Abstract
Background
Men have a higher risk for cardiovascular disease (CVD) early in life, while women have a higher risk later in life. The sex-related differences in CVD risk, especially by age, could be related to sphingolipid metabolism. We compared plasma sphingolipid concentrations and its increase by age in men and women.
Methods
Plasma concentrations of 13 types of sphingolipids were measured by liquid chromatography-tandem mass spectrometry in a random subsample of 328 men and 372 women of Dutch and South-Asian Surinamese ethnic origin, participating in the HELIUS study. Sphingolipid concentrations were compared between men and women by age group (18–39, 40–55, and 56–70 years). Multiple linear regression was used to determine sex differences in age trends in sphingolipids stratified by ethnicity. Analyses were performed without adjustment and adjusted for body mass index (BMI) and waist circumference.
Results
At age 18–39 years, sphingolipid concentrations were lower in women than those in men, but at age 56–70 years this was reversed. At higher age, women showed higher concentrations than men. In line, we observed a more rapid increase of sphingolipid concentrations by age in women than in men. The observed sex differences were not explained by BMI or waist circumference. Patterns of sex differences were similar across ethnic groups, although the strength of associations differed.
Conclusions
Mean sphingolipid concentrations increase more rapidly with age in women than in men. Therefore, plasma lipid concentrations of sphingolipids, although lower in women than in men at younger age, are higher in women than in men at older age.
Similar content being viewed by others
Introduction
Each year, 41 million people die from non-communicable diseases (NCDs) globally, this accounts for 71% of all deaths [1]. Two major classes of NCDs are cardiovascular diseases (CVDs) and type 2 diabetes (T2D) [1], which both show different distributions in prevalence between men and women [2]. Men, for instance, have a higher risk for CVD early in life, while women have a higher risk later in life [3, 4]. Despite these observed differences, mechanisms potentially linking these sex-related differences to CVD risk remain understudied.
Mechanisms related to the previously observed sex differences in body fat may partly explain observed differences in prevalence of CVD and T2D. Men tend to store higher amounts of ectopic and visceral fat than women, while women are relatively protected from ectopic fat storage [5, 6]. With age, hormonal changes occur, such as a drop in oestrogen levels in women after menopause. These hormonal changes affect deposition of ectopic and visceral fat [7]. While both men and women tend to store higher amounts of visceral fat with increasing age, the amount of visceral fat is shown to increase by 200% in men, but with 400% in women [8].
A mechanism that may play a role is the increased formation of potentially toxic lipid intermediates such as sphingolipids, due to the increase of ectopic fat. The higher availability of free fatty acids increases for example ceramide (a class of sphingolipids) synthesis. Sphingolipid levels have been shown to be associated with various diseases [9,10,11,12], including CVD [13,14,15,16,17], T2D [18,19,20,21,22,23], and metabolic syndrome [24, 25]. Where long-chain (dihydro) ceramides have been positively associated with T2D and CVD risk, very-long-chain ceramides and more complex sphingolipids including lactosylceramides have been negatively associated [23, 26]. Moreover, enzymes of sphingolipid synthesis are potential targets to reduce CVD risk [27], and the inhibition of glycosphingolipid biosynthesis has for instance been shown to decrease atherosclerosis in mice [28]. Previous studies already showed that men have higher levels of circulating ceramides than premenopausal women [29], while ceramide levels increase more rapidly in post-menopausal women than in men [30]. Sphingolipids could, thus, potentially explain not only the higher prevalence of CVD in men compared to women, but also the accelerated occurrence of CVD in post-menopausal women. How other classes of sphingolipids, e.g., more complex glucosylceramides and lactosylceramides, increase by age in both men and women has not been determined yet.
In our cross-sectional study, we describe the differences in mean sphingolipid concentrations across age groups between 18- and 70-year-old Dutch and South-Asian Surinamese men and women living in Amsterdam, the Netherlands. In addition, we explored age trends in sphingolipids and determined whether age trends in sphinogolipids differ by sex.
