Abstract
In the realm of studying joint-related diseases, there is a continuous quest for more accurate and representative models. Recently, regenerative medicine and tissue engineering have seen a growing interest in utilizing organoids as powerful tools for studying complex biological systems in vitro. Organoids, three-dimensional structures replicating the architecture and function of organs, provide a unique platform for investigating disease mechanisms, drug responses, and tissue regeneration. The surge in organoid research is fueled by the need for physiologically relevant models to bridge the gap between traditional cell cultures and in vivo studies. Osteochondral organoids have emerged as a promising avenue in this pursuit, offering a better platform to mimic the intricate biological interactions within bone and cartilage. This review explores the significance of osteochondral organoids and the need for their development in advancing our understanding and treatment of bone and cartilage-related diseases. It summarizes osteochondral organoids’ insights and research progress, focusing on their composition, materials, cell sources, and cultivation methods, as well as the concept of organoids on chips and application scenarios. Additionally, we address the limitations and challenges these organoids face, emphasizing the necessity for further research to overcome these obstacles and facilitate orthopedic regeneration.
Similar content being viewed by others
Introduction
Several joint-related diseases decrease individuals’ quality of life and impose a substantial burden on societies and healthcare systems worldwide [1], including non-union fractures [2], osteosarcoma [3], osteoporosis [4], osteoarthritis (OA) [5], ankylosing spondylitis [6], gout [7], and rheumatoid arthritis (RA) [8]. In these pathological conditions, mainly OA, the structure of the joint is altered, and the cartilage and subchondral bone go through degradation and remodeling, respectively [9, 10]. An osteochondral unit that contains articular cartilage and subchondral bone, covers the joint surface and is responsible for its movement and transmission of load-bearing weight over it [11]. Hence, investigating its structure and composition can aid in joint disease.
On the one hand, cartilage with its limited self-repair ability makes treatments challenging and inadequate [12]. On the other hand, bone, as a main section of the joint, is capable of regenerating its minor defects. However, this self-repair is ineffective for more extensive fractures and remains an obstacle for orthopedic physicians [13]. In this regard, tissue engineering methods in regenerative medicine may be an efficient alternative for osteochondral diseases and injuries. Developing a tissue-engineered system to study the development of joint, and related pathological conditions and drug monitoring is utterly beneficial [14]. Therefore, “osteochondral organoids” emerged as in vitro models to increase our knowledge of the interaction between those two tissues in both physiological and pathological conditions.
The term “organoid” refers to a three-dimensional culture system derived from stem cells or tissue-resident progenitor cells that captures the complex architecture, cellular composition, and functionality of the modeled tissues [15]. Their possible self-renewal and self-organization capability can make them physiologically relevant models for developmental biology, disease modeling, and drug testing in vitro [16]. To date, organoids have been successfully generated for various organs such as kidney [17], intestine [18], colon [19], brain [20], and liver [21].
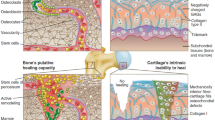
While bone and cartilage organoids have been developed to enhance our understanding of joint diseases, they do have limitations when replicating the complex hierarchical structure and interactions between different cell populations found in natural joints [22, 23]. Osteochondral organoids emerged as advantageous models in the regenerative orthopedic field by mimicking the origin environment and by incorporating different cell types, such as chondrocytes and osteoblasts, to better reflect the complex interactions between different cell populations within the osteochondral unit [24]. Moreover, these organoids can be personalized using patient-derived cells, allowing for studying individual-specific disease mechanisms and personalized medicine approaches [25]. They also open up new possibilities for regenerative medicine, as they may potentially be transplanted into damaged joints to induce tissue repair and regeneration. Here in, we will introduce joint structure characteristics, and summarize the cell sources and material for the generation of osteochondral organoids models, their application prospects, and the current shortcomings in this field Fig. 1.
Structure of osteochondral unit
The synovial joint emerges as a central player in various pathophysiological processes, particularly in osteochondral (OC) regeneration. OC unit constitutes a sophisticated structure crucial for facilitating joint motion and maintaining flexibility [26]. This joint is enveloped by a synovial membrane [27] and comprises two primary elements that originate from the mesoderm layer during embryonic development: the articular cartilage and the subchondral bone [28, 29] (Fig. 2). The joint cavity is filled with synovial fluid that contains signaling factors and provides nutrients for avascular cartilage [30]. Moreover, a capsule of ligaments and tendons surrounds the joint, contributing to its stability [31]. Emerging insights into the OC interface, the region between subchondral bone and hyaline cartilage, underscore its significance in maintaining joint structural integrity [32]. The crosstalk between cartilage and subchondral bone components makes the joint a complex functional unit [33]. Diffusion and vascular channels facilitate the communication between these two sections. The vascularization in the bone matrix affects the mediators produced by both bone and cartilage sections and affects OC units [34]. These interactions are necessary for osteochondral unit development and their alteration will impact joint pathobiology [11]. The proximity of its layers allows for the maintenance of homeostasis through precisely controlled regulatory pathways, enabling effective molecular and biochemical communication between tissues and adaptive responses to environmental cues [35]. The present cells in the joint including chondrocytes, osteocytes, synoviocytes, synovial fibroblasts, and tissue-resident macrophages produce transcription and growth factors to modulate the interaction between the cell-cell and cell-microenvironment [36].
Moreover, OC units are prone to various conditions and injuries, such as OA [37]. Therefore, understanding their structure and interactions is essential for diagnosing and treating disorders, as well as for developing strategies for joint tissue engineering.
Structure of articular cartilage
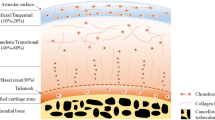
Articular cartilage is located on the external boundary of movable joints and serves as a superficially lubricated cushion that minimizes friction between adjacent bones [38]. This avascular connective tissue plays a vital role in the mechanical loading transition into the deep subchondral bone plate while facilitating smooth bone movement [39]. The articular cartilage’s unique structure and functions make it a key determinant of joint health, and its role in OC unit dynamics is indispensable [40]. During embryonic development, articular cartilage is derived from the mesoderm [41] and exhibits a nuanced macro and microstructure, consisting of four distinct zones: calcified, deep, middle, and superficial zone (Fig. 3) [42]. The thin layer of articular cartilage comprises chondrocytes, dense extracellular matrix, and fluid-filled spaces known as lacunae [43]. The extracellular matrix itself is a complex biochemical microenvironment that contains various proteins and glycosaminoglycans, which regulate the functions of many cells and affect the stiffness and load-bearing of cartilage [44]. This organization contributes to the overall biomechanical properties of the joint. There are several diseases related to articular structure. However, when the articular cartilage is damaged, its ability to undergo repair is limited [45]. The architectural intricacies highlight the significance of considering the hierarchical structure of articular cartilage in the context of osteochondral research.
Structure of subchondral bone
The subchondral bone contributes to joint stability and provides structural, mechanical, and nutritional support [46]. It is anatomically divided into subchondral cortical plate and subchondral trabecular (cancellous) bone [47]. Subchondral cortical bone is a thin layer lying immediately underneath the calcified cartilage and is responsible for mechanical support (Fig. 3) [48]. Beneath that, subchondral cancellous bone is metabolically active and has porosity features that adjust to local mechanical influences [49]. Generally, subchondral bone provides structural support to the joint and contributes to its overall stability preventing bone deformation, collectively creating a robust and flexible system [50]. Any abnormalities or changes in the subchondral bone can significantly affect joint health and function [51]. For instance, in OA, subchondral bone goes through remodeling, and bone spurs (osteophyte) grow on it [52]. Together, the articular cartilage and subchondral bone work in harmony to ensure proper joint function.
Designing of mini-joint (osteochondral) organoids
Advances in three-dimensional (3D) cell culture methods represent a powerful tool that offers many advantages to 2D systems [53]. Moreover, individual monocultures are not capable of modeling the crosstalk between different tissues that are essential for joint homeostasis [54]. By combining different tissue organoids, such as bone or cartilage organoids in biological models, we can reduce the gap between individual cells and a whole organ, which is important for improving research outcomes [55]. Although there is myriad research about bone and cartilage organoids [56,57,58,59], we have limited sources for OC organoids. These systems require a more complex environment to grow two tissues with different compositions and structures. Generally, three different methods are used to engineer these organoids, which involve initiating the culture with either osteogenic medium, chondrogenic medium, or utilizing two different plates simultaneously (Table 1). To develop an effective 3D joint model, it is crucial to choose the right cell source, biomaterial, and other essential factors based on the specific goals of the application. Figure 2 illustrates the cell source and biomaterials for OC organoids.
