1468–78.
Bonomo Y. Early onset of drinking increases alcohol use in adulthood. Evid Based Ment Health. 2005;8(4):98.
Pitkänen T, Lyyra AL, Pulkkinen L. Age of onset of drinking and the use of alcohol in adulthood: a follow-up study from age 8–42 for females and males. Addiction. 2005;100(5):652–61.
Alcoholism NIoAAa. Underage drinking 2022. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking. Accessed 10 Jan 2023.
Spear LP. Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci. 2018;19(4):197–214.
Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, et al. Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcohol Clin Exp Res. 2019;43(9):1806–22.
Mokdad AH, Forouzanfar MH, Daoud F, Mokdad AA, El Bcheraoui C, Moradi-Lakeh M, et al. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10036):2383–401.
Amodeo LR, Wills DN, Sanchez-Alavez M, Nguyen W, Conti B, Ehlers CL. Intermittent voluntary ethanol consumption combined with ethanol vapor exposure during adolescence increases drinking and alters other behaviors in adulthood in female and male rats. Alcohol. 2018;73:57–66.
Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev. 2014;45:1–8.
Jury NJ, Pollack GA, Ward MJ, Bezek JL, Ng AJ, Pinard CR, et al. Chronic ethanol during adolescence impacts corticolimbic dendritic spines and behavior. Alcohol Clin Exp Res. 2017;41(7):1298–308.
Nentwig TB, Starr EM, Chandler LJ, Glover EJ. Absence of compulsive drinking phenotype in adult male rats exposed to ethanol in a binge-like pattern during adolescence. Alcohol. 2019;79:93–103.
Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29(7):1139–45.
Spanagel R. Animal models of addiction. Dialogues Clin Neurosci. 2017;19(3):247–58.
Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013;4(6):624–30.
Vengeliene V, Noori HR, Spanagel R. The use of a novel drinkometer system for assessing pharmacological treatment effects on ethanol consumption in rats. Alcohol Clin Exp Res. 2013;37(Suppl 1):E322–8.
Timme NM, Linsenbardt D, Timm M, Galbari T, Cornwell E, Lapish C. Alcohol-preferring P rats exhibit aversion-resistant drinking of alcohol adulterated with quinine. Alcohol. 2020;83:47–56.
Vengeliene V, Bilbao A, Spanagel R. The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice. Alcohol. 2014;48(3):313–20.
Foo JC, Vengeliene V, Noori HR, Yamaguchi I, Morita K, Nakamura T, et al. Drinking levels and profiles of alcohol addicted rats predict response to nalmefene. Front Pharmacol. 2019;10:471.
Foo JC, Meinhardt MW, Skorodumov I, Spanagel R. Alcohol solution strength preference predicts compulsive-like drinking behavior in rats. Alcohol Clin Exp Res. 2022;46(9):1710–9.
Hopf FW, Lesscher HM. Rodent models for compulsive alcohol intake. Alcohol. 2014;48(3):253–64.
Foo JC, Noori HR, Yamaguchi I, Vengeliene V, Cosa-Linan A, Nakamura T, et al. Dynamical state transitions into addictive behaviour and their early-warning signals. Proc Biol Sci. 2017. https://doi.org/10.1098/rspb.2017.0882.
Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16.
Towers EB, Bakhti-Suroosh A, Lynch WJ. Females develop features of an addiction-like phenotype sooner during withdrawal than males. Psychopharmacology. 2021;238(8):2213–24.
Bell RL, Rodd-Henricks ZA, Kuc KA, Lumeng L, Li TK, Murphy JM, et al. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on the intake of ethanol by male and female periadolescent alcohol-preferring (P) rats. Alcohol. 2003;29(3):137–48.
Torres OV, Walker EM, Beas BS, O’Dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcohol Clin Exp Res. 2014;38(1):108–15.
Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science. 1967;156(3777):942–3.
Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav. 2017;152:61–7.
Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J Clin Invest. 1998;101(12):2677–85.
Cailhol S, Mormède P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25(4):594–9.
Logrip ML, Oleata C, Roberto M. Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology. 2017;114:123–34.
Kirson D, Oleata CS, Parsons LH, Ciccocioppo R, Roberto M. CB1 and ethanol effects on glutamatergic transmission in the central amygdala of male and female msp and wistar rats. Addict Biol. 2018;23(2):676–88.