Materials and methods
Population
Baseline data from the Healthy Life in an Urban Setting (HELIUS) study, collected between 2011 and 2015, was used. HELIUS is a multi-ethnic cohort study among six ethnic groups living in Amsterdam. A detailed description of the design is available elsewhere [31, 32]. In brief, participants were randomly sampled from the municipal register, stratified by ethnicity. Questionnaires, physical examinations, and biological samples were obtained [31]. Full data were collected among 22,165 participants, from whom we selected those of Dutch and South-Asian Surinamese ethnicity (n = 7607), because the current study includes secondary analyses of data collected as part of a HELIUS sub-study aimed at studying causes of incident T2D among high-risk South-Asian populations and sphingolipids were not determined in other ethnicities. We then excluded participants who did not provide permission for data linkage or storage of biological material (n = 671) and those who had less than two vials of EDTA-plasma available in the biobank (n = 186). In addition, participants with T2D based on self-report, increased fasting glucose (≥ 7.0 mmol/L), increased HbA1c (≥ 48 mmol/mol), or use of glucose lowering medication were excluded (n = 773). From the 5977 participants (3972 of Dutch and 2005 of South-Asian Surinamese origin) who remained in the study, we took a random sample of 350 participants per ethnic group in whom metabolites were determined using the sample function in the R statistical software package. The Institutional Review Board of the Amsterdam Medical Center approved the HELIUS study (MREC 10/100# 17.10.1729). All participants provided written informed consent.
Measurements
Ethnicity was defined by the individual’s country of birth combined with the parental countries of birth. Dutch ethnicity was assigned to participants born in the Netherlands, with both parents born in the Netherlands. South-Asian Surinamese ethnicity was assigned to participants born in Suriname with at least one parent born in Suriname (1st generation) or born in the Netherlands with both parents born in Suriname (2nd generation) combined with self-reported South-Asian ethnic origin.
Body mass index (BMI) was determined by dividing measured body weight (kg) by height squared (m2). Weight and height were measured in barefoot subjects wearing light clothes only. Waist circumference was measured using a tape measure at the level midway between the lowest rib margin and the iliac crest. All anthropometric measures were taken in duplicate, and the mean was used in the analyses. If the discrepancy between the duplicate measures differed more than 0.5 cm for height, more than 0.5 kg for weight, or more than 1 cm for waist circumference, a third measurement was taken. The two measures which were most similar were used to calculate the mean.
The total reported fat intake and total energy intake were derived from an ethnic-specific food frequency questionnaire (FFQ) which was taken among a subsample of the HELIUS cohort, as described in detail elsewhere [33]. The FFQ data were available for 259 participants of our study sample, of whom 58 participants were Dutch men, 47 South-Asian men, 67 Dutch women, and 87 South-Asian women. Menopause was derived from the questionnaire based on lack of menstruation for a year or longer (not for reasons such as pregnancy, breastfeeding, or using birth control).
Blood was collected after a fasting period of at least 10 h. Sphingolipids were measured in plasma by liquid chromatography-tandem mass spectrometry (LC-tMS) as described previously [23]. We adjusted for amino acids in sensitivity analyses. These were determined in plasma by LC-tMS as described previously [34].
Statistical analyses
First, the normal distribution of variables was checked by plotting histograms and evaluating skewness and kurtosis. Baseline characteristics and sphingolipid concentrations were examined among men and women stratified by ethnicity. We calculated means and standard deviations (SD) for continuous normally distributed variables, medians, and interquartile ranges for continuous non-normally distributed variables and numbers of observations and percentages for categorical variables. Baseline characteristics were not tested for statistical differences [35]. Waist circumference was missing for one participant and imputed with an expectation-maximization algorithm. Sex differences in sphingolipid concentrations stratified by age (categories 18–39, 40–55, and 56–70 years) were studied by multiple linear regression within each age group.