Cells for osteochondral organoids
The phenotype of the cell source involved in the formation of both articular cartilage and the subchondral bone is intricately linked to the osteochondral organoid development [60, 61]. Pluripotent stem cells (PSCs), such as induced pluripotent stem cells (iPSCs), as well as progenitor cells, and adult stem cells (ASCs), can produce organoids (Table 1) and possess the ability to generate bone, cartilage, or/and osteochondral tissue [62, 63]. All of these cell types have their advantages, and choosing the cells depends on the purpose and application of the organoid.
Mesenchymal stem cells
To begin with, several research groups proposed various approaches for mesenchymal stem cells (MSCs) differentiation into bone tissue [64,65,66]. In native bone microenvironments, MSCs are recruited to form osteoblasts [22] and they can be utilized in vitro studies to give rise to the bony part of organoids. MSCs’ advantageous characteristics such as stemness [67], proliferation [68], and differentiation capacity [69] allow researchers to use them, and their anti-inflammatory [70], and antiapoptotic abilities [71] can make them compatible options for bone organoids. For instance, following the implantation of organoids, the present inflammatory signals polarize MSCs towards an anti-inflammatory and pro-trophic phenotype to aid in tissue recovery [72]. These cells can suppress the expression of genes that promote cell death and contribute to their therapeutic effects [73]. Moreover, they are entirely able to produce and release critical growth factors and cytokines [74]. In addition, the cartilaginous section of OC organoids can be generated from MSCs [75]. They are capable of differentiating into specialized cells developing from mesoderm such as cartilage [76]. These cells are present in multiple tissues, and their chondrogenic potential advances cartilage tissue engineering [77].
The first OC organoid strategy was developed from human bone marrow derived-MSC (BMSCs). They were initially micromass cultured for four weeks. Transforming growth factor-beta (TGFβ1), dexamethasone, and ascorbic acid were used for differentiating MSC. The resulting structures, known as “cartilage beads,” had hyaline cartilage characteristics. Moreover, culturing MSCs in a mineralization inductive medium, successfully resulted in a mineralized bone-like collar around the cartilage. Considerable calcification was a consequence of synthesized collagen type I (COL1), sialoprotein, and osteocalcin (OCN) [64]. Moreover, MSCs-loaded scaffolds demonstrated an osteoconductive environment favorable for bone healing [78]. In particular, loaded umbilical cord MSCs-biomaterial were used in a more recent strategy to form both cartilage and bone in two separate dishes spontaneously. Upregulation of multiple osteogenesis signaling pathways confirmed the commitment of MSCs to osseous lineage and their efficient regulation of mineralized microenvironment [66]. Moreover, MSCs displayed the higher relative gene expression of collagen type II (COL2) and SRY-Box Transcription Factor 9 (SOX9) in 3D cultures [66].
MSCs produced from iPSCs also hold great generative potential for joint-related disorders, showing promise in OC repair [79]. In another research, iPSC-derived MSC was first cultured in an osteogenic environment and afterward, maintained in the cartilaginous medium for 21 days to promote cartilage development on the surface. The chondral outer region of the osteochondral organoid exhibited abundant deposition of COL2, similar to the superficial zone. However, compared to the cartilage organoids, the co-cultured OC organoids demonstrated lower expression of aggrecan (ACAN), and the levels of COL2 and SOX9, were just the same [80].
Induced-pluripotent stem cells
There has been a growing focus on iPSCs due to the limitations associated with MSCs in terms of their regenerative capabilities [81]. MSCs have been used in some clinical bone regeneration. However, they have critical shortcomings, such as heterogeneity, differentiation potential, and migratory capacity [82, 83]. An alternative approach for generating in vitro OC models involves using iPSCs [84]. These cells are able to create unlimited cell sources for bone and cartilage regeneration and maintain the genetic background [85]. As mentioned before, in embryonic development, bone and cartilage rise from mesodermal origin. Therefore, in some approaches, recapitulating an intermediate step to generate mesodermal cells is necessary [86].
There are two research that applied mice iPSCs to develop organoids successfully [87, 88]. In the first one, Limraksasin et al. used a stepwise protocol, beginning with the administration of trans-retinoic acid to iPSCs to achieve mesodermal lineage commitment. In the next step, the pre-somatic mesoderm was differentiated into osteoblast via an osteogenic growth medium in a 3D sphere culture. After 10 days, the medium was replaced with a chondrogenic one and maintained for 21 days. The former medium results in the development of some cartilage-like tissue, which stimulates both osteogenesis and chondrogenesis gene expression. Cultivation in the later medium leads to a substantial area of cartilage tissue, with a significant increase in the chondrogenic gene expression. This induction additionally enhanced the commitment of mesodermal lineage, as demonstrated by the sequential expression of mesoderm marker genes. The cartilage-like tissues primarily emerged in the exterior layer, where a cluster of cells with chondrocyte morphology were found in lacunae. Manipulation of the induction protocol can alter the bone-to-cartilage ratio in model [87]. This indicates that iPSC-derived cells were not restricted to bony fate and maintained their potential to transdifferentiate in the cartilage pathway, which was confirmed in another study [80]. In contrast, the O’Connor research group initiated their strategy with chondrogenic induction of murine iPSCs by differentiating them in micromass culture and subsequently cultured in chondrogenic media for 45 days. These cells produced a cartilaginous matrix with s-GAGs and Col 2 and 6 that remained in the center of the organoids. They also demonstrated chondrogenic gene expression including Acan, Col 2, proteoglycan 4 (Prg4), and Sox9. Subsequently, the cell pellet was cultured in osteogenic media for 28 days [88]. Through this method, mature chondrocytes were triggered to differentiate into osteoblasts which consistently indicated the long-term potential of their iPSC source [89].
Moreover, human iPSC-derived chondrocytes could shape cartilage microtissues and form zonal structures [61]. Some articular cartilage-associated mRNA expression levels were significantly higher than the bony part of the organoid. However, SOX9, COL2, and COL1 were no different between these two parts [61]. Regardless of the vast opportunities iPSCs offer for cartilage regeneration, their application is limited regarding their expenses and recapitulating vivo functionality [90]. The iPSC-derived organoids are incapable of demonstrating the natural environments and have limitations in self-organizing with their lack of scalability [91, 92]. It is essential for researchers to carefully weigh the benefits and shortcomings of MSCs and iPSCs to reach their goals and obtain superior results.
Tissue resident cells
Another potential cell source for generating OC organoid is from joint-resident cells [93]. For instance, Periosteum-derived cells (PDC) can be the origin of osseous sections of organoids and hold noticeable promise for advancing regenerative medicine and tissue engineering applications in the field of orthopedics [61]. Periosteum has a connective bilayer texture that covers the bone surface contains osteoprogenitor cells and is responsible for providing nutrients, osteogenesis, and bone repair [94]. PDCs are involved in osteogenic development, homeostasis, and repair and exhibit strong potential for bone tissue regeneration due to their proliferative and osteogenic differentiation capabilities [95]. The periosteum, located within a mechanically dynamic environment, serves as a specialized microenvironment conducive to the maintenance and proliferation of pluripotent stem cells [96]. In comparison with BMSCs, the periosteum resident stem cells have a larger capacity to repair bone tissue [58]. PDCs-derived organoids have been successfully developed and demonstrated the mineralized part of the osteochondral-like tissue. After 21 days of culturing in a chondrogenic medium, they showed hypertrophic gene markers and formed a microtissue that got implanted and shaped the bony section of the organoid [61]. All in all, these organoids, irrespective of their origin, offer unprecedented means to study osteochondral tissue in vitro.
Cell-free osteochondral constructs
Cell-free osteochondral strategies do not contain living cells and are composed of biomaterial scaffolds that are designed to replicate the native extracellular matrix of OC tissue [97]. Due to the challenges of creating artificial biomaterials that reflect the chemical and topographical features of cellular environments [98], there is growing interest in using naturally derived ECM as a biological scaffold. This ECM scaffold is obtained through decellularization, which aims to eliminate native cells and genetic components like DNA and RNA while preserving its biochemical and biomechanical properties [99]. Recellularizing the decellularized ECM with patient cells, makes it possible to generate effective personalized tissues [100]. These scaffolds have been used clinically in various organs and successfully promoted tissue regeneration [101]. Acellular osteochondral ECM should preserve the connection of the bone-to-cartilage border and be affordable and biodegradable to restore OA and other defects [102, 103]. Rowland et al. applied a decellularized scaffold to develop joint organoids in a spatiotemporal controlled condition via site-specific, tunable, and inducible protein delivery systems. This construct serves as a valuable tool platform to monitor inflammatory signaling in osteochondral repair [104]. In addition, in a recent study, an efficient decellularized OC sheet was repopulated by BM-MSCs and demonstrated largely preserved interface integrity between cartilage and bone in the joint structure. Following the implantation of this scaffold, effective cell penetration, proliferation, and differentiation into osteoblasts and chondrocytes occurred, as well as ECM secretion were observed [105]. To achieve optimal results with decellularized tissues, it is essential to carefully control scaffold degradation properties and the simultaneous formation of cartilage and bone. Time plays a crucial role in this process, as the unpredictable degradation of decellularized scaffolds may not provide sufficient time for the development of mechanically competent tissue, especially within the challenging conditions of joint pathology [106].