Li J, Chen P, Han X, Zuo W, Mei Q, Bian EY, et al. Differences between male and female rats in alcohol drinking, negative affects and neuronal activity after acute and prolonged abstinence. Int J Physiol Pathophysiol Pharmacol. 2019;11(4):163–76.
Hitzemann R, Bergeson SE, Berman AE, Bubier JA, Chesler EJ, Finn DA, et al. Sex differences in the brain transcriptome related to alcohol effects and alcohol use disorder. Biol Psychiatry. 2022;91(1):43–52.
Sneddon EA, Masters BM, Ream KD, Fennell KA, DeMedio JN, Cash MM, et al. Sex chromosome and gonadal hormone contributions to binge-like and aversion-resistant ethanol drinking behaviors in Four Core Genotypes mice. Front Psychiatry. 2023;14:1098387.
Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–37.
Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology. 2014;231(8):1831–9.
Radke AK, Held IT, Sneddon EA, Riddle CA, Quinn JJ. Additive influences of acute early life stress and sex on vulnerability for aversion-resistant alcohol drinking. Addict Biol. 2020;25(6): e12829.
Fulenwider HD, Nennig SE, Price ME, Hafeez H, Schank JR. Sex differences in aversion-resistant ethanol intake in mice. Alcohol Alcohol. 2019;54(4):345–52.
Arnold ME, Butts AN, Erlenbach TR, Amico KN, Schank JR. Sex differences in neuronal activation during aversion-resistant alcohol consumption. Alcohol Clin Exp Res. 2023;47(2):240–50.
Dhaher R, McConnell KK, Rodd ZA, McBride WJ, Bell RL. Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacol Biochem Behav. 2012;102(4):540–8.
Spear LP. Adolescent alcohol exposure: are there separable vulnerable periods within adolescence? Physiol Behav. 2015;148:122–30.
Bell RL, Hauser SR, Liang T, Sari Y, Maldonado-Devincci A, Rodd ZA. Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology. 2017;122:201–43.
Towner TT, Applegate DT, Varlinskaya EI, Werner DF. Impact of adolescent intermittent ethanol exposure on social investigation, social preference, and neuronal activation in cFos-LacZ male and female rats. Front Pharmacol. 2022;13: 841657.
Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk DJ. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci U S A. 2016;113(19):E2730–9.
Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35(1):111–39.
McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32(7):2241–7.
Mong JA, Cusmano DM. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150110.
Rosenwasser AM. Circadian clock genes: non-circadian roles in sleep, addiction, and psychiatric disorders? Neurosci Biobehav Rev. 2010;34(8):1249–55.
Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20(2):173–9.
Logan RW, Williams WP, McClung CA. Circadian rhythms and addiction: mechanistic insights and future directions. Behav Neurosci. 2014;128(3):387–412.
Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin Exp Res. 2013;37(4):558–65.
Füllgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86(2):320–6.
Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5.
Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7:34.
McClintock MK. Estrous synchrony and its mediation by airborne chemical communication (Rattus norvegicus). Horm Behav. 1978;10(3):264–75.
Heinz A, Kiefer F, Smolka MN, Endrass T, Beste C, Beck A, et al. Addiction research consortium: losing and regaining control over drug intake (ReCoDe)-From trajectories to mechanisms and interventions. Addict Biol. 2020;25(2): e12866.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. Financial support for this work was provided by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 402170461 (A05)—TRR 265, Heinz et al. [69] to RS and Project-ID: ME 5279/3-1 to MWM as well as the Bundesministerium für Bildung und Forschung (BMBF) funded E:med program: Target-OXY (FKZ: 031L0190A) to RS and JCF, and the BMBF-funded SysMedSUDs consortium (FKZ: 01ZX1909A) to RS.
Author information
Authors and Affiliations
Contributions
JCF drafted the manuscript and analyzed and interpreted the data. IS conducted experiments, collected and processed the data. RS conceived/designed the work, interpreted the results and revised the paper. MWM conceived/designed the work, interpreted the results and revised the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Experimental procedures were conducted according to the ethical guidelines for the care and use of laboratory animals. Experiments were approved by the local animal care committee (Regierungspräsidium Karlsruhe, Germany: AZ:35-9185.81/G-227/20).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Foo, J.C., Skorodumov, I., Spanagel, R. et al. Sex- and age-specific effects on the development of addiction and compulsive-like drinking in rats. Biol Sex Differ 14, 44 (2023). https://doi.org/10.1186/s13293-023-00529-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13293-023-00529-4