Second, we analyzed the association of metabolites with age. We checked the linearity of the association by plotting scatterplots in the total population and stratified by ethnicity. The multiplicative interaction of age with ethnicity was checked by adding an interaction term between age and ethnicity with sphingolipids as the outcome. This was done because BMI may reflect different levels of intra-abdominal fat storage in European than South-Asian populations [36], which may also have implications for the use of non-oxidative pathways. A multiplicative interaction between age and ethnicity was observed for five of the thirteen included sphingolipids (GlcCer(d18:2), GlcCer(d18:1), LacCer(d18:2), LacCer(d18:1), and Cer(d18:1)). Analyses were thus stratified by ethnicity in all analyses. A multiplicative interaction term between age and sex was used to investigate whether the association between sphingolipids and age differed by sex.
All models were run both unadjusted and adjusted for measures of body fat distribution (BMI and waist circumference). We adjusted for cholesterol- and blood pressure-lowering medication in sensitivity analyses, as the use may affect sphingolipid concentrations [29, 37]. We also checked whether the amount of substrate available influenced the results, by adjusting for important substrates for sphingolipids including amino acids (serine, alanine, and glycine), and fat and energy intake in the subset of the population with FFQ data available. Finally, we excluded participants with CVD at baseline. In post-hoc analyses, we adjusted for menopause.
All analyses were conducted using IBM SPSS Statistics 23. Graphs were plotted in RStudio version 3.6.1 using the visreg package. Tests were two-sided, and p values < 0.05 were considered statistically significant. Analyses were not adjusted for multiple testing as our study was of exploratory nature [38], but the consistency of findings was considered to avoid chance findings.
Results
Mean age in the 18–39 year group ranged from 28.4 (SD 5.7) among South-Asian Surinamese men to 31.3 (SD 4.8) among Dutch men, that in the 40–55 year group from 46.8 (SD 4.4) among Dutch men to 47.8 (SD 4.3) among South-Asian Surinamese women, and that in the 56–70 year group from 59.9 (SD 4.1) among South-Asian Surinamese women to 62.0 (SD 4.5) among Dutch men. Mean BMI ranged from 22.5 (SD 3.1) in Dutch women aged 18–39 years to 26.7 (SD 4.6) in South-Asian Surinamese women aged 55–70 years (Table 1). Mean waist circumference was lowest among Dutch women aged 18–39 years old with a mean of 79.5 (SD 9.0) and highest in 55–70-year-old men with a mean of 97.6 (SD 11.3).
Dihydroceramide (Cer(d20:1), Cer(d18:2), Cer(d18:1), Cer(d18:0), Cer(d17:1), and Cer(d16:1)) concentrations were generally lower in women than in men in the 18–39 years age group, although mostly not statistically significantly different (Table 2). The sphingolipid concentrations were generally, however, statistically significantly higher in women than in men in the older age groups, especially in the 56–70 years group. The age-adjusted difference in women compared to that in men for Cer(d18:2) was for instance − 123.4 (95% CI − 244; − 2.3) nmol/L in the 18–39 years age group and 208.2 (95% CI 37.2; 379.2) nmol/L in the 56−70 years age group. The more complex sphingolipids (GlcCer(d18:2), GlcCer(d18:2), LacCer(d18:2), LacCer(d18:1), CTH(d18:1), and CTH(d20:1)) showed similar patterns, but were already higher in women in the 18− 39 years age groups in the South-Asian Surinamese with a further increase in the difference with men in older age groups. Patterns of sex differences in mean sphingolipid concentrations remained similar after adjustment for BMI and waist circumference.
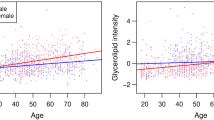
Most sphingolipids increased with age in both men and women (Fig. 1; Table 3). Cer(d18:1) for instance increased with 52.28 nmol/L (95% CI 26.56; 78.00) per year in Dutch men. However, no clear trends were observed for CTH(d18:1) and LacCer(d18:1). Most plasma concentrations of sphingolipids increased more with age in women than in men, although only statistically significantly differed for GlcCer(d18:2), LacCer(d18:2), and Cer(d18:2). Figure 1 shows that plasma concentrations of sphingolipids are generally lower in young adult women than in men, but higher in women than in men from the age of approximately 45 years. The patterns in the associations of sphingolipids and age did not change after adjusting for BMI and waist circumference. Although the strength of the associations differed by ethnicity, patterns of differences in age trends between men and women were similar.