Osteochondral targeted biomaterials, biomolecules, and physical factors
In addition to selecting appropriate cells, scaffolds and signaling factors (biochemical, chemo-physical, and physical signals) are crucial in tissue engineering. The generated organoid is expected to have a high resemblance to the natural tissue in chemical, physical, and functional aspects to succeed in research studies [107]. Biomaterials with/without growth factors present promising platforms to get the most out of cells’ capacities by obtaining microenvironments to achieve spatial complexity [108]. The complex hierarchical OC unit needs the application of both bone and cartilage-associated biomaterials for the repair and regeneration of defects.
Although some experiments have been developed in the scaffold-free environment [64, 88], others utilized polymers, bioceramics, and extracellular matrix (ECM)-derived materials (Table 1). Generally, a suitable natural or synthetic scaffold should fulfill these requirements: biocompatibility, bioactivity, protective mechanical strength, the capacity of adherence morphology, proliferation and/or differentiation of the embedded cells, ability to imitate the native ECM, bio-integration, and biodegradability [44, 109]. In addition, designing a scaffold for osteochondral engineering requires osteo-inductivity, osteo-conductivity, and mechanical properties such as appropriate pore size and surface roughness [110, 111]. In the following paragraphs, we will mainly summarize the suitable microenvironment for OC organoid engineering.
Bone-associated biomaterials, biomolecules, and physical factors
There are various growth factors associated with bone differentiation and regeneration, including parathyroid hormone-(PTH), insulin-like growth factor (IGFs), platelet-derived growth factor (PDGF), and bone morphogenetic proteins (BMP) [112]. These factors modulate cell migration, adhesion, proliferation, differentiation, and survival [113]. A proper scaffold can facilitate growth factor secretion that triggers the osteogenesis pathway [114]. In a time-dependent strategy, the O’Connor group applied BMP-2, a particular growth factor in the osteogenesis medium [88]. BMP-2 has a regenerative effect on bone defects and is capable of increasing the expression of alkaline phosphatase (ALP), and Runt-related transcription factor 2 (RUNX2). Therefore, it has been used for differentiating stem cells toward osteogenic lineage [115]. BMP2-included scaffolds can be applied to generate in vivo bone-related organoids for destructed bone tissue [116]. However, O’Connor et al. induced an osteochondral organoid in a scaffold and bioreactor-free system. In the final 28 days of the 3D culture, they induced osteogenic media, and their model appeared to have a dense mineralized outer layer rich in COL6. Moreover, the higher expression of OCN, ALP, RUNX2, bone sialoprotein (BSP), and COL1 genes confirmed the existence of the osseous outer [88]. The extracellular matrix is responsible for arranging the local distribution of growth factors by regulating their concentration and duration. Hence, producing a suitable matrix is vitally necessary for bone tissue engineering [117].
Bioceramics are one of the most practicable materials in bioengineering methods. They are well-known for their oxidation resistance, high mechanical strength, and biocompatibility [118]. Due to their porous structure, they can easily integrate with bone and due to their osteo-inductive properties can be utilized to recover osteochondral defects optimistically [119]. For instance, Li et al. used hydroxyapatite nanorod (HANR) which is an osteo-inductive bioceramic nanoparticle to produce OC organoid model [80]. This particular nanomaterial is synthesized from calcium hydroxide and ortho-phosphoric acid. It bears a resemblance to the mineral parts of bone tissue and is an effective substance in bone tissue engineering [120]. After treating cells and their produced ECM with Ascorbic acid, HANRs were added to induce osteogenesis for 21 days in a vitamin D3-contained medium. This HANR-included ECM helped them to generate a highly mineralized bony core and cartilage shell that showed higher level expression of bone-associated proteins including ALP, OCN, and RUNX2 which confirmed the increased osteogenesis efficiency of HANR-containing matrix [80].
Gelatin-based microcrystal is another biomaterial used in generating osteochondral organoid strategies. Gelatin which is a natural collagen-derived biopolymer, is widely used in tissue engineering, through diverse strategies, and supports cell growth with its biodegradability and biocompatibility [121]. Microcryogel is a small-scale scaffold that benefits organoid engineering by providing 3D microniche to load cell and growth factors and is utterly practicable in cell therapy due to its capability to get injected [122]. Various cells can get loaded on the microcryogel to generate a cell-laden construct with an oriented differentiation pathway [123]. Their structure enhances self-assembly toward a prearranged shape in 3D culture. This porous material appeared to support the stemness of MSCs, improve their secretion, reduce their senescence, and enhance cell-ECM interaction [124]. Yang et al., predifferentiated the microcryogel via hydroxyapatite (HYP) to develop osteogenic (OS) microcryogel. Their model demonstrated sufficient cell proliferation, interaction surface, and cytocompatibility. First, they seeded the cells on the microcryogel and induced differentiation via the osteogenic medium. Then, customized a meshed frame with defined space and loaded the OS-microcryogel at the bottom layer. Increased ALP, RUNX2, and calcium deposition affirmed the potential of OS-microcryogel in improving MSC differentiation. It also provides an environment for several blood vessels to grow, unlike chondrogenic-microcryogel. The organoid demonstrated correct interactions and cytokine secretion in vivo. This scaffold was superior to growth-factor-based methods in some aspects: the porous composition, cell viability, function protection, and fitting the defect size due to its small size [66].
Physical force is another compelling element in the OC organoids generation process. The osteochondral unit is subject to mechanical pressure [125]. Particularly, cartilage is frequently exposed to various mechanical forces, such as tension and shear stress [126]. Limraksasin et al. used an ultra-low attachment micro space plate to induce osteogenesis by subjecting it to shaking force. Physical force positively impacted osteogenesis and cell condensation, facilitating the self-organizing process of cells to form the organoid. This structure features a calcified inner region surrounded by a rich osteoblastic layer containing COL I [87].
Cartilaginous-associated biomaterials, biomolecules, and physical factors
In harmony with bone differentiation, the natural cartilaginous microenvironment requires various growth factors. These biomolecules orchestrate the pivotal pathways responsible for chondrogenic proliferation, differentiation, and apoptosis [127]. TGFs-β family is one of the effective growth factors in chondrogenic development. A combination of this growth factor with micromass, a 3D culture that provides a chondrogenic environment similar to embryonic development, has been suggested for studying chondrogenesis [128]. Accordingly, in a study by O’Connor, a scaffold-free micromass environment was utilized to form iPSC cells pellet and after digestion, the chondrogenic medium was added that contained TGF-β3 88. The chondrogenic center of the organoid was rich in sulfated glycosaminoglycans (s-GAGs) and COL2 and had activated pathways of ACAN; a resistant factor to compressive loads [129], proteoglycan 4 (Prg4); a joint/boundary lubricant [130], and Sox9; a major chondrocyte transcription factor [131]. In addition, their chondrogenic matrix was resistant to the pluripotent state and remarkably prevented cell reprogramming pathway [88]. Notably, the 3D chondrogenic culture environment demonstrated a lower capacity for undergoing osteogenic differentiation [66] highlighting the prominent role of matrix in preventing the reinduction of differentiated iPSCs. However, unlike the native structure, the shelly region in this study was observed in the center of the construct.
Alternatively, several research groups utilized scaffold materials that facilitate the regenerative capacity of cartilage. A suitable scaffold for cartilage cultivation should possess proper physical properties; stiffness, bio integration, flexibility, structural features; porosity, permeability, and functional traits; adhesion, proliferation, and differentiation capability [23, 55]. Furthermore, a 3D scaffold provides an environment for cartilage to produce and secrete the necessary cytokine and other proteins, and it also prevents dedifferentiation to fibroblast-like cells [132]. These biomaterials can be both natural and artificial, and each of them has its advantages and disadvantages. Natural ones such as hyaluronic acid (HA) have similarities to native tissue environments [133]. However, they have some limitations, including inflexibility, time-limited functionality, and low stability [134]. Therefore, synthetic scaffolds showed conspicuous efficiency. As mentioned earlier, HANR-included culture was used in a study to generate OC organoids. In combination with a cartilage-mediated medium that contained BMP6 and TGF- β3, subsequently, they led to a chondral shell in the last 21 days of cultivation. The exterior region appeared to have a high level of COL2 and GAG [80]. However, fabricated scaffolds may have insufficient biological properties and unexpected breakdowns. Consequently, this highlighted the superiority of combined natural and synthetic biomaterials [134].
To use both of these scaffolds, loaded umbilical cord MSCs-biomaterial were used in a more recent strategy to spontaneously form both cartilage and bone in two separate dishes. Yang et al. designed their experiment by mixing gelatin and 6% hydroxyapatite (HA) to fabricate a microcryogel suitable for chondrogenesis, that appeared to be effective in cell adherence, differentiation, and survival. After seeding the cells and embedding them on a poly (lactic-co-glycolic acid)/gelatin scaffold, the chondrogenic differentiation process began by using a TGF-β-included medium. This scaffold specifically provided a chondrogenic-specific environment that allowed cells to secrete GAG and express a sufficient level of COL2 and SOX9 and averted the osteogenic markers expression [66]. Nevertheless, the biophysical and biochemical aspects of organoid models, affect their functional effectiveness and their resemblance to native tissue.
Application of osteochondral organoids
Organoid cultures provide tremendous advantages, including the ability to generate from both healthy and diseased cell sources [135]. They can be expanded over extended periods, ensuring to preservation of their genetic stability [136, 137]. Furthermore, these cultures can be cryopreserved to generate biobanks for future research [138]. Compared with 2D culture, 3D organoids have more resemblance to physiological conditions and provide a platform to manipulate signaling pathways and perform genome editing [139]. As such, these cultures have been used for various applications including drug discovery, developmental biology, personalized diagnostics, and cell therapy (Fig. 4).
Study of bone and cartilage development and bone–cartilage crosstalk
3D cell-cultured methods are superior to animal and 2D models in various aspects. They can shape diverse cell types in a complex microstructure, allowing for demonstration of the cell-cell and cell-microenvironment interaction in all three dimensions [140]. In addition, manipulating the defined gradient concentration of growth factors, cytokines, essential nutrients, and waste products is more pragmatic in 3D cultures, compared to 2D monolayer [141]. Osteochondral organoids elucidated the molecular biology involved in the development, thus offering a comprehensive framework for studying the underlying mechanisms of articular cartilage and OC joint.
Several research investigated the crosstalk between bone and cartilage and their development through tissue-engineered approaches [142,143,144]. As we mentioned before, the cells and their produced messenger biomolecules such as growth factors affect other cells and their microenvironment in the complex joint structure. Therefore, almost all studies could directly/indirectly demonstrate the interaction between bone and cartilage. For instance, in Limraksasin’s study, not only the initiation osteogenic medium induced the osteogenic part, but also chondrogenic induction of iPSC enhanced the osteogenic markers such as Col1 and Osterix (Osx). Moreover, they demonstrated that endochondral ossification is regulated by some critical transcription factors, including both Sox9 and Osx [87]. Endochondral ossification is one of the most studied processes describing bone formation [58]. O’Connor et al. demonstrated the natural progression of the cartilage-to-bone interface during development in iPSC-derived organoids successfully. They illustrated that mature chondrocyte cells directly differentiate into osteocytes and osteoblasts to create bone tissue [88]. However, the complex molecular and cellular mechanisms may be further inquired to clarify endochondral ossification and other interactions.
Study of diseases models
The use of disease-specific organoids will facilitate the analysis of the cascade of molecular, cellular, and biomechanical signals and seek new treatments for degenerative joint diseases to improve patient care and outcomes [145]. Cartilage degradation, inflammation, and joint stiffness can be studied in 3D cultures [146]. 3D models also have the potential for exploring patient-specific genetic risk factors [88]. Disease-specific organoids can help to identify promising novel therapies and provide patient-derived platforms for drug screening that shed light on personalized medicine [147]. As mentioned earlier, OA is one of the most studied joint-related diseases, thus, generating OA organoids can be beneficial in the orthopedic field. For instance, Abraham et al. harvested diseased cells to study OA pathobiology and evaluate its potential treatments [93]. It is noteworthy that Interleukin-1β (IL-1β) is widely used as a pro-inflammatory cytokine to induce most joint diseases [148]. It is involved in cartilage destruction and inhibition of chondrogenic ECM formation in OA [149].
These models facilitate investigating diseases in numerous aspects. To illustrate, microRNA signaling is one of the alterations associated with OA progression [150] and their dysregulation can be studied in OA organoid models [151]. The advances in genetic engineering enable understanding of multiple biological phenotypes through 3D models [152]. Van Hoolwerff et al. studied mutation of genes encoding osteoprotegerin which is a critical protein in OA and their potential as hallmarks of this disease. They utilized organoid models to show that the mutations can directly affect chondrocytes and osteoblasts [153].
Drug testing programs
Animal models and 2D cell cultures have been used to deepen our knowledge of joint-related disorders, disease-modifying OA drugs (DMOADs) discovery, and to assure safety before clinical trials with human subjects [154]. However, due to the intrinsic species differences between human and animal models and ethical concerns, several obstacles appeared in testing novel drugs, investigating the metabolism pathway, and examining side effects [155]. 2D cell cultures are unable to recapitulate the heterogeneity of in vivo disease and unable to represent the in vivo physiological condition [156]. Therefore, organoid technology evolved as a potential approach to facilitate drug testing process [157]. Pharmaceutical companies can utilize 3D organoids for drug screening, as well as for evaluating drug metabolism, toxicity, and side effects [158]. This approach enables the delivery of precise data and facilitates the adaptation of studies for high-throughput performance [159]. Moreover, patient-derived organoids, with their maintained genetic heterogeneity, are superior platforms for personalized evaluation [160]. A shorter detection cycle, lack of organ toxicity, and cost-efficiency are some advantages of screening drugs on these cell cultures [161].
Tissue-engineered models broaden new opportunities for customized drug validation of genetic disorders [162], inflammatory diseases [93], and cancer [163]. Abraham et al. developed an organoid for testing an anti-inflammatory agent as a regenerative therapy for OA. The effects of Adenosine A2A receptor (A2AR) agonist were evaluated in an OA organoid. Although it successfully upregulates two transcription factors that reduce inflammation, it could not enhance differentiation and regeneration [93]. The recent development of DNA nanostructures can progress novel drug design and delivery systems with remarkable editability and biocompatibility features and may improve OC organoids [66]. There is a greater emphasis on utilizing bone or cartilage organoids for drug screening [164, 165]; however, the number of studies exploring osteochondral organoids as platforms for drug testing remains limited in the scientific literature. The various techniques used for organoid production have developed in very recent years, and further improvements are required to advance the accuracy, precision, and efficiency of drug monitoring of osteochondral-related diseases.
Osteochondral chip models
Organoid-on-a-chips are miniature systems that mimic the physiological and functional aspects of a particular organ/tissue by controlling tissue-specific microenvironments such as fluid flow, the culture condition, and interactions [163, 166, 167]. With the mechanical stimuli and bioactive cues, these models can be superior to organoids due to their high controllability. Biosensors, fabrication material, proper scaffolds, and cell sources assisted researchers in generating organoid-on-chips [168]. Chips-based joint models can be applied to advance our knowledge of joint pathology and the progress of promising novel treatments [169, 170]. Therefore, demonstrating OA phenotype and evaluating DMOAD in these systems is expected as ordinary (Table 2). In one study, iPSCs surrounded with gelatin scaffolds in a dual-flow bioreactor, and consequently, the generated OC chips faced IL-1 𝛽 treatment to show OA condition. This provided a real circumstance to learn the crosstalk between bone and cartilage along with screening Celecoxib, a commonly prescribed drug [171]. Additionally, MSCs have been used to engineer a more complex construct: osteochondral among other tissues including, adipose and synovial-like fibrous. A methacrylate gelatin hydrogel scaffold was applied to create a 3D environment and the efficiency of Naproxen and four underdeveloped drugs, including fibroblast growth factor 18, IL-1RA, sclerostin, and SM04690 were tested for OA treatments [170]. Although various chip models appeared as potential drug screening applications, they are still incapable of recapitulating the exact physiology of the natural tissues in mechanical studies [1].
Challenges of osteochondral organoids
While OC organoids simulate some of the critical aspects of the joint, their use in biomedical applications on a large scale is still limited by our current inability to fabricate a functional and structural unit of OC, maintain scalability, and cost-effectiveness as much as their safety [172,173,174]. Their application in biomedical treatments depends on organoid size, shape, cell composition, and survival. The generation of an osteochondral unit with the seamless gradient of the bone part containing nerve, blood vessel, and mineralized ECM, and a cartilaginous part as aneural, avascular, and non-mineralized, is not controllable. Managing these aspects to reach the optimal condition may be challenging, considering the time element and the value of long-term preservation [175].
Mimicking the whole osteochondral unit with its diverse cell sources such as osteoblast, osteocyte, chondrocyte, synoviocytes, and the microenvironment is complicated [176]. On other words, the simultaneous differentiation of cells in bony and cartilaginous parts of organoids using a set of different cells, biomaterials, and bioactive factors is noncontrollable. Furthermore, three-dimensional organoids often lack essential organ-specific cells, such as tissue-resident macrophages that play crucial roles in the immune responses against infections and diseases [177, 178]. Enhanced homeostatic mechanisms of macrophages can be used as a long-lasting treatment for OA [179]. Therefore, their presence in organoid models can be beneficial. However, current methods lack communication between the immune and musculoskeletal systems which is crucial for regulating tissue regeneration [180].