Sphingolipids which concentrations increase faster by age in women than men. The sphingolipids are shown of which the plasma concentrations increase faster by age in women than in men. A significant interaction by sex means a statistically significant multiplicative interaction between age and sex with sphingolipid concentrations as the outcome at a P value < 0.05. Analyses were stratified by ethnicity. Red asterisk denotes statistically significant association between age and sphingolipid in women at a P value < 0.05. Blue asterisk denotes statistically significant association between age and sphingolipid in men a P value < 0.05
Sensitivity analyses with additional adjustment for use of cholesterol- or blood pressure-lowering medication, plasma amino acid (serine, alanine, glycine) concentrations, and energy or fat intake did not alter the results (data not shown). The sensitivity analyses excluding participants with CVD also did not change our interpretations (data not shown). Additional adjustment for menopause did, overall, not alter our interpretation (Supplementary Table 1). Menopause, however, mediated the associations between age and Cer(d18:0) and Cer(m18:0).
Discussion
Our study shows that plasma levels of sphingolipids are generally lower among women than men in younger age categories, while they are higher among women than men in older age categories. Most sphingolipid concentrations increase by age in both men and women, but increases by age are larger in women than in men. Adiposity levels decreased the strength of observations, but did not impact the observed patterns of sex differences.
Studies on sex differences in sphingolipid concentrations showed conflicting results. A study by Sui et al. suggests that lactosylceramide concentrations are higher among women than men [19], whereas a study by Ishikawa et al. suggested generally similar levels of sphingolipids in both sexes [39], and a study by Weir et al. higher ceramide concentrations among men than women [29]. These studies, however, did not consider that the difference in concentrations by sex may differ by age, and the included study populations in the studies by Ishikawa et al. and Weir et al. were approximately 10 years younger than those in the study by Sui et al [19, 29, 39]. A study by Mielke et al. did consider age groups and showed that ceramide and dihydroceramides concentrations were higher in women than in men and increased more strongly in women than in men by age [30]. Although the study by Mielke et al. was limited to participants over 55 years of age [30], this finding is in line with our study. We added to these findings that the slope of the association is such that in younger age groups sphingolipid concentrations may be higher among men than women. Moreover, we are the first to report on sex differences in 1-deoxyceramides, glucosylceramides, and globotriaosylceramides, for which patterns of sex differences and age trends were similar to the dihydroceramides.
The higher levels of sphingolipids in (especially older) women when compared to men may partly be explained by a drop in oestrogen levels after menopause in women [40]. The drop in oestrogen levels may lead to a change in body fat distribution; however, adjustments for adiposity levels suggest that differences in body fat distribution are, apparently, not strongly related to sex differences in sphingolipid concentrations. Although it was in contrast with our hypothesis, sphingolipids are associated with the amount of visceral fat [41], and (especially younger) men are more likely to store visceral fat than women. Perhaps, measures of adiposity used in our study (BMI and waist circumference) do not properly reflect the difference in amounts of visceral fat between men and women [42]. However, post-hoc adjustments for menopause also did not support a major role for specific changes after menopause. Menopause only mediated the associations between age and the saturated (18:0) sphingolipid species in our analyses, while especially the mono-unsaturated species showed steeper increases in women than in men. Other mechanisms may also explain the observed sex difference in sphingolipid concentrations. One of the obvious differences between men and women are sex steroid differences. Already in 1985, studies showed that estradiol levels were associated with reduced concentrations of sphingolipids, but only in women [43], possibly by downregulation of key enzymes for de novo ceramide synthesis such as serine-palmitoyltransferase and ceramide synthase. Another possible candidate mechanism includes oxidative stress leading to inflammation and higher sphingolipid concentrations, which could for instance lead to CVD and T2D [12]. Generally, levels of oxidative stress were observed to be lower among women than men, but higher among post-menopausal women [44]. Finally, the higher sphingolipid concentrations among women than men may also be explained by differences in lipid lipoprotein metabolism, especially the higher levels of high density lipoproteins (HDL) particles in women [45], since lipoproteins transport insoluble lipids such as sphingolipids in the circulation.