In vivo tissue-engineered grafts show limited capacity to regenerate the damaged tissue due to poor integration with host cartilage and the failure to retain structural integrity after insertion, resulting in reduced mechanical function [181, 182]. Moreover, they are not capable of achieving the same complexity of the interfacial tissue size and gradient structure as native organs and lack the crucial directional cues and physical, structural, and mechanical properties [183]. The mechanical properties of articular cartilage are highly divergent in different layers, and recapitulating this complexity is effortful [184].
The limited self-repairing capacity of cartilage makes restoration of its mechanical properties challenging [185]. Lack of certain zones impairs load-bearing capacity, affects biomechanical properties, and impedes joint health [186].
As previously indicated, specific existing techniques for organoid generation rely on costly growth factors, making large-scale production prohibitively [187]. Their short half-life, expensive costs, and weak portability limit growth factors’ practicality in organoid development [188]. Additionally, some methods necessitate the incorporation of engineered biomaterials to establish controllable conditions. These strategies require scaffolds that mimic the native architecture and function precisely [189]. In other words, the main challenge is to determine the combination of different scaffolds, cells, and biomaterials that perfectly create an OC microenvironment that enhances tissue growth and closely mimics the native tissue environment. However, researchers face challenges in monitoring and controlling every aspect of the development or/and implantation processes to develop similar organoids to natural tissues [190]. It is noteworthy that future ethical research is required to study organoid implantation in humans [191]. Furthermore, generating vascular networks within osteochondral organoids to support nutrient and oxygen diffusion throughout the structure is a critical challenge that needs to be addressed for long-term viability [192]. Overcoming these technical limitations can make organoid technology a remarkably effective biomedical clinical tool.
Conclusion and perspective
The inaccessibility of in vivo human samples and differences between animal models and human biology are the noticeable obstacles in studying joint development and diseased states [193]. The development of 3D organoids requires suitable cell origin, effective biomaterial, and controlled conditions. The specific type of model created may vary depending on the desired application and the researchers’ goals. Although these models offer several advantages, some drawbacks need to be addressed.
By subjecting osteochondral organoids to controlled mechanical stimulation, tissue maturation can be improved, and the development of physiologically relevant mechanical properties can be promoted [194]. Improvement of nutrient and waste exchange within osteochondral organoids directly affects their survival [195]. Therefore, establishing vascularization strategies, such as incorporating endothelial cells or bioactive factors, can improve the functionality and viability of organoid models [196].
Creating a multi-organoid platform that offers high physiological and clinical relevance for comprehensive mechanistic studies and preclinical assessment of potential DMOADs and disease-modifying antirheumatic drugs (DMARDs) can be a promising approach for the most common joint-related disease, OA and RA. A practical method to link these organoids and facilitate their mutual communication is through their integration into an organoid-on-a-chip system or co-culturing [170]. This can enhance the mimicry of native osteochondral tissue and promote cross-talk between different cell populations [197]. Moreover, using advanced biomaterials as well as technologies can provide a conducive microenvironment for osteochondral organoid development and maturation [198]. For instance, leveraging bioprinting technologies can precisely pattern multiple cell types and extracellular matrix components with tunable properties that create biomimetic osteochondral organoids [199, 200].
In conclusion, osteochondral organoids offer enormous promise in advancing our understanding of OC tissue development, disease mechanisms, and therapeutic application. They have the potential to revolutionize the field of musculoskeletal research and contribute to improved treatments for joint-related disorders such as osteoarthritis and cartilage injuries. Further research is required to generate physiologically relevant osteochondral organoids that are operational in regenerative medicine.
Data availability
Not applicable.
References
Huang J, Zhang L, Lu A, Liang C. Organoids as innovative models for bone and Joint diseases. Cells. 2023;12:1590.
Wildemann B, et al. Non-union bone fractures. Nat Reviews Disease Primers. 2021;7:57.
Yoshida A. Osteosarcoma: old and new challenges. Surg Pathol Clin. 2021;14:567–83.
Barnsley J, et al. Pathophysiology and treatment of osteoporosis: challenges for clinical practice in older people. Aging Clin Exp Res. 2021;33:759–73.
Yu H, Huang T, Lu WW, Tong L, Chen D. Osteoarthritis pain. Int J Mol Sci. 2022;23:4642.
Zhu W, et al. Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res. 2019;7:22.
Fields TR. The challenges of approaching and managing gout. Rheumatic Disease Clin. 2019;45:145–57.
Conigliaro P, et al. Challenges in the treatment of rheumatoid arthritis. Autoimmun rev. 2019;18:706–13.
Chen B, Huang W, Liao J. MDPI. 2023;12:5103.
Allen K, Thoma L, Golightly Y. Epidemiology of osteoarthritis. Osteoarthr Cartil. 2022;30:184–95.
Lepage SI, et al. Beyond cartilage repair: the role of the osteochondral unit in joint health and disease. Tissue Eng Part B: Reviews. 2019;25:114–25.
Trengove A, Di Bella C, O’Connor AJ. The challenge of cartilage integration: understanding a major barrier to chondral repair. Tissue Eng Part B: Reviews. 2022;28:114–28.
Xue N, et al. Bone tissue engineering in the treatment of bone defects. Pharmaceuticals. 2022;15:879.
Zhou Z et al. Engineering Innervated Musculoskeletal tissues for regenerative orthopedics and Disease modeling. Small. 2024:2310614.
Huch M, Knoblich JA, Lutolf MP, Martinez-Arias A. The hope and the hype of organoid research. Development. 2017;144:938–41.
Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19:671–87.
Takasato M, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–8.
Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5.
Crespo M, et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med. 2017;23:878–84.
Bagley JA, Reumann D, Bian S, Lévi-Strauss J, Knoblich JA. Fused cerebral organoids model interactions between brain regions. Nat Methods. 2017;14:743–51.
Takebe T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–4.
Chen S, Chen X, Geng Z, Su J. The horizon of bone organoid: a perspective on construction and application. Bioactive Mater. 2022;18:15–25.
Yu Y, Wang J, Li Y, Chen Y, Cui W. Cartilaginous organoids: advances, applications, and perspectives. Adv NanoBiomed Res. 2023;3:2200114.
Wu JY, Vunjak-Novakovic G. Bioengineering Human cartilage–bone tissues for modeling of Osteoarthritis. Stem Cells Dev. 2022;31:399–405.
Lee S-Y, Koo I-S, Hwang HJ, Lee DW. In Vitro three-dimensional (3D) cell culture tools for spheroid and organoid models. SLAS Discovery. 2023.
Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18:419–33.
Li N, et al. Synovial membrane mesenchymal stem cells: past life, current situation, and application in bone and joint diseases. Stem Cell Res Ther. 2020;11:1–12.
Breeland G, Sinkler MA, Menezes RG. StatPearls [Internet]. StatPearls Publishing; 2022.
Wang T, et al. Enhanced chondrogenesis from human embryonic stem cells. Stem cell Res. 2019;39:101497.
Bhattaram P, Chandrasekharan U. in Seminars in Cell & Developmental Biology. Elsevier; 86–93.
Kaya DÖ. Comparative kinesiology of the human body. Elsevier; 2020:115–47.
Wang X, et al. Identification of an ultrathin osteochondral interface tissue with specific nanostructure at the human knee joint. Nano Lett. 2022;22:2309–19.
Findlay DM, Kuliwaba JS. Bone–cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res. 2016;4:1–12.
Sharma AR, Jagga S, Lee S-S, Nam J-S. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci. 2013;14:19805–30.
Oliveira Silva M, Gregory JL, Ansari N, Stok KS. Molecular signaling interactions and transport at the osteochondral interface: a review. Front cell Dev Biology. 2020;8:750.
Li Z, Huang Z, Bai L. Cell interplay in osteoarthritis. Front cell Dev Biology. 2021;9:720477.
Jacob G, Shimomura K, Nakamura N. Osteochondral injury, management and tissue engineering approaches. Front Cell Dev Biology. 2020;8:580868.
Żylińska B, et al. Structure and pathologies of articular cartilage. vivo. 2021;35:1355–63.
Charlier E, et al. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol. 2019;165:49–65.
Lyndin M et al. Morphofunctional features of articular cartilage structure. Folia Med Cracov. 2019:59.
Li T, Chen S, Pei M. Contribution of neural crest-derived stem cells and nasal chondrocytes to articular cartilage regeneration. Cell Mol Life Sci. 2020;77:4847–59.
Mansour JM. Biomechanics of cartilage. Kinesiology: Mech Pathomechanics Hum Mov. 2003;2:66–79.
Gilliland KO, Kernick ET. Musculoskeletal tissues and anatomy. Clinical Foundations of Musculoskeletal Medicine: A Manual for Medical Students. 2021:11–21.
Haghwerdi F, et al. Application of bone and cartilage extracellular matrices in articular cartilage regeneration. Biomed Mater. 2021;16:042014.
Armiento AR, Alini M, Stoddart MJ. Articular fibrocartilage-why does hyaline cartilage fail to repair? Adv Drug Deliv Rev. 2019;146:289–305.
Stewart HL, Kawcak CE. The importance of subchondral bone in the pathophysiology of osteoarthritis. Front Veterinary Sci. 2018;5:178.
Ajami S, et al. Spatial links between subchondral bone architectural features and cartilage degeneration in osteoarthritic joints. Sci Rep. 2022;12:6694.
Mastbergen S, et al. Subchondral bone changes after joint distraction treatment for end stage knee osteoarthritis. Osteoarthr Cartil. 2022;30:965–72.
Zhu X, Chan YT, Yung PS, Tuan RS, Jiang Y. Subchondral bone remodeling: a therapeutic target for osteoarthritis. Front cell Dev Biology. 2021;8:607764.
Gupta SD, Workman J, Finnilä MA, Saarakkala S, Thambyah A. Subchondral bone plate thickness is associated with micromechanical and microstructural changes in the bovine patella osteochondral junction with different levels of cartilage degeneration. J Mech Behav Biomed Mater. 2022;129:105158.
Aho O-M, Finnilä M, Thevenot J, Saarakkala S, Lehenkari P. Subchondral bone histology and grading in osteoarthritis. PLoS ONE. 2017;12:e0173726.
Orava H, et al. Changes in subchondral bone structure and mechanical properties do not substantially affect cartilage mechanical responses–A finite element study. J Mech Behav Biomed Mater. 2022;128:105129.
Jensen C, Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci. 2020;7:33.
Silva-Pedrosa R, Salgado AJ, Ferreira PE. Revolutionizing disease modeling: the emergence of organoids in cellular systems. Cells. 2023;12:930.
Koh Y-G, et al. Optimal mechanical properties of a scaffold for cartilage regeneration using finite element analysis. J Tissue Eng. 2019;10:2041731419832133.
Abe K, et al. Engraftment of allogeneic iPS cell-derived cartilage organoid in a primate model of articular cartilage defect. Nat Commun. 2023;14:804.
Kleuskens MW et al. Neo-cartilage formation using human nondegenerate versus osteoarthritic chondrocyte‐derived cartilage organoids in a viscoelastic hydrogel. J Orthop Research®. 2023.
Nilsson Hall G, et al. Developmentally engineered callus organoid bioassemblies exhibit predictive in vivo long bone healing. Adv Sci. 2020;7:1902295.
Tam WL, et al. Human pluripotent stem cell-derived cartilaginous organoids promote scaffold-free healing of critical size long bone defects. Stem Cell Res Ther. 2021;12:1–16.
Roa-Linares VC, Escudero-Flórez M, Vicente-Manzanares M, Gallego-Gómez. J. C. Host cell targets for unconventional antivirals against RNA viruses. Viruses. 2023;15:776.
Hall GN, et al. Patterned, organoid-based cartilaginous implants exhibit zone specific functionality forming osteochondral-like tissues in vivo. Biomaterials. 2021;273:120820.
Solorio LD, et al. Spatially organized differentiation of mesenchymal stem cells within biphasic microparticle-incorporated high cell density osteochondral tissues. Adv Healthc Mater. 2015;4:2306–13.
Lozito TP, et al. Three-dimensional osteochondral microtissue to model pathogenesis of osteoarthritis. Stem Cell Res Ther. 2013;4:1–6.
Muraglia A, et al. Formation of a chondro-osseous rudiment in micromass cultures of human bone-marrow stromal cells. J Cell Sci. 2003;116:2949–55.
Chang S-J, et al. Cytokine levels and neuropsychological function among patients with Attention-Deficit/Hyperactivity disorder and atopic diseases. J Personalized Med. 2022;12:1155.
Yang Z, et al. In situ self-assembled organoid for osteochondral tissue regeneration with dual functional units. Bioactive Mater. 2023;27:200–15.
Jauković A, et al. Modulating stemness of mesenchymal stem cells from exfoliated deciduous and permanent teeth by IL-17 and bFGF. J Cell Physiol. 2021;236:7322–41.
Naji A, et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76:3323–48.
Hoang DH, et al. Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum-and xeno-free condition. Front Mol Biosci. 2020;7:119.
Liu H, et al. Immunomodulatory effects of mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles in rheumatoid arthritis. Front Immunol. 2020;11:1912.
Zhang Y, et al. An in vitro comparative study of multisource derived human mesenchymal stem cells for bone tissue engineering. Stem Cells Dev. 2018;27:1634–45.
Han Y, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Therapy. 2022;7:92.
Kossl J, et al. Antiapoptotic properties of mesenchymal stem cells in a mouse model of corneal inflammation. Stem Cells Dev. 2021;30:418–27.
Pittenger MF, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regenerative Med. 2019;4:22.
Hosseini S, Taghiyar L, Safari F, Baghaban Eslaminejad M. Regenerative medicine applications of mesenchymal stem cells. Cell Biology and Translational Medicine: Approaches for Diverse Diseases and Conditions. 2018;2:115–141.
Urlić I, Ivković A. Cell sources for cartilage repair—biological and clinical perspective. Cells. 2021;10:2496.
Deng Z et al. Narrative review of the choices of stem cell sources and hydrogels for cartilage tissue engineering. Annals Translational Med. 2020:8.
Andalib N, Kehtari M, Seyedjafari E, Motamed N, Matin MM. In vivo bone regeneration using a bioactive nanocomposite scaffold and human mesenchymal stem cells. Cell Tissue Banking. 2021:1–11.
Chijimatsu R et al. Characterization of mesenchymal stem cell-like cells derived from human iPSCs via neural crest development and their application for osteochondral repair. Stem Cells Int. 2017:2017.
Li ZA, et al. Articular tissue-mimicking organoids derived from mesenchymal stem cells and induced pluripotent stem cells. Organoids. 2022;1:135–48.
Barui S, Ghosh D, Laurencin CT. Osteochondral regenerative engineering: challenges, state-of-the-art and translational perspectives. Regenerative Biomaterials. 2023;10:rbac109.
Costa LA, et al. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell Mol Life Sci. 2021;78:447–67.
Zhou T, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:1–24.
Rana D, Kumar S, Webster TJ, Ramalingam M. Impact of induced pluripotent stem cells in bone repair and regeneration. Curr Osteoporos Rep. 2019;17:226–34.
Nakamura A, et al. Bio-3D printing iPSC-derived human chondrocytes for articular cartilage regeneration. Biofabrication. 2021;13:044103.
Ferretti E, Hadjantonakis A-K. Mesoderm specification and diversification: from single cells to emergent tissues. Curr Opin Cell Biol. 2019;61:110–6.
Limraksasin P, et al. In vitro fabrication of hybrid bone/cartilage complex using mouse induced pluripotent stem cells. Int J Mol Sci. 2020;21:581.
O’Connor SK, Katz DB, Oswald SJ, Groneck L, Guilak F. Formation of osteochondral organoids from murine induced pluripotent stem cells. Tissue Eng Part A. 2021;27:1099–109.
Rolian C. Endochondral ossification and the evolution of limb proportions. Wiley Interdisciplinary Reviews: Dev Biology. 2020;9:e373.
Zhang M, et al. Recapitulation of cartilage/bone formation using iPSCs via biomimetic 3D rotary culture approach for developmental engineering. Biomaterials. 2020;260:120334.
Turhan AG, et al. iPSC-derived organoids as therapeutic models in regenerative medicine and oncology. Front Med. 2021;8:728543.
Lee H, Son M-Y. Current challenges associated with the use of human induced pluripotent stem cell-derived organoids in regenerative medicine. Int J Stem Cells. 2021;14:9.
Abraham DM, et al. Self-assembling human skeletal organoids for disease modeling and drug testing. J Biomedical Mater Res Part B: Appl Biomaterials. 2022;110:871–84.
Zhang W, et al. Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J Orthop Translation. 2022;33:41–54.
Cao R et al. Characterization and potential of periosteum-derived cells: an overview. Front Med. 2023:10.
Jeyaraman M et al. Osteogenic and chondrogenic potential of periosteum-derived mesenchymal stromal cells: do they hold the key to the future? Pharmaceuticals. 2021;14:1133.
Chen R, Pye JS, Li J, Little CB, Li J. J. Multiphasic scaffolds for the repair of osteochondral defects: outcomes of preclinical studies. Bioactive Mater. 2023;27:505–45.