Ceramides have been implicated in the development of age-related diseases including T2D and cardiovascular disease, for instance by inducing insulin resistance, formation of plaques, pro-inflammatory properties, and apoptosis [13,14,15,16,17,18,19,20,21,22]. We showed that ceramide concentrations increase with age, more in women than in men. The complex sphingolipids, associated with decreased T2D risk [22, 23], also increased with age, which may be explained by the fact that ceramides are precursors for these more complex sphingolipids. Nonetheless, age may not affect all sphingolipid concentrations similarly since sphingolipid metabolic pathways are highly complex and contain many different enzymes which may be differently affected by age [46]. This is also underscored by the observed increase of specific sphingolipid species concentration with age in women than in men, since the steeper increase was especially observed for the d18:2 sphingolipid species. The d18:1 sphingolipid species are formed by condensation of palmitoyl-CoA (C16:0) with serine by serine palmitoyl transferase (SPT) followed by DEGS-dependent desaturation of the sphinganine backbone at the dihydroceramide stage. The d18:2 sphingolipid species are formed similarly, but palmitoleic acid (C16:1) condenses with serine, later followed by DEGS desaturation. We speculate that the higher d18:2 levels in women could be caused by a higher dietary intake of palmitoleic acid or a higher stearoyl-CoA desaturase (SCD1) activity, which forms the n-9 double bond in activated saturated fatty acids (C16:0). Whether this increase in d18:2 species is linked to disease and what mechanism causes this remains to be established.
Our study is not exempt from limitations. First, our study is a secondary analysis of existing data. In the sampling procedure participants with T2D were excluded from the study. This may have affected our results since sphingolipids are associated with T2D [20], underestimating the mean concentrations across groups. This may especially have affected the results for men and the South-Asian Surinamese participants, since T2D is more prevalent among men and those of South-Asian descent [47, 48]. Further, our study included only participants of two ethnic groups, and findings need to be replicated among participants from other ethnic backgrounds, especially since the strength of associations differed by ethnicity. Nevertheless, patterns of differences were consistent across both ethnic groups although the sex difference in age-related increase of sphingolipids was more apparent among the Dutch. In addition, sensitivity analyses that also excluded participants with baseline CVD, also more prevalent among those of South-Asian descent than in the majority Dutch, did not affect our results. Next, the cross-sectional design of our study is a limitation. We did not follow participants over time, but cross-sectionally grouped our study population by age. The results may thus reflect a cohort effect. Characteristics of older participants may differ from younger participants, which is especially important if characteristics of women have changed differently over time than those of men. This seems unlikely, since a linear association between age and sphingolipids was observed; this is only likely if characteristics have changed gradually over time. Nevertheless, longitudinal studies are needed to confirm our findings.
Plasma sphingolipid levels increase with age in both men and women. While sphingolipids are lower in young women than men, the sphingolipids increase more rapidly with age in women than men, leading to higher sphingolipid levels in women than men at higher age. A better understanding of sex differences in age-related trajectories of sphingolipids is important since sphingolipids have repeatedly been associated with age-related diseases. Future studies may investigate whether the observed changes in sphingolipid concentrations by age are reflective of other processes and may serve as biomarkers for disease risk or are a target in itself to reduce CVD risk. This understanding may help in developing targeted interventions and to identify biomarkers for disease risk.
Availability of data and materials
The HELIUS data are owned by the Academic Medical Center (AMC) in Amsterdam, The Netherlands. Any researcher can request the data by submitting a proposal to the HELIUS Executive Board as outlined at http://www.heliusstudy.nl/en/researchers/collaboration. Requests for further information and proposals can be submitted to the Scientific Coordinator and Data Manager of HELIUS, at info@heliusstudie.nl. The HELIUS Executive Board will check proposals for compatibility with the general objective, ethical approvals, and informed consent forms of the HELIUS study, and potential overlap with ongoing work affiliated with HELIUS. There are no other restrictions to obtaining the data, and all data requests will be processed in the same manner.
References
World Health Organization. Noncommunicable diseases. 2018 https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases. Accessed 12 Nov 2019.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. https://doi.org/10.1002/ijc.29210..