Burroughs L, et al. Discovery of synergistic material-topography combinations to achieve immunomodulatory osteoinductive biomaterials using a novel in vitro screening method: the ChemoTopoChip. Biomaterials. 2021;271:120740.
Neishabouri A, Soltani Khaboushan A, Daghigh F, Kajbafzadeh A-M, Majidi Zolbin M. Decellularization in tissue engineering and regenerative medicine: evaluation, modification, and application methods. Front Bioeng Biotechnol. 2022;10:805299.
Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. Biomed Res Int. 2017:2017.
Giobbe GG, et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun. 2019;10:5658.
Tian G, et al. Cell-free decellularized cartilage extracellular matrix scaffolds combined with interleukin 4 promote osteochondral repair through immunomodulatory macrophages: in vitro and in vivo preclinical study. Acta Biomater. 2021;127:131–45.
Fu J-N, et al. Scaffold-based tissue engineering strategies for osteochondral repair. Front Bioeng Biotechnol. 2022;9:812383.
Rowland CR, et al. Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs. Biomaterials. 2018;177:161–75.
Taghiyar L, Asadi H, Baghaban Eslaminejad M. A bioscaffold of decellularized whole osteochondral sheet improves proliferation and differentiation of loaded mesenchymal stem cells in a rabbit model. Cell Tissue Banking. 2023:1–14.
Marzi J, et al. Marker-independent monitoring of in vitro and in vivo degradation of Supramolecular polymers Applied in Cardiovascular in situ tissue Engineering. Front Cardiovasc Med. 2022;9:885873.
Wei W, Dai H. Articular cartilage and osteochondral tissue engineering techniques: recent advances and challenges. Bioactive Mater. 2021;6:4830–55.
D’Costa K, et al. Biomaterials and culture systems for development of organoid and organ-on-a-chip models. Ann Biomed Eng. 2020;48:2002–27.
Shimojo AAM et al. Scaffolds for tissue engineering: a state-of-the-art review concerning types, properties, materials, processing, and characterization. Racing Surface: Antimicrob Interface Tissue Eng. 2020:647–76.
Wang Z, et al. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliv Rev. 2021;174:504–34.
Zhou J, et al. Study on the influence of scaffold morphology and structure on osteogenic performance. Front Bioeng Biotechnol. 2023;11:1127162.
Hsu EL, Stock SR. Growth factors, carrier materials, and bone repair. Bone Regulators Osteoporos Therapy. 2020:121–56.
Toosi S, Behravan J. Osteogenesis and bone remodeling: a focus on growth factors and bioactive peptides. BioFactors. 2020;46:326–40.
Oliveira ÉR, et al. Advances in growth factor delivery for bone tissue engineering. Int J Mol Sci. 2021;22:903.
Min SK, Kim M, Park J-B. Bone morphogenetic protein 2–enhanced osteogenic differentiation of stem cell spheres by regulation of Runx2 expression. Experimental Therapeutic Med. 2020;20:1–1.
Dai K, et al. Generation of rhBMP-2-induced juvenile ossicles in aged mice. Biomaterials. 2020;258:120284.
Lin W, et al. Osteomodulin positively regulates osteogenesis through interaction with BMP2. Cell Death Dis. 2021;12:147.
Zahir A, Mahmood U, Nazir A, Hussain T, Abid S. in Medical Textiles from Natural Resources. Elsevier; 2022:43–86.
Pina S, Rebelo R, Correlo VM, Oliveira JM, Reis RL. Bioceramics for osteochondral tissue engineering and regeneration. Osteochondral Tissue Engineering: Nanatechnol Scaffolding-Related Developments Translation. 2018:53–75.
Yu F, et al. Biomimetic hydroxyapatite nanorods promote bone regeneration via accelerating osteogenesis of BMSCs through T cell-derived IL-22. ACS Nano. 2022;16:755–70.
Skopinska-Wisniewska J, Tuszynska M, Olewnik-Kruszkowska E. Comparative study of gelatin hydrogels modified by various cross-linking agents. Materials. 2021;14:396.
Marsico G, Martin-Saldaña S, Pandit A. Therapeutic biomaterial approaches to alleviate chronic limb threatening ischemia. Adv Sci. 2021;8:2003119.
Zhang X, et al. Implanted 3D gelatin microcryogel enables low-dose cell therapy for osteoarthritis by preserving the viability and function of umbilical cord MSCs. Chem Eng J. 2021;416:129140.
Zhang Z, et al. Injectable conductive micro-cryogel as a muscle stem cell carrier improves myogenic proliferation, differentiation and in situ skeletal muscle regeneration. Acta Biomater. 2022;151:197–209.
Ansari S, Khorshidi S, Karkhaneh A. Engineering of gradient osteochondral tissue: from nature to lab. Acta Biomater. 2019;87:41–54.
Belluzzi E, et al. Human cartilage biomechanics: experimental and theoretical approaches towards the identification of mechanical properties in healthy and osteoarthritic conditions. Processes. 2023;11:1014.
Fathi-Achachelouei M, Keskin D, Bat E, Vrana NE, Tezcaner A. Dual growth factor delivery using PLGA nanoparticles in silk fibroin/PEGDMA hydrogels for articular cartilage tissue engineering. J Biomedical Mater Res Part B: Appl Biomaterials. 2020;108:2041–62.
Zuliani CC et al. Micromass cultures are effective for differentiation of human amniotic fluid stem cells into chondrocytes. Clinics. 2018:73.
Hayes AJ, Melrose J. Aggrecan, the primary weight-bearing cartilage proteoglycan, has context-dependent, cell-directive properties in embryonic development and neurogenesis: aggrecan glycan side chain modifications convey interactive biodiversity. Biomolecules. 2020;10:1244.
Qiao Z, et al. Proteoglycan 4 predicts tribological properties of repaired cartilage tissue. Theranostics. 2020;10:2538.
Lefebvre V, Angelozzi M, Haseeb A. SOX9 in cartilage development and disease. Curr Opin Cell Biol. 2019;61:39–47.
Eltom A, Zhong G, Muhammad A. Scaffold techniques and designs in tissue engineering functions and purposes: a review. Adv Mater Sci Eng. 2019:2019.
Agarwal G, Agiwal S, Srivastava A. Hyaluronic acid containing scaffolds ameliorate stem cell function for tissue repair and regeneration. Int J Biol Macromol. 2020;165:388–401.
Wasyłeczko M, Sikorska W, Chwojnowski A. Review of synthetic and hybrid scaffolds in cartilage tissue engineering. Membranes. 2020;10:348.
Kim J, Koo B-K, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–84.
Fujii M, Matano M, Nanki K, Sato T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat Protoc. 2015;10:1474–85.
Fujii M, Sato T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat Mater. 2021;20:156–69.
Gunti S, Hoke AT, Vu KP. London Jr, N. R. Organoid and spheroid tumor models: techniques and applications. Cancers. 2021;13:874.
Zhou Z, Cong L, Cong X. Patient-derived organoids in precision medicine: drug screening, organoid-on-a-chip and living organoid biobank. Front Oncol. 2021;11:762184.
Cacciamali A, Villa R, Dotti S. 3D cell cultures: evolution of an ancient tool for new applications. Front Physiol. 2022;13:836480.
Fontoura JC, et al. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater Sci Engineering: C. 2020;107:110264.
Scotti C, et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci. 2013;110:3997–4002.
Stüdle C, et al. Spatially confined induction of endochondral ossification by functionalized hydrogels for ectopic engineering of osteochondral tissues. Biomaterials. 2018;171:219–29.
Whelan IT, et al. A microphysiological model of bone development and regeneration. Biofabrication. 2023;15:034103.
Kim S, Cho AN, Min S, Kim S, Cho SW. Organoids for advanced therapeutics and disease models. Adv Ther. 2019;2:1800087.
Bartolotti I, Roseti L, Petretta M, Grigolo B, Desando G. A roadmap of in vitro models in osteoarthritis: a focus on their biological relevance in regenerative medicine. Journal of clinical medicine. 2021;10:1920.
Tang X-Y, et al. Human organoids in basic research and clinical applications. Signal Transduct Target Therapy. 2022;7:168.
Wahafu P, Xu A, Zhao B, Tuo Y, Yang J. Circ_0005526 contributes to interleukin-1β-induced chondrocyte injury in osteoarthritis via upregulating transcription factor 4 by interacting with miR-142-5p. Bioengineered. 2022;13:8407–18.
Li M, et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022;140:23–42.
Liu H et al. MicroRNA expression in osteoarthritis: a meta-analysis. Clin Experimental Med. 2023:1–13.
Sun Y, Wu Q, Dai K, You Y, Jiang W. Generating 3D-cultured organoids for pre-clinical modeling and treatment of degenerative joint disease. Signal Transduct Target Therapy. 2021;6:380.