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet (London, England). 2010;375:9733:2215–22. https://doi.org/10.1016/s0140-6736(10)60484-9.
Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol. 2013;168(2):934–45. https://doi.org/10.1016/j.ijcard.2012.10.046..
Elbers JM, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276(2):E317–25. https://doi.org/10.1152/ajpendo.1999.276.2.E317..
Nordstrom A, Hadrevi J, Olsson T, Franks PW, Nordstrom P. Higher Prevalence of Type 2 Diabetes in men than in women Is associated with differences in visceral fat mass. J Clin Endocrinol Metab. 2016;101(10):3740–6. https://doi.org/10.1210/jc.2016-1915.
Bairey Merz CN, Ramineni T, Leong D. Sex-specific risk factors for cardiovascular disease in women-making cardiovascular disease real. Curr Opin Cardiol. 2018;33(5):500–5. https://doi.org/10.1097/hco.0000000000000543..
Hunter GR, Gower BA, Kane BL. Age related shift in visceral fat. Int J Body Compos Res. 2010;8(3):103–8.
Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19(3):175–91. https://doi.org/10.1038/nrm.2017.107..
Stiban J. Introduction: Enigmas of Sphingolipids. Adv Exp Med Biol. 2019;1159:1–3. https://doi.org/10.1007/978-3-030-21162-2_1..
Matanes F, Twal WO, Hammad SM. Sphingolipids as biomarkers of disease. Adv Exp Med Biol. 2019;1159:109–38. https://doi.org/10.1007/978-3-030-21162-2_7..
Borodzicz S, Czarzasta K, Kuch M, Cudnoch-Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015;14(1):55. https://doi.org/10.1186/s12944-015-0053-y.
Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37(25):1967–76. https://doi.org/10.1093/eurheartj/ehw148.
Meeusen JW, Donato LJ, Bryant SC, Baudhuin LM, Berger PB, Jaffe AS. Plasma ceramides. Arterioscler Thromb Vasc Biol. 2018;38(8):1933–9. https://doi.org/10.1161/atvbaha.118.311199..
Uchida Y, Uchida Y, Kobayashi T, Shirai S, Hiruta N, Shimoyama E, et al. Detection of ceramide, a risk factor for coronary artery disease, in human coronary plaques by fluorescent angioscopy. Circ J. 2017;81(12):1886–93. https://doi.org/10.1253/circj.CJ-17-0363.
Edsfeldt A, Duner P, Stahlman M, Mollet IG, Asciutto G, Grufman H, et al. Sphingolipids contribute to human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol. 2016;36(6):1132–40. https://doi.org/10.1161/atvbaha.116.305675..
Cheng JM, Suoniemi M, Kardys I, Vihervaara T, de Boer SP, Akkerhuis KM, et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis. 2015;243(2):560–6. https://doi.org/10.1016/j.atherosclerosis.2015.10.022..
Neeland IJ, Singh S, McGuire DK, Vega GL, Roddy T, Reilly DF, et al. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: the Dallas Heart Study. Diabetologia. 2018;61(12):2570–9. https://doi.org/10.1007/s00125-018-4720-1.
Sui J, He M, Wang Y, Zhao X, He Y, Shi B. Sphingolipid metabolism in type 2 diabetes and associated cardiovascular complications. Exp Ther Med. 2019;18(5):3603–14. https://doi.org/10.3892/etm.2019.7981..
Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A, et al. Plasma 1-deoxysphingolipids are predictive biomarkers for type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2015;3(1):e000073. https://doi.org/10.1136/bmjdrc-2014-000073.
Zuellig RA, Hornemann T, Othman A, Hehl AB, Bode H, Guntert T, et al. Deoxysphingolipids, novel biomarkers for type 2 diabetes, are cytotoxic for insulin-producing cells. Diabetes. 2014;63(4):1326–39. https://doi.org/10.2337/db13-1042..
Jensen PN, Fretts AM, Yu C, Hoofnagle AN, Umans JG, Howard BV, et al. Circulating sphingolipids, fasting glucose, and impaired fasting glucose: the Strong Heart Family Study. EBioMedicine. 2019;41:44–9. https://doi.org/10.1016/j.ebiom.2018.12.046..
Muilwijk M, SMI G, Celis-Morales C, Hof MH, Ghauharali-van der Vlugt K, Beers-Stet FS, et al. Contributions of amino acid, acylcarnitine and sphingolipid profiles to type 2 diabetes risk among South-Asian Surinamese and Dutch adults. BMJ Open Diabetes Res Care. 2020;8(1):e001003. https://doi.org/10.1136/bmjdrc-2019-001003.
Choromańska B, Myśliwiec P, Razak Hady H, Dadan J, Myśliwiec H, Chabowski A, et al. Metabolic syndrome is associated with ceramide accumulation in visceral adipose tissue of women with morbid obesity. Obesity. 2019;27(3):444–53. https://doi.org/10.1002/oby.22405..
Brice SE, Cowart LA. Sphingolipid metabolism and analysis in metabolic disease. Adv Exp Med Biol. 2011;721:1–17. https://doi.org/10.1007/978-1-4614-0650-1_1..
Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Völzke H, et al. Ceramide remodeling and risk of cardiovascular events and mortality. J Am Heart Assoc. 2018;7(10):e007931. https://doi.org/10.1161/JAHA.117.007931..
Park J-W, Park W-J, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta. 2014;1841(5):671–81. https://doi.org/10.1016/j.bbalip.2013.08.019.
Chatterjee S, Bedja D, Mishra S, Amuzie C, Avolio A, Kass DA, et al. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E-/- mice and rabbits fed a high-fat and -cholesterol diet. Circulation. 2014;129(23):2403–13. https://doi.org/10.1161/CIRCULATIONAHA.113.007559..
Weir JM, Wong G, Barlow CK, Greeve MA, Kowalczyk A, Almasy L, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res. 2013;54(10):2898–908. https://doi.org/10.1194/jlr.P035808.
Mielke MM, Bandaru VV, Han D, An Y, Resnick SM, Ferrucci L, et al. Demographic and clinical variables affecting mid- to late-life trajectories of plasma ceramide and dihydroceramide species. Aging cell. 2015;14(6):1014–23. https://doi.org/10.1111/acel.12369..
Snijder MB, Galenkamp H, Prins M, Derks EM, Peters RJG, Zwinderman AH, et al. Cohort profile: the Healthy Life in an Urban Setting (HELIUS) study in Amsterdam, The Netherlands. BMJ Open. 2017;7(12):e017873. https://doi.org/10.1136/bmjopen-2017-017873.
Stronks K, Snijder MB, Peters RJG, Prins M, Schene AH, Zwinderman AH. Unravelling the impact of ethnicity on health in Europe: the HELIUS study. BMC Public Health. 2013;13:402. https://doi.org/10.1186/1471-2458-13-402.
Beukers MH, Dekker LH, de Boer EJ, Perenboom CW, Meijboom S, Nicolaou M, et al. Development of the HELIUS food frequency questionnaires: ethnic-specific questionnaires to assess the diet of a multiethnic population in The Netherlands. Eur J Clin Nutr. 2015;69(5):579–84. https://doi.org/10.1038/ejcn.2014.180..
Casetta B, Tagliacozzi D, Shushan B, Federici G. Development of a method for rapid quantitation of amino acids by liquid chromatography-tandem mass spectrometry (LC-MSMS) in plasma. Clin Chem Lab Med. 2000;38(5):391–401. https://doi.org/10.1515/cclm.2000.057..
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107. https://doi.org/10.1002/sim.3697.
Eastwood SV, Tillin T, Wright A, Heasman J, Willis J, Godsland IF, et al. Estimation of CT-derived abdominal visceral and subcutaneous adipose tissue depots from anthropometry in Europeans, South Asians and African Caribbeans. PloS one. 2013;8(9):e75085. https://doi.org/10.1371/journal.pone.0075085.
Spijkers LJ, van den Akker RF, Janssen BJ, Debets JJ, De Mey JG, Stroes ES, et al. Hypertension is associated with marked alterations in sphingolipid biology: a potential role for ceramide. PloS one. 2011;6(7):e21817. https://doi.org/10.1371/journal.pone.0021817.