Menche C, Farin HF. Strategies for genetic manipulation of adult stem cell-derived organoids. Exp Mol Med. 2021;53:1483–94.
van Hoolwerff M et al. Mutation in the CCAL1 locus accounts for bidirectional process of human subchondral bone turnover and cartilage mineralization. Rheumatology. Oxford; 2022.
Kitaeva KV, Rutland CS, Rizvanov AA, Solovyeva VV. Cell culture based in vitro test systems for anticancer drug screening. Front Bioeng Biotechnol. 2020;8:322.
Brancato V, Oliveira JM, Correlo VM, Reis RL, Kundu SC. Could 3D models of cancer enhance drug screening? Biomaterials. 2020;232:119744.
Benam KH, et al. Engineered in vitro disease models. Annu Rev Pathol. 2015;10:195–262.
Xu H, et al. Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Experimental Hematol Oncol. 2018;7:1–12.
Du Y, et al. Development of a miniaturized 3D organoid culture platform for ultra-high-throughput screening. J Mol Cell Biol. 2020;12:630–43.
O’Connell L, Winter DC. Organoids: past learning and future directions. Stem Cells Dev. 2020;29:281–9.
Kondo J, Inoue M. Application of cancer organoid model for drug screening and personalized therapy. Cells. 2019;8:470.
Nie X, et al. Novel organoid model in drug screening: past, present, and future. Liver Res. 2021;5:72–8.
Wei X, et al. Germline lysine-specific demethylase 1 (LSD1/KDM1A) mutations confer susceptibility to multiple myeloma. Cancer Res. 2018;78:2747–59.
Subramaniam D, et al. Suppressing STAT5 signaling affects osteosarcoma growth and stemness. Cell Death Dis. 2020;11:149.
Zhao D, Saiding Q, Li Y, Tang Y, Cui W. Bone organoids: recent advances and Future challenges. Adv Healthc Mater. 2023:2302088.
Lin W, Wang M, Xu L, Tortorella M, Li G. Cartilage organoids for cartilage development and cartilage-associated disease modeling. Front Cell Dev Biology. 2023;11:1125405.
Wang Y, Qin J. Advances in human organoids-on-chips in biomedical research. Life Med. 2023;2:lnad007.
Singh YP, Moses JC, Bhardwaj N, Mandal BB. Overcoming the dependence on animal models for osteoarthritis therapeutics–the promises and prospects of in vitro models. Adv Healthc Mater. 2021;10:2100961.
Hu Y, et al. Bone/cartilage organoid on-chip: construction strategy and application. Bioactive Mater. 2023;25:29–41.
Rothbauer M, et al. Establishment of a human three-dimensional chip-based chondro-synovial coculture joint model for reciprocal cross talk studies in arthritis research. Lab Chip. 2021;21:4128–43.
Li Z, et al. Human mesenchymal stem cell-derived miniature Joint System for Disease modeling and drug testing. Adv Sci. 2022;9:2105909.
Lin Z, et al. Osteochondral tissue chip derived from iPSCs: modeling OA pathologies and testing drugs. Front Bioeng Biotechnol. 2019;7:411.
Andrews MG, Kriegstein AR. Challenges of organoid research. Annu Rev Neurosci. 2022;45:23–39.
Bose S, Clevers H, Shen X. Promises and challenges of organoid-guided precision medicine. Med. 2021;2:1011–26.
Huang Y, et al. Research progress, challenges, and breakthroughs of organoids as disease models. Front Cell Dev Biology. 2021;9:740574.
Yi SA, Zhang Y, Rathnam C, Pongkulapa T, Lee K. B. Bioengineering approaches for the advanced organoid research. Adv Mater. 2021;33:2007949.
Semenistaja S, Skuja S, Kadisa A, Groma V. Healthy and osteoarthritis-affected joints facing the cellular crosstalk. Int J Mol Sci. 2023;24:4120.
Fernandes TL, Gomoll AH, Bueno DF. Macrophage: a potential target on cartilage regeneration. Front Immunol. 2020;11:480818.
Hofer M, Lutolf MP. Engineering organoids. Nat Reviews Mater. 2021;6:402–20.
Menarim BC, MacLeod JN, Dahlgren LA. Bone marrow mononuclear cells for joint therapy: the role of macrophages in inflammation resolution and tissue repair. World J Stem Cells. 2021;13:825.
Xiong Y, et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Military Med Res. 2022;9:65.
Angotzi GN, et al. Integrated micro-devices for a lab-in-organoid technology platform: current status and future perspectives. Front NeuroSci. 2022;16:842265.
Hsia GSP, Esposito J, da Rocha LA, Ramos SLG, Okamoto OK. Clinical application of human induced pluripotent stem cell-derived organoids as an alternative to organ transplantation. Stem Cells Int. 2021;2021:1–16.
Ansari M. Bone tissue regeneration: biology, strategies and interface studies. Prog Biomater. 2019;8:223–37.
Eschweiler J, et al. The biomechanics of cartilage—An overview. Life. 2021;11:302.
Davis S, Roldo M, Blunn G, Tozzi G, Roncada T. Influence of the mechanical environment on the regeneration of osteochondral defects. Front Bioeng Biotechnol. 2021;9:603408.
Choe R, Devoy E, Jabari E, Packer JD, Fisher JP. Biomechanical aspects of osteochondral regeneration: implications and strategies for three-dimensional bioprinting. Tissue Eng Part B: Reviews. 2022;28:766–88.
Ren X, Zhao M, Lash B, Martino MM, Julier Z. Growth factor engineering strategies for regenerative medicine applications. Front Bioeng Biotechnol. 2020;7:469.
Zhao Z, et al. Organoids. Nat Reviews Methods Primers. 2022;2:94.
Morouço P, Fernandes C, Lattanzi W. Challenges and innovations in osteochondral regeneration: insights from biology and inputs from bioengineering toward the optimization of tissue engineering strategies. J Funct Biomaterials. 2021;12:17.
Matthys OB, Silva AC, McDevitt TC. Engineering human organoid development ex vivo—challenges and opportunities. Curr Opin Biomedical Eng. 2020;13:160–7.
de Jongh D, Massey EK, Bunnik EM. Organoids: a systematic review of ethical issues. Stem Cell Res Ther. 2022;13:337.
Scheinpflug J, et al. Journey into bone models: a review. Genes. 2018;9:247.
Heydari Z, et al. Organoids: a novel modality in disease modeling. Bio-design Manuf. 2021;4:689–716.
Charelli LE, Ferreira JP, Naveira-Cotta CP, Balbino TA. Engineering mechanobiology through organoids‐on‐chip: a strategy to boost therapeutics. J Tissue Eng Regen Med. 2021;15:883–99.
Zhao X, et al. Review on the vascularization of organoids and organoids-on-a-C hip. Front Bioeng Biotechnol. 2021;9:637048.
Li A et al. Vascularization of a Bone Organoid Using Dental Pulp Stem Cells. Stem Cells International. 2023:2023.
Zahmatkesh E, et al. Evolution of organoid technology: lessons learnt in co-culture systems from developmental biology. Dev Biol. 2021;475:37–53.
Park S, Cho S-W. Bioengineering toolkits for potentiating organoid therapeutics. Adv Drug Deliv Rev. 2024:115238.
Ren Y et al. Developments and opportunities for 3D bioprinted organoids. Int J Bioprinting. 2021:7.
Maharjan S et al. Advanced 3D imaging and organoid bioprinting for biomedical research and therapeutic applications. Adv Drug Deliv Rev. 2024:115237.
Shi X, Zhou J, Zhao Y, Li L, Wu H. Gradient-regulated hydrogel for interface tissue engineering: steering simultaneous osteo/chondrogenesis of stem cells on a chip. Adv Healthc Mater. 2013;2:846–53.
Lin H, Lozito TP, Alexander PG, Gottardi R, Tuan RS. Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1β. Mol Pharm. 2014;11:2203–12.
Mondadori C, et al. Recapitulating monocyte extravasation to the synovium in an organotypic microfluidic model of the articular joint. Biofabrication. 2021;13:045001.
Tuerlings M, et al. Development of a human osteochondral construct on a microfluidic chip–to advance functional studies of osteoarthritis risk genes. Osteoarthr Cartil. 2021;29:S108–9.
Pirosa A, et al. An in vitro chondro-osteo-vascular triphasic model of the osteochondral complex. Biomaterials. 2021;272:120773.
Acknowledgements
The present research was financially supported by the Royan Institute and the Iranian Council of Stem Cell Research and Technology (ICSCR).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
MF: Conceptualization, writing original draft preparation; MGH and BF: writing review and editing; LT: project administration and editing; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Faeed, M., Ghiasvand, M., Fareghzadeh, B. et al. Osteochondral organoids: current advances, applications, and upcoming challenges. Stem Cell Res Ther 15, 183 (2024). https://doi.org/10.1186/s13287-024-03790-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03790-5