Li G, Taljaard M, Van den Heuvel ER, Levine MAH, Cook DJ, Wells GA, et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol. 2016;46(2):746–55. https://doi.org/10.1093/ije/dyw320..
Ishikawa M, Tajima Y, Murayama M, Senoo Y, Maekawa K, Saito Y. Plasma and serum from nonfasting men and women differ in their lipidomic profiles. Biol Pharm Bull. 2013;36(4):682–5. https://doi.org/10.1248/bpb.b12-00799..
Colleluori G, Chen R, Napoli N, Aguirre LE, Qualls C, Villareal DT, et al. Fat mass follows a U-shaped distribution based on estradiol levels in postmenopausal women. Front Endocrinol (Lausanne). 2018;9:315. https://doi.org/10.3389/fendo.2018.00315.
Samad F, Badeanlou L, Shah C, Yang G. Adipose tissue and ceramide biosynthesis in the pathogenesis of obesity. Adv Exp Med Biol. 2011;721:67–86. https://doi.org/10.1007/978-1-4614-0650-1_5..
Kim HI, Kim JT, Yu SH, Kwak SH, Jang HC, Park KS, et al. Gender differences in diagnostic values of visceral fat area and waist circumference for predicting metabolic syndrome in Koreans. J Korean Med Sci. 2011;26(7):906–13. https://doi.org/10.3346/jkms.2011.26.7.906..
Vozella V, Basit A, Piras F, Realini N, Armirotti A, Bossù P, et al. Elevated plasma ceramide levels in post-menopausal women: a cross-sectional study. Aging (Albany NY). 2019;11(1):73–88. https://doi.org/10.18632/aging.101719.
Kander MC, Cui Y, Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med. 2017;21(5):1024–32. https://doi.org/10.1111/jcmm.13038..
Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847–70. https://doi.org/10.1016/j.cll.2006.07.006..
Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286(32):27855–62. https://doi.org/10.1074/jbc.R111.254359..
Meeks KA, Freitas-Da-Silva D, Adeyemo A, Beune EJ, Modesti PA, Stronks K, et al. Disparities in type 2 diabetes prevalence among ethnic minority groups resident in Europe: a systematic review and meta-analysis. Intern Emerg Med. 2016;11(3):327–40. https://doi.org/10.1007/s11739-015-1302-9..
Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278–316. https://doi.org/10.1210/er.2015-1137.
Acknowledgements
We are most grateful to the participants of the HELIUS study and the management team, research nurses, interviewers, research assistants, and other staff who have taken part in gathering the data of this study.
Funding
This work was supported by the European Union Health Programme 2014-2020 grant number 664609 HP-PJ-2014. The HELIUS study is conducted by the Academic Medical Center Amsterdam and the Public Health Service of Amsterdam. Both organizations provided core support for HELIUS. The HELIUS study is also funded by the Dutch Heart Foundation, the Netherlands Organization for Health Research and Development (ZonMw), the European Union (FP-7), and the European Fund for the Integration of non-EU immigrants (EIF). This work was additionally supported by a grant from the ZonMw Gender and Health Program (grant number 84920008).
Author information
Authors and Affiliations
Contributions
MM and IvV contributed to the conception and design of the work. MM, SG, FMV, and IvV contributed to data collection. MM and NC contributed to the analysis of data. All authors contributed to the interpretation of the results. MM and NC drafted the manuscript. SG, FMV, and IvV critically revised the manuscript. MM is the guarantor of the work. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of the Amsterdam Medical Center approved the HELIUS study (MREC 10/100# 17.10.1729). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Association of sphingolipids with age, additionally adjusted for menopause.
Additional file 2:
Sphingolipid concentrations by age, stratified by sex and ethnicity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Muilwijk, M., Callender, N., Goorden, S. et al. Sex differences in the association of sphingolipids with age in Dutch and South-Asian Surinamese living in Amsterdam, the Netherlands. Biol Sex Differ 12, 13 (2021). https://doi.org/10.1186/s13293-020-00353-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13293-020-00353